A Pilot Study on Structural Changes of Choroidal Vasculature Following Intravitreal Anti-VEGF Injection in Neovascular Age-Related Macular Degeneration: Faricimab vs Ranibizumab
Abstract
1. Introduction
2. Materials and Methods
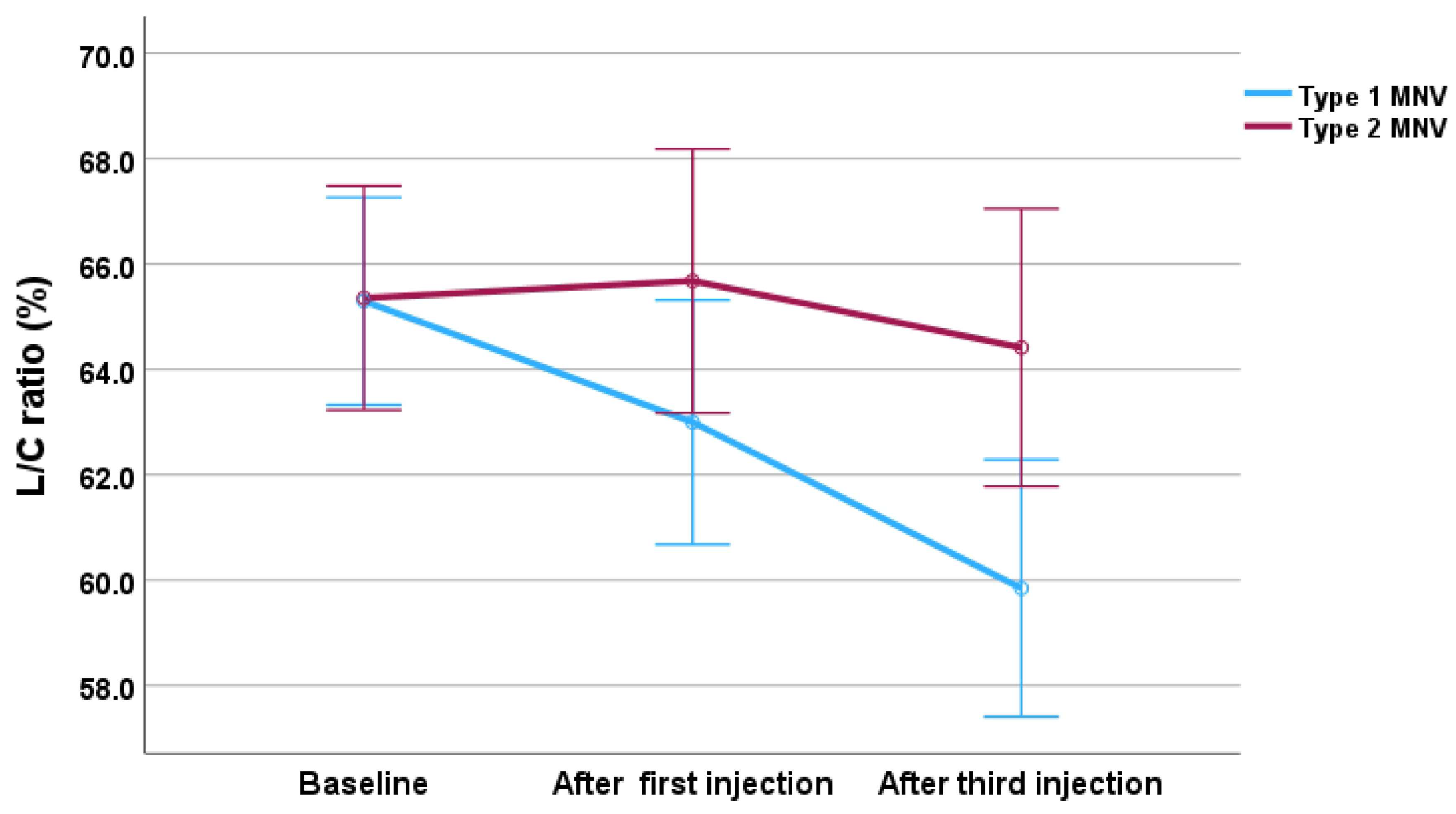
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ang-1 | angiopoietin 1 |
| Ang-2 | angiopoietin-2 |
| BCVA | best-corrected visual acuity |
| CCT | central choroidal thickness |
| FA | fluorescein angiography |
| ICGA | indocyanine green angiography |
| IRF | intraretinal fluid |
| IQR | interquartile range |
| LCA | luminal choroidal area |
| L/C ratio | the ratio of luminal area to choroidal area |
| MNV | macular neovascularization |
| nAMD | neovascular age-related macular degeneration |
| OCT | optical coherence tomography |
| RPE | retinal pigment epithelium |
| SCA | stromal choroidal area |
| SRF | subretinal fluid |
| TCA | total choroidal area |
| Tie-2 | tyrosine kinase with immunoglobulin and epidermal growth factor homology domains 2 |
| VEGF | vascular endothelial growth factor |
References
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef]
- Mathis, T.; Holz, F.G.; Sivaprasad, S.; Yoon, Y.H.; Eter, N.; Chen, L.-J.; Koh, A.; de Souza, E.C.; Staurenghi, G. Characterisation of macular neovascularisation subtypes in age-related macular degeneration to optimise treatment outcomes. Eye 2023, 37, 1758–1765. [Google Scholar] [CrossRef]
- Al-Zamil, W.M.; Yassin, S.A. Recent developments in age-related macular degeneration: A review. Clin. Interv. Aging 2017, 12, 1313–1330. [Google Scholar] [CrossRef] [PubMed]
