Impact of the Different Corneal Storage Flasks on Endothelial Cell Loss During Cultivation—A Retrospective Analysis
Abstract
1. Introduction
2. Materials and Methods
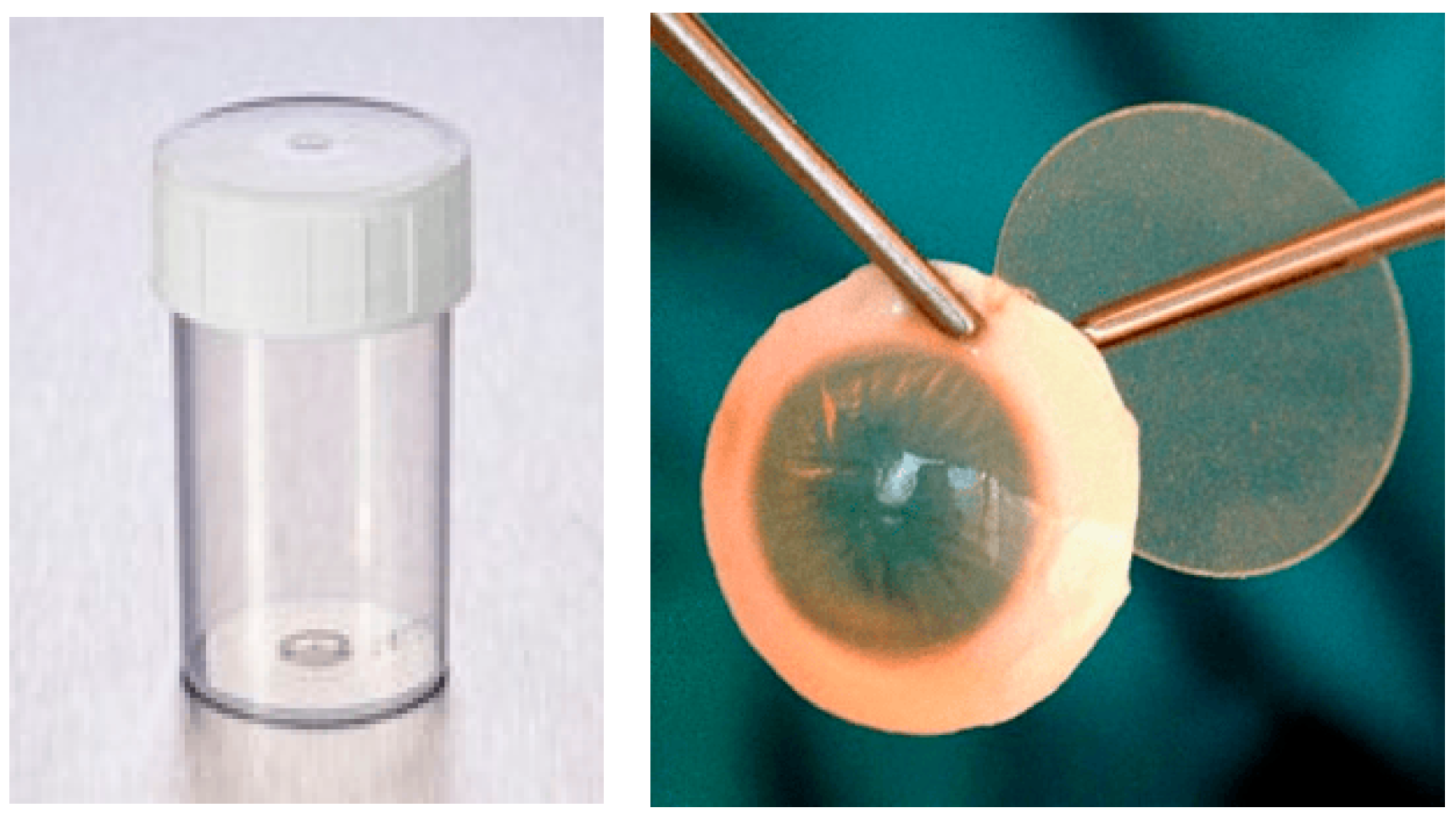
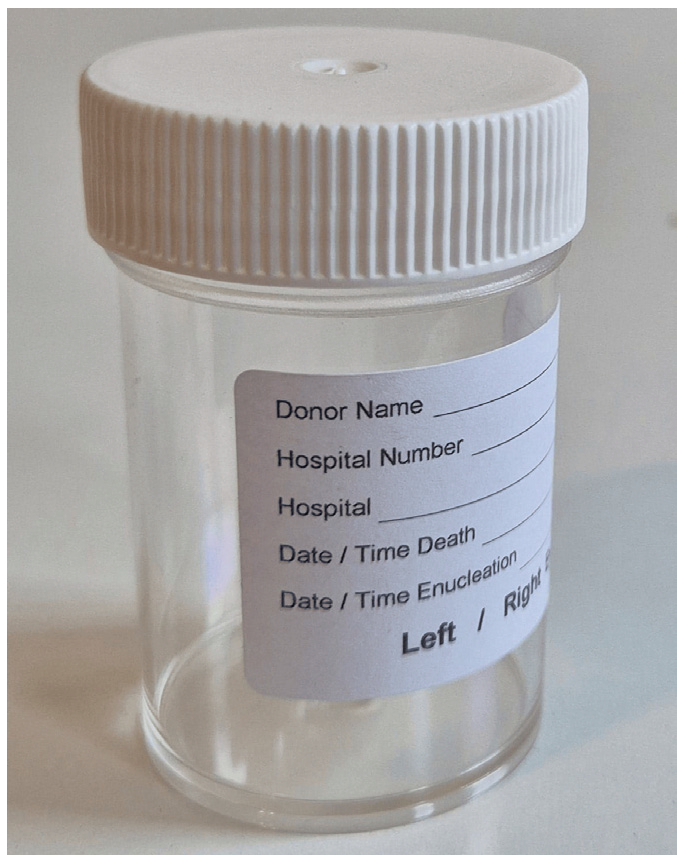
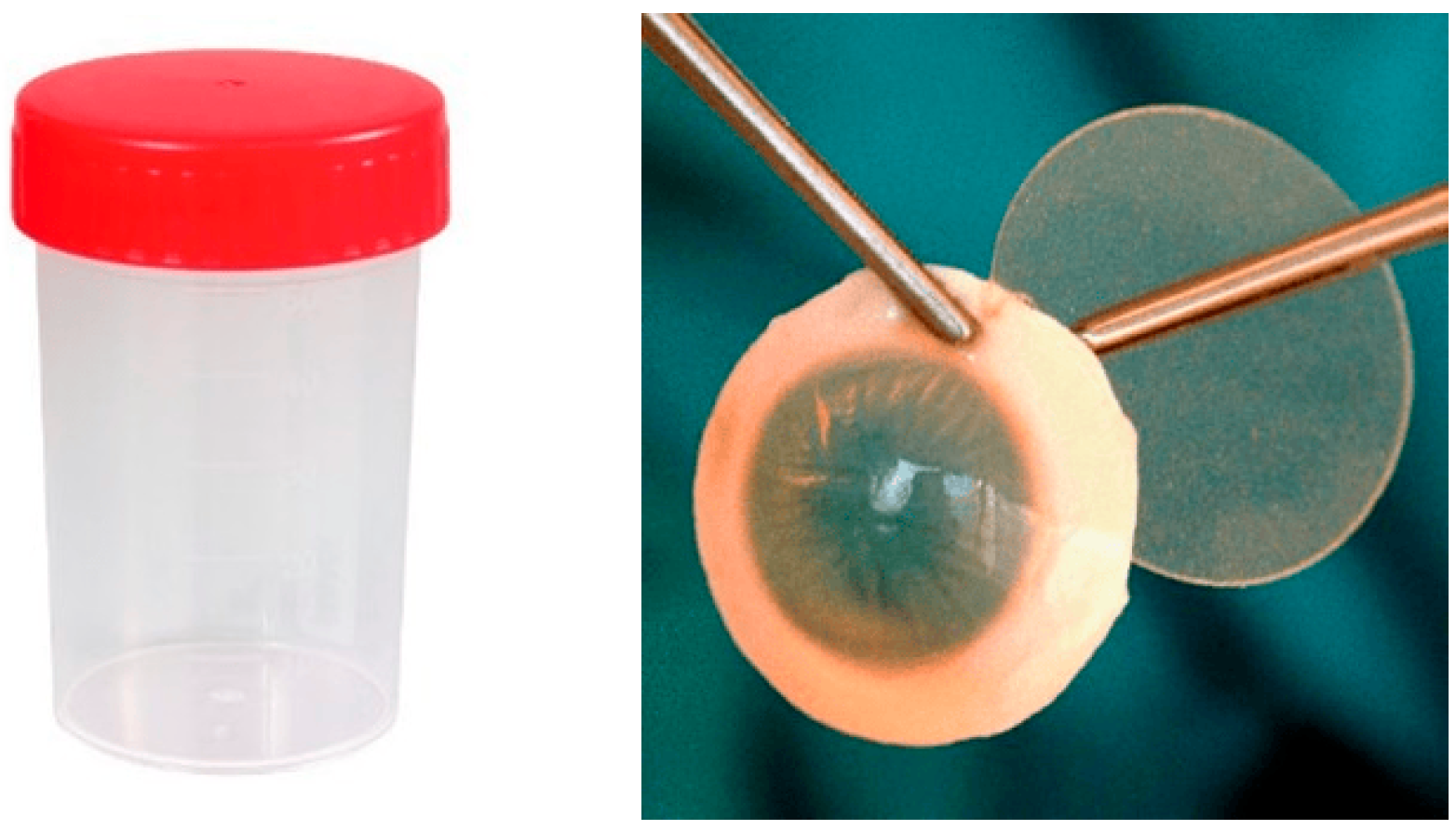
- Standard container (Figure 1):
- 2.