- Liberski, S.; Wichrowska, M.; Kocięcki, J. Aflibercept versus Faricimab in the Treatment of Neovascular Age-Related Macular Degeneration and Diabetic Macular Edema: A Review. Int. J. Mol. Sci. 2022, 23, 9424. [Google Scholar] [CrossRef] [PubMed]
- Penha, F.M.; Masud, M.; Khanani, Z.A.; Thomas, M.; Fong, R.D.; Smith, K.; Chand, A.; Khan, M.; Gahn, G.; Melo, G.B.; et al. Review of real-world evidence of dual inhibition of VEGF-A and ANG-2 with faricimab in NAMD and DME. Int. J. Retin. Vitr. 2024, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Ferro Desideri, L.; Barra, F.; Ferrero, S.; Traverso, C.E.; Nicolò, M. Clinical efficacy and safety of ranibizumab in the treatment of wet age-related macular degeneration. Expert Opin. Biol. Ther. 2019, 19, 735–751. [Google Scholar] [CrossRef]
- Sonoda, S.; Sakamoto, T.; Yamashita, T.; Shirasawa, M.; Uchino, E.; Terasaki, H.; Tomita, M. Choroidal structure in normal eyes and after photodynamic therapy determined by binarization of optical coherence tomographic images. Investig. Opthalmol. Vis. Sci. 2014, 55, 3893–3899. [Google Scholar] [CrossRef]
- Sadeghi, E.; Valsecchi, N.; Rahmanipour, E.; Ejlalidiz, M.; Hasan, N.; Vupparaboina, K.K.; Ibrahim, M.N.; Rasheed, M.A.; Baek, J.; Iannetta, D.; et al. Choroidal biomarkers in age-related macular degeneration. Surv. Ophthalmol. 2025, 70, 167–183. [Google Scholar] [CrossRef]
- Ambiya, V.; Goud, A.; Rasheed, M.A.; Gangakhedkar, S.; Vupparaboina, K.K.; Chhablani, J. Retinal and choroidal changes in steroid-associated central serous chorioretinopathy. Int. J. Retin. Vitr. 2018, 4, 11. [Google Scholar] [CrossRef]
- Foo, V.H.X.; Gupta, P.; Nguyen, Q.D.; Chong, C.C.Y.; Agrawal, R.; Cheng, C.-Y.; Yanagi, Y. Decrease in Choroidal Vascularity Index of Haller’s layer in diabetic eyes precedes retinopathy. BMJ Open Diabetes Res. Care 2020, 8, e001295. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, L.; Chen, M.; Liu, L. Choroidal vascular changes in age-related macular degeneration: A protocol for systematic review and meta-analysis. Medicine 2020, 99, e23200. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, A.; Benatti, E.; Cozzi, M.; Erba, S.; Vaishnavi, S.; Vupparaboina, K.K.; Staurenghi, G.; Chhablani, J.; Gillies, M.; Viola, F. Choroidal Structural Changes Correlate With Neovascular Activity in Neovascular Age Related Macular Degeneration. Investig. Opthalmol. Vis. Sci. 2018, 59, 3836–3841. [Google Scholar] [CrossRef] [PubMed]
- Hirai, H.; Yamashita, M.; Ijuin, N.; Jimura, H.; Nishi, T.; Ogata, N.; Ueda, T. Evaluation of Choroidal Structure in Type 1 Macular Neovascularization Using Different Optical Coherence Tomography Analyses: Scale Bar and Binarization. J. Clin. Med. 2024, 13, 1383. [Google Scholar] [CrossRef]
- Pellegrini, M.; Bernabei, F.; Mercanti, A.; Sebastiani, S.; Peiretti, E.; Iovino, C.; Casini, G.; Loiudice, P.; Scorcia, V.; Giannaccare, G. Short-term choroidal vascular changes after aflibercept therapy for neovascular age-related macular degeneration. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 911–918. [Google Scholar] [CrossRef]
- Boscia, G.; Pozharitskiy, N.; Grassi, M.O.; Borrelli, E.; D’aDdario, M.; Alessio, G.; Boscia, F.; Viggiano, P. Choroidal remodeling following different anti-VEGF therapies in neovascular AMD. Sci. Rep. 2024, 14, 1941. [Google Scholar] [CrossRef]