3. Statistical Analysis
4. Results
4.1. Demographics
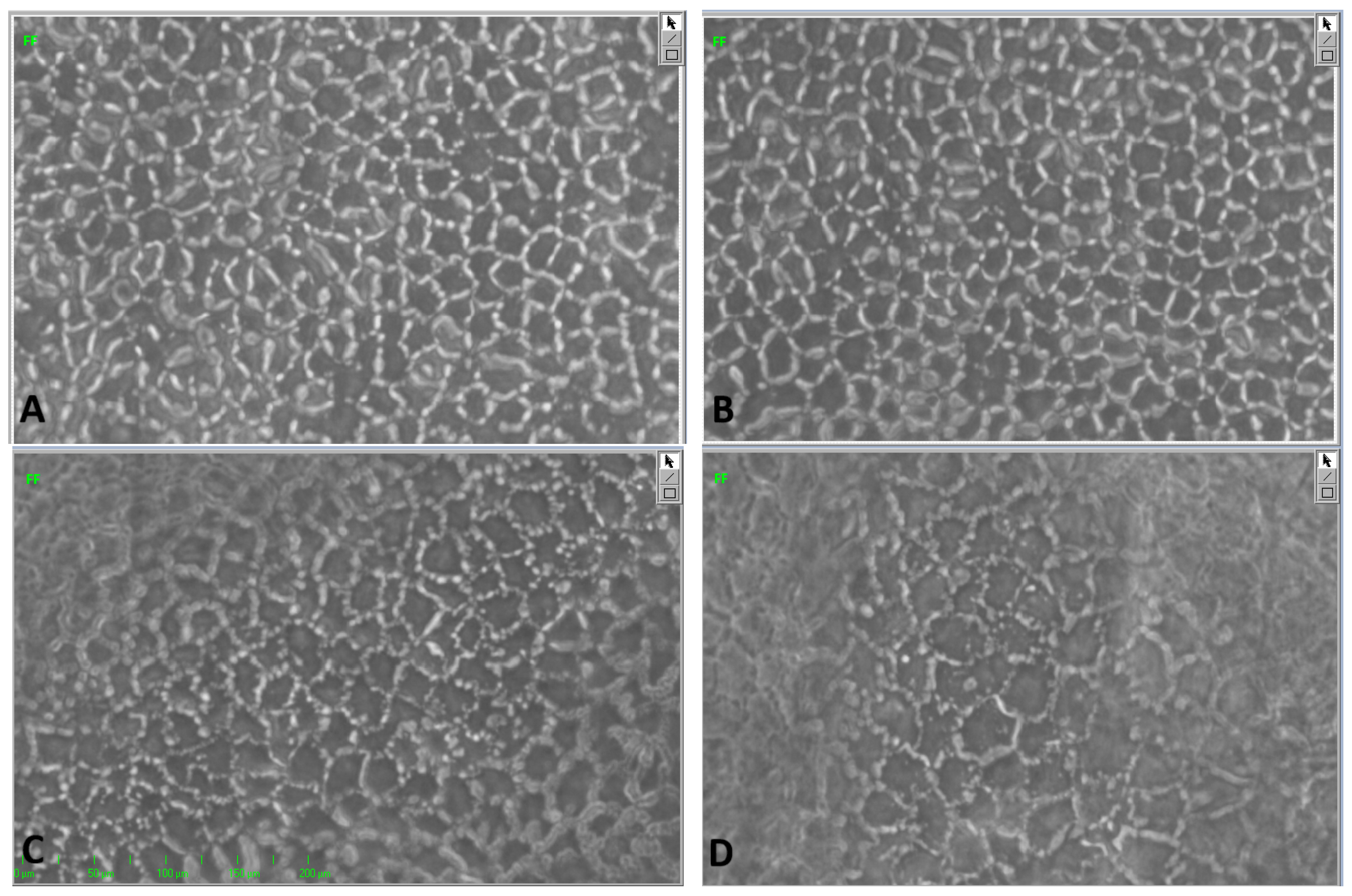
4.2. ECD1
- G4 (15 mL without corneal holder) exhibited a significantly higher proportion of subthreshold corneas (p < 0.001).