- Iida, T.; Gomi, F.; Yasukawa, T.; Yamashiro, K.; Honda, S.; Maruko, I.; Kataoka, K. Japanese clinical guidelines for neovascular age-related macular degeneration. Jpn. J. Ophthalmol. 2025, 69, 639–660. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Moon, B.-H.; Kim, Y.; Kim, S.-Y. Twenty Years of Anti-Vascular Endothelial Growth Factor Therapeutics in Neovascular Age-Related Macular Degeneration Treatment. Int. J. Mol. Sci. 2023, 24, 13004. [Google Scholar] [CrossRef]
- Stapor, P.C.; Sweat, R.S.; Dashti, D.C.; Betancourt, A.M.; Murfee, W.L. Pericyte dynamics during angiogenesis: New insights from new identities. J. Vasc. Res. 2014, 51, 163–174. [Google Scholar] [CrossRef]
- Ribatti, D.; Nico, B.; Crivellato, E. The role of pericytes in angiogenesis. Int. J. Dev. Biol. 2011, 55, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Takakura, N.; Huang, X.-L.; Naruse, T.; Hamaguchi, I.; Dumont, D.J.; Yancopoulos, G.D.; Suda, T. Critical role of the TIE2 endothelial cell receptor in the development of definitive hematopoiesis. Immunity 1998, 9, 677–686. [Google Scholar] [CrossRef]
- Mueller, S.B.; Kontos, C.D. Tie1: An orphan receptor provides context for angiopoietin-2/Tie2 signaling. J. Clin. Investig. 2016, 126, 3188–3191. [Google Scholar] [CrossRef]
- Maisonpierre, P.C.; Suri, C.; Jones, P.F.; Bartunkova, S.; Wiegand, S.J.; Radziejewski, C.; Compton, D.; McClain, J.; Aldrich, T.H.; Papadopoulos, N.; et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 1997, 277, 55–60. [Google Scholar] [CrossRef]
- Akwii, R.G.; Sajib, M.S.; Zahra, F.T.; Mikelis, C.M. Role of Angiopoietin-2 in Vascular Physiology and Pathophysiology. Cells 2019, 8, 471. [Google Scholar] [CrossRef]
- Ng, D.S.; Yip, Y.W.; Bakthavatsalam, M.; Chen, L.J.; Ng, T.K.; Lai, T.Y.; Pang, C.P.; Brelén, M.E. Elevated angiopoietin 2 in aqueous of patients with neovascular age related macular degeneration correlates with disease severity at presentation. Sci. Rep. 2017, 7, 45081. [Google Scholar] [CrossRef]
- Spaide, R.F. Optical Coherence Tomography Angiography Signs of Vascular Abnormalization with Antiangiogenic Therapy for Choroidal Neovascularization. Am. J. Ophthalmol. 2015, 160, 6–16. [Google Scholar] [CrossRef]
- Wada, I.; Nakao, S.; Fukuda, Y.; Shiose, S.; Takeda, A.; Kannan, R.; Sonoda, K.-H. Persistence of vascular empty sleeves in choroidal neovascularization after VEGF therapy in both animal models and humans. Graefe’s Arch. Clin. Exp. Ophthalmol. 2023, 261, 2189–2197. [Google Scholar] [CrossRef]
- Kim, M.; Allen, B.; Korhonen, E.A.; Nitschké, M.; Yang, H.W.; Baluk, P.; Saharinen, P.; Alitalo, K.; Daly, C.; Thurston, G.; et al. Opposing actions of angiopoietin-2 on Tie2 signaling and FOXO1 activation. J. Clin. Investig. 2016, 126, 3511–3525. [Google Scholar] [CrossRef]
- Wong, D.T.; Aboobaker, S.; Maberley, D.; Sharma, S.; Yoganathan, P. Switching to faricimab from the current anti-VEGF therapy: Evidence-based expert recommendations. BMJ Open Ophthalmol. 2025, 10, e001967. [Google Scholar] [CrossRef]