- G6 showed a trend toward significance with a clinically relevant difference of 22% (p = 0.088).
4.3. ECD2
- G1 and G6 demonstrated essentially stable endothelial counts.
- All other groups showed mild ECL without statistical significance (range: 160–327 cells/mm2).
- Significantly higher rates of ECL to less than 2000 cells/mm2 in G4 (p = 0.008) and G5 (p = 0.001).
- No significant trend in G2 and G3 as well as no ECL in G6.
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anitha, V.; Tandon, R.; Shah, S.G.; Radhakrishnan, N.; Singh, S.; Murugesan, V.; Patwardhan, V.; Ravindran, M. Corneal blindness and eye banking: Current strategies and best practices. Indian J. Ophthalmol. 2023, 71, 3142–3148. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.Y.; Kong, X.; Wolle, M.; Gasquet, N.; Ssekasanvu, J.; Mariotti, S.P.; Bourne, R.; Taylor, H.; Resnikoff, S.; West, S. Global trends in blindness and vision impairment resulting from corneal opacity 1984–2020: A meta-analysis. Ophthalmology 2023, 130, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Wei, C.; Ma, L.; Yu, Y.; Zhang, T.; Shi, W. The ocular immunological alterations in the process of high-risk corneal transplantation rejection. Exp. Eye Res. 2024, 245, 109971. [Google Scholar] [CrossRef] [PubMed]
- Hori, J.; Yamaguchi, T.; Keino, H.; Hamrah, P.; Maruyama, K. Immune privilege in corneal transplantation. Prog. Retin. Eye Res. 2019, 72, 100758. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wong, Y.L.; Walkden, A. Current perspectives on corneal transplantation. Clin. Ophthalmol. 2022, 16, 631–646. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, T.; Tuli, S.S.; Steigleman, W.A.; Shah, A.A. Overview of corneal transplantation for the nonophthalmologist. Transpl. Direct 2023, 9, e1434. [Google Scholar] [CrossRef] [PubMed]
- Flockerzi, E.; Turner, C.; Seitz, B.; Adamiak-Kalinowska, J.; Aisenbrey, S.; Al Ashi, N.; Auffarth, G.; Baatz, H.; Baier, C.; Bahlmann, D.; et al. Descemet’s membrane endothelial keratoplasty is the predominant keratoplasty procedure in Germany since 2016: A report of the DOG-section cornea and its keratoplasty registry. Br. J. Ophthalmol. 2024, 108, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Romano, V.; Passaro, M.L.; Ruzza, A.; Parekh, M.; Airaldi, M.; Levis, H.J.; Ferrari, S.; Costagliola, C.; Semeraro, F.; Ponzin, D.; et al. Quality assurance in corneal transplants: Donor cornea assessment and oversight. Surv. Ophthalmol. 2024, 69, 465–482. [Google Scholar] [CrossRef] [PubMed]
- Shehab, A.; Gram, N.; Ivarsen, A.; Hjortdal, J. The importance of donor characteristics, post-mortem time and preservation time for use and efficacy of donated corneas for posterior lamellar keratoplasty. Acta Ophthalmol. 2022, 100, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Schön, F.; Gericke, A.; Bu, J.B.; Cursiefen, C.; Bachmann, B.; Kruse, F.E.; Seitz, B.; Meller, D.; Riss, S.; Yoeruek, E.; et al. How to predict the suitability for corneal donorship? J. Clin. Med. 2021, 10, 3426. [Google Scholar] [CrossRef] [PubMed]
- Board of the Federal German Medical Association. Decision of the Federal Medical Association on the first circumscribed continuation of the guideline for the acquisition of donor corneas and for guiding an eye cornea bank. Dtsch. Arztebl. 2018, 115, A-262. [Google Scholar] [CrossRef]