- Brinkmann, M.; Müller, T.; Köster, M.; Schweighofer, J.; Danckwardt, M.; Giannaccare, G.; Marolo, P.; Borrelli, E.; Reibaldi, M.; El-Shabrawi, Y.; et al. Optical Coherence Tomography Angiography Flow Signal in Non-Treatment-Naïve Patients with Neovascular Age-Related Macular Degeneration Treated with Faricimab. Medicina 2025, 61, 260. [Google Scholar] [CrossRef]
- Cheung, C.M.G.; Lim, J.I.; Priglinger, S.; Querques, G.; Margaron, P.; Patel, S.; Souverain, A.; Willis, J.R.; Yang, M.; Guymer, R. Anatomic Outcomes with Faricimab vs Aflibercept in Head-to-Head Dosing Phase of the TENAYA/LUCERNE Trials in Neovascular Age-related Macular Degeneration. Ophthalmology 2025, 132, 519–526. [Google Scholar] [CrossRef]
- Hatamnejad, A.; Dadak, R.; Orr, S.; Wykoff, C.; Choudhry, N. Systematic review of efficacy and meta-analysis of safety of ranibizumab biosimilars relative to reference ranibizumab anti-VEGF therapy for nAMD treatment. BMJ Open Ophthalmol. 2023, 8, e001205. [Google Scholar] [CrossRef] [PubMed]
- Toto, L.; Ruggeri, M.L.; Evangelista, F.; Trivigno, C.; D’aloisio, R.; De Nicola, C.; Viggiano, P.; Doronzo, E.; Di Nicola, M.; Porreca, A.; et al. Choroidal and Retinal Imaging Biomarkers in Different Types of Macular Neovascularization. J. Clin. Med. 2023, 12, 1140. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, J.; Forte, P.; Forte, G.; Eandi, C.M. Faricimab efficacy in type 1 macular neovascularization: AI-assisted quantification of pigment epithelium detachment (PED) volume reduction over 12 months in Naïve and switch eyes. Int. J. Retin. Vitr. 2025, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Mukai, R.; Kataoka, K.; Tanaka, K.; Miyara, Y.; Maruko, I.; Nakayama, M.; Watanabe, Y.; Yamamoto, A.; Wakatsuki, Y.; Onoe, H.; et al. Three-month outcomes of faricimab loading therapy for wet age-related macular degeneration in Japan. Sci. Rep. 2023, 13, 8747. [Google Scholar] [CrossRef]

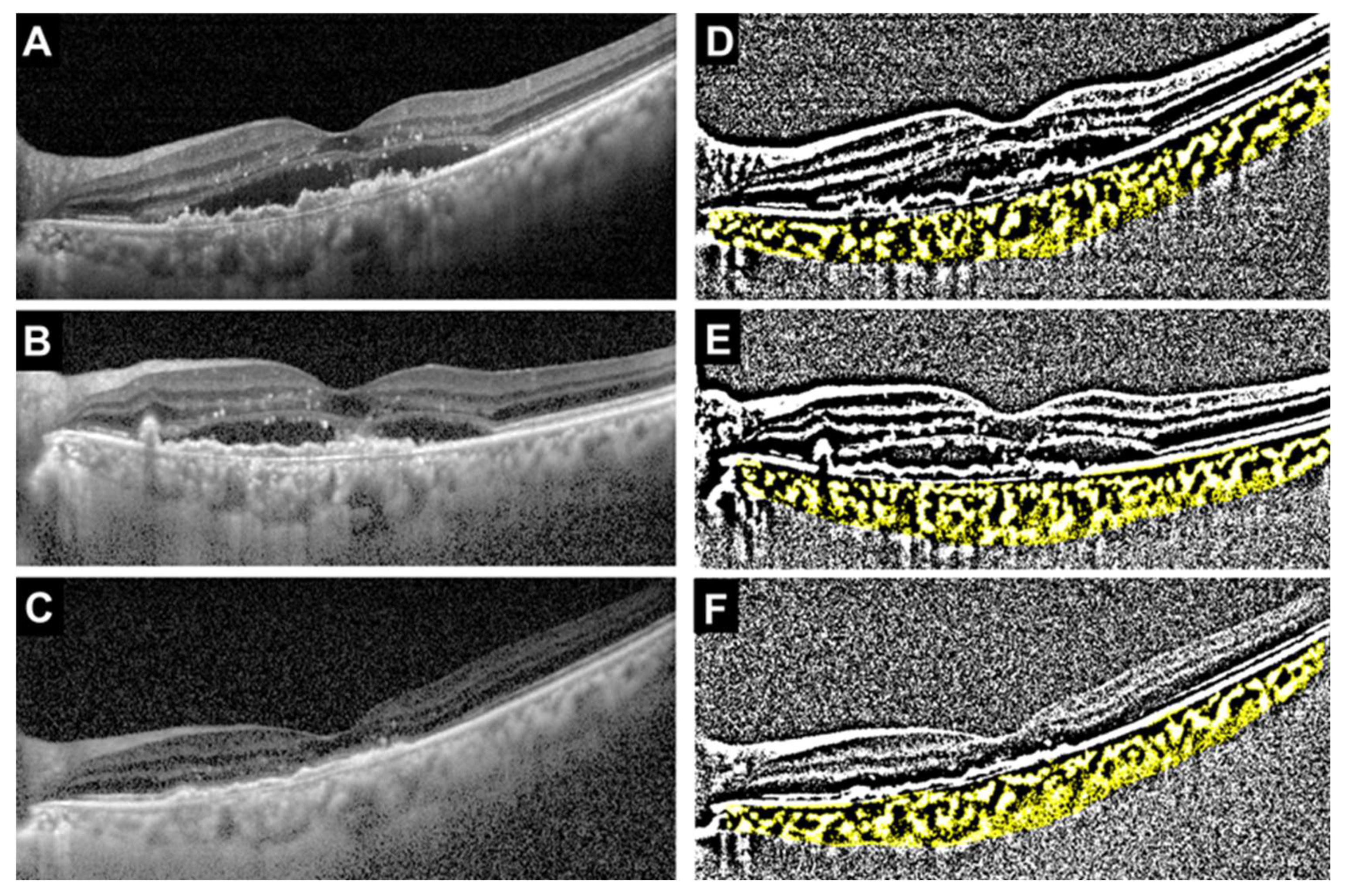
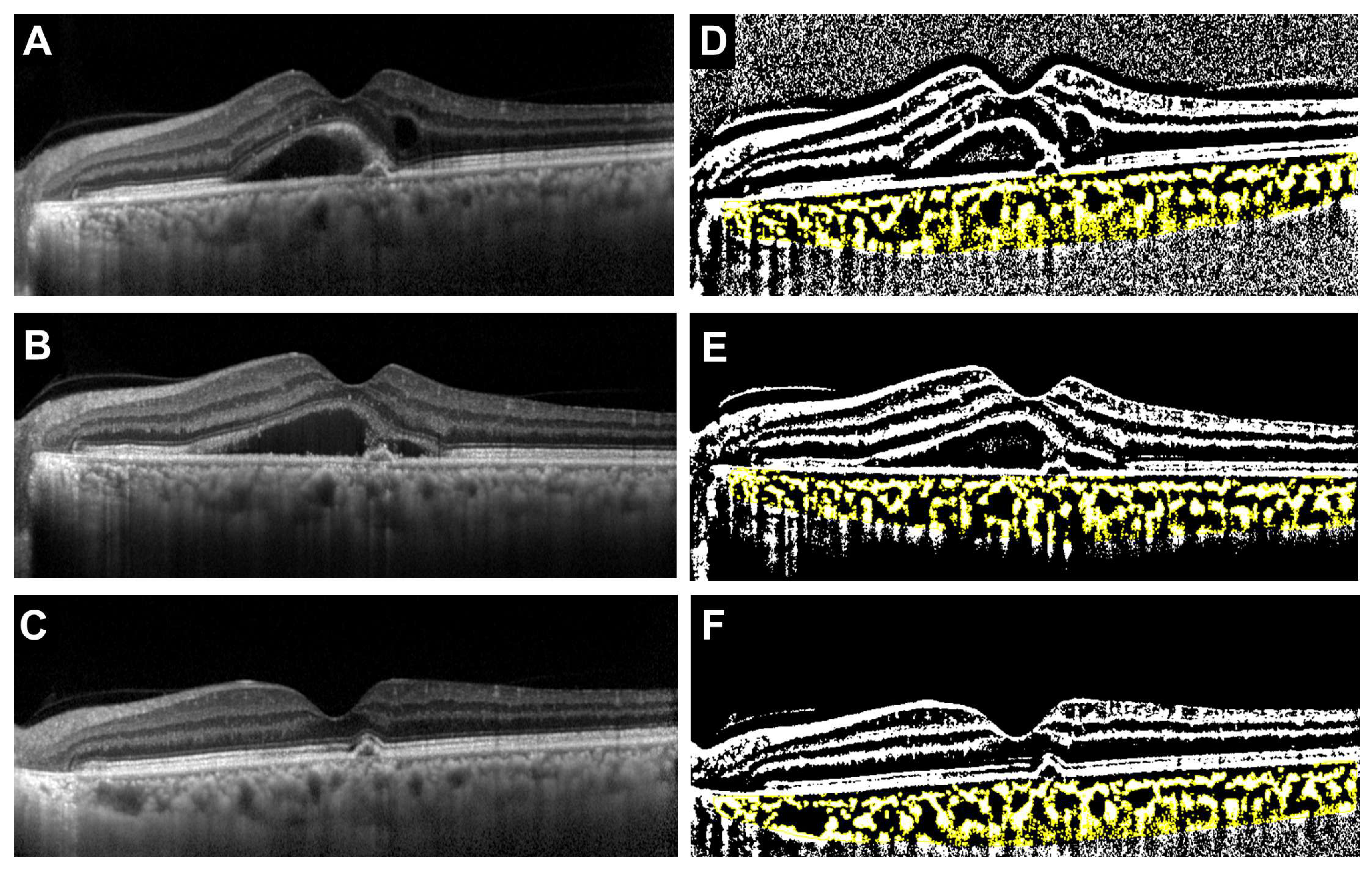

| Age (IQR) | 77 (68–82) |
| Sex (%) | male: 20 (71) female: 8(29) |
| Cardiovascular disease (%) | 10 (36) |
| Type of MNV | |
| Type 1 (%) | 17 (61) |
| Polyps (%, in Type 1 MNV) | 9 (53) |
| Type 2 (%) | 11 (39) |
| drusen (%) | 21 (75) |
| IRF (%) | 11 (39) |
| SRF (%) | 27 (96) |
| BCVA (logMAR, IQR) | 0.30 (0.15–0.52) |
| CCT (μm, IQR) | 230 (142–302) |
| TCA (mm2, IQR) | 1.33 (1.03–1.80) |
| LCA (mm2, IQR) | 0.84 (0.63–1.16) |
| SCA (mm2, IQR) | 0.47 (0.37–0.64) |
| L/C ratio (%, IQR) | 64.7 (62.4–66.1) |
| Faricimab (n = 13) | Ranibizumab (n = 15) | p | |
|---|---|---|---|
| Age (IQR) | 69 (65–78) | 81 (75–83) | 0.021 † |
| Sex (%) | male: 9 (69) female: 4 (31) | male: 11 (73) female: 4 (27) | >0.999 ‡ |
| Cardiovascular disease (%) | 3 (23) | 7 (47) | 0.25 ‡ |
| Type of MNV | |||
| Type 1 (%) | 7 (54) | 10 (67) | 0.70 ‡ |
| Polyps (%, in Type 1 MNV) | 5 (71) | 4 (40) | 0.34 ‡ |
| Type 2 (%) | 6 (46) | 5 (33) | - |
| drusen (%) | 8 (62) | 13 (87) | 0.20 ‡ |
| IRF (%) | 2 (15) | 9 (60) | 0.024 ‡ |
| SRF (%) | 13 (100) | 14 (93) | >0.999 ‡ |
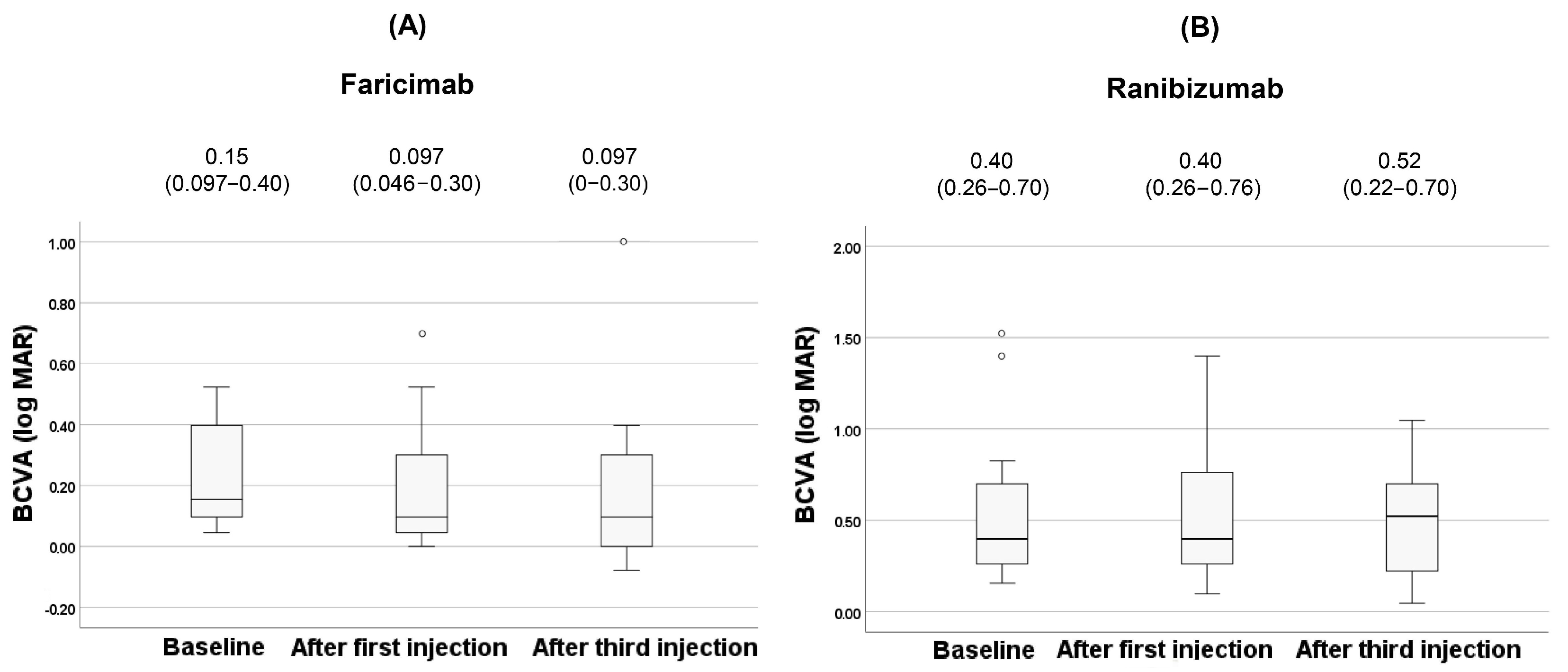
| BCVA (logMAR, IQR) | 0.15 (0.097–0.40) | 0.40(0.26–0.70) | 0.012 † |
| CCT (μm, IQR) | 209 (188–274) | 287 (118–328) | 0.93 † |
| TCA (mm2, IQR) | 1.30 (1.19–1.76) | 1.42 (0.95–1.82) | 0.79 † |
| LCA (mm2, IQR) | 0.83 (0.80–1.14) | 0.91 (0.58–1.16) | 0.68 † |
| SCA (mm2, IQR) | 0.44 (0.39–0.63) | 0.50 (0.35–0.64) | 0.93 † |
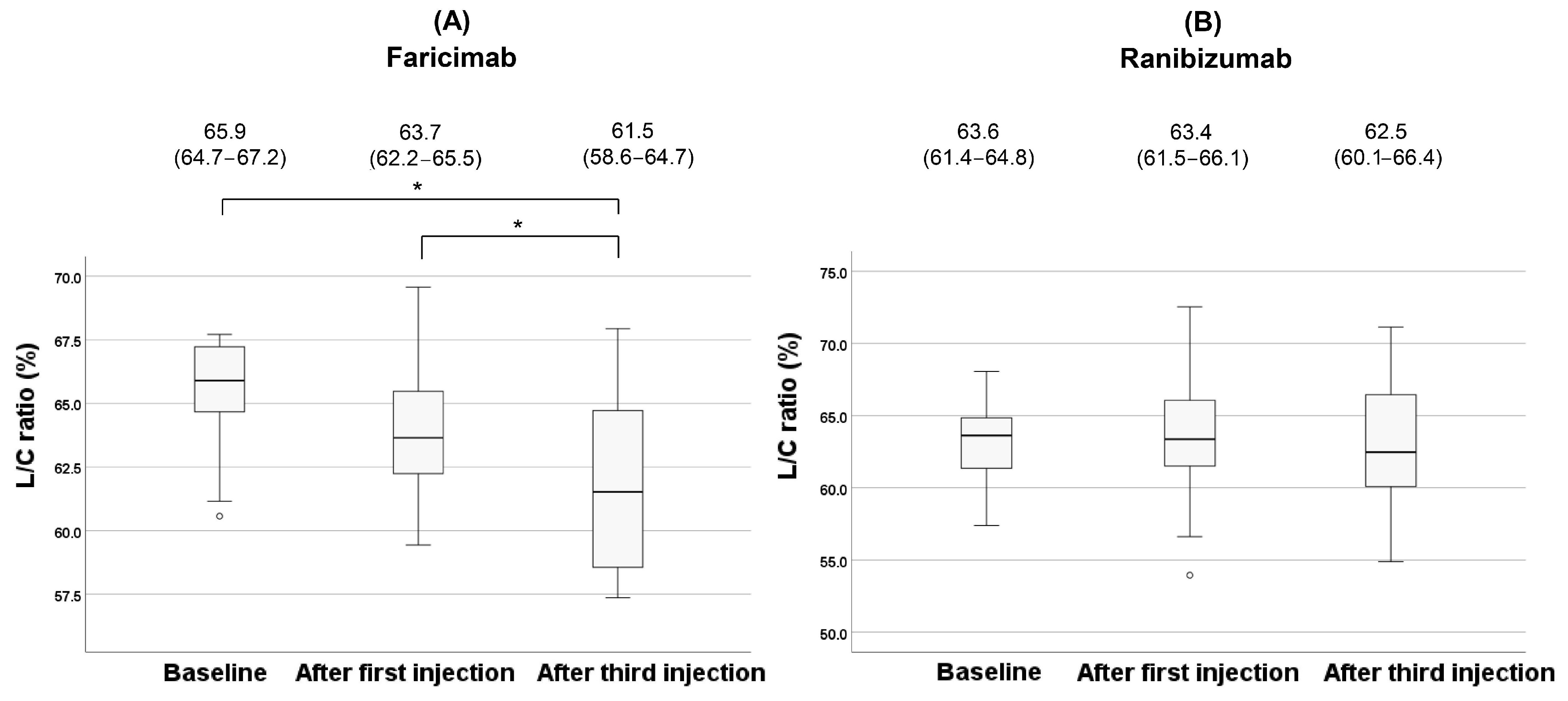
| L/C ratio (%, IQR) | 65.9 (64.7–67.2) | 63.6 (61.4–64.8) | 0.029 † |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishiyama, T.; Hirai, H.; Miyata, K.; Nishi, T.; Ueda, T.; Kase, S. A Pilot Study on Structural Changes of Choroidal Vasculature Following Intravitreal Anti-VEGF Injection in Neovascular Age-Related Macular Degeneration: Faricimab vs Ranibizumab. J. Clin. Med. 2025, 14, 7257. https://doi.org/10.3390/jcm14207257
Nishiyama T, Hirai H, Miyata K, Nishi T, Ueda T, Kase S. A Pilot Study on Structural Changes of Choroidal Vasculature Following Intravitreal Anti-VEGF Injection in Neovascular Age-Related Macular Degeneration: Faricimab vs Ranibizumab. Journal of Clinical Medicine. 2025; 14(20):7257. https://doi.org/10.3390/jcm14207257
Chicago/Turabian StyleNishiyama, Takeyuki, Hiromasa Hirai, Kimie Miyata, Tomo Nishi, Tetsuo Ueda, and Satoru Kase. 2025. "A Pilot Study on Structural Changes of Choroidal Vasculature Following Intravitreal Anti-VEGF Injection in Neovascular Age-Related Macular Degeneration: Faricimab vs Ranibizumab" Journal of Clinical Medicine 14, no. 20: 7257. https://doi.org/10.3390/jcm14207257
APA StyleNishiyama, T., Hirai, H., Miyata, K., Nishi, T., Ueda, T., & Kase, S. (2025). A Pilot Study on Structural Changes of Choroidal Vasculature Following Intravitreal Anti-VEGF Injection in Neovascular Age-Related Macular Degeneration: Faricimab vs Ranibizumab. Journal of Clinical Medicine, 14(20), 7257. https://doi.org/10.3390/jcm14207257