- Böhnke, M. A new system for storing donor corneas. Klin. Monbl. Augenheilkd. 1991, 198, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Filev, F.; Stein, M.; Schultheiss, M.; Yoeruek, E.; Bartz-Schmidt, K.U.; Szurman, P.; Roters, S.; Röck, T.; Siebelmann, S.; Cursiefen, C.; et al. Semiautomatic assessment of endothelial density and morphology in organ-cultured corneas—Potential predictors for transplantation suitability and clinical outcome? Graefes Arch. Clin. Exp. Ophthalmol. 2023, 261, 2593–2602. [Google Scholar] [CrossRef] [PubMed]
- European Directorate for the Quality of Medicines & HealthCare of the Council of Europe. Guide to the Quality and Safety of Tissues and Cells for Human Application, 5th ed.; EDQM; Council of Europe: Strasbourg, France, 2022. [Google Scholar]
- EEBA Technical Guidelines Special Interest Group. Technical Guidelines for Ocular Tissue; European Eye Bank Association: Brussels, Belgium, 2020. [Google Scholar]
- Pilger, D.; Torun, N.; Maier, A.-K.B.; Schroeter, J.; Bartz-Schmidt, K.U.; Röck, T.; Yoeruek, E.; Szurman, P.; Roters, S.; Siebelmann, S.; et al. Pseudophakic corneal donor tissue in Descemet membrane endothelial keratoplasty (DMEK): Implications for cornea banks and surgeons. BMJ Open Ophthalmol. 2020, 5, e000524. [Google Scholar] [CrossRef] [PubMed]
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G.; Dumollard, J.M.; Apte, R.; Williams, K.; et al. Global survey of corneal transplantation and eye banking. JAMA Ophthalmol. 2016, 134, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Pels, E.; Schuchard, Y. Organ-culture preservation of human corneas. Doc. Ophthalmol. 1983, 56, 147–153. [Google Scholar] [CrossRef] [PubMed]



| Groups | Total Cornea Count | Death-to-Explantation Interval [hour] | Mean Age [year] | Male/ Female | Phakic/ Pseudophakic |
|---|---|---|---|---|---|
| G1 | 283 | 35.8 ± 16.6 | 73 ± 11 | 176/107 | 165/117 |
| G2 | 10 | 29.5 ± 16.3 | 72 ± 10 | 8/2 | 6/4 |
| G3 | 44 | 30.0 ± 19.5 | 72 ± 13 | 27/17 | 23/20 |
| G4 | 16 | 39.2 ± 21.0 | 79 ± 5 | 4/12 | 4/12 |
| G5 | 16 | 26.2 ± 10.5 | 75 ± 9 | 6/10 | 4/12 |
| G6 | 14 | 23.2 ± 14.2 | 68 ± 12 | 8/6 | 10/4 |
| Total | 383 | 34.3 ± 17.3 | 73 ± 11 | 229/154 | 212/169 |
| Groups | Total Cornea Count | Corneal Count with ECD1 > 2000 cells/mm2 | Corneal Count with ECD1 < 2000 cells/mm2 (%) | Chi-Square Test Compared with G1 |
|---|---|---|---|---|
| G1 | 283 | 201 | 82 (29%) | - |
| G2 | 10 | 6 | 4 (40%) | 0.451 |
| G3 | 44 | 31 | 13 (30%) | 0.938 |
| G4 | 16 | 4 | 12 (75%) | <0.001 |
| G5 | 16 | 13 | 3 (19%) | 0.377 |
| G6 | 14 | 13 | 1 (7%) | 0.075 |
| Groups | Total Cornea Count | Mean ± SD ECD1 [cells/mm2] | Mean ± SD ECD2 [cells/mm2] | Mean ± SD ECL [cells/mm2] | ECL [%] | t-Test |
|---|---|---|---|---|---|---|
| G1 | 283 | 2480 ± 637 | 2460 ± 544 | −20 ± 552 | −1% | 0.125 |
| G2 | 10 | 2441 ± 360 | 2217 ± 463 | −223 ± 473 | −9% | 0.397 |
| G3 | 44 | 2514 ± 657 | 2308 ± 839 | −206 ± 795 | −8% | 0.127 |
| G4 | 16 | 2420 ± 570 | 2093 ± 396 | −327 ± 446 | −14% | 0.282 |
| G5 | 16 | 2351 ± 514 | 2191 ± 554 | −160 ± 509 | −7% | 0.418 |
| G6 | 14 | 2569 ± 381 | 2613 ± 245 | 44 ± 254 | 2% | 0.597 |
| Groups | Corneal Count with ECD1 > 2000 cells/mm2 | Corneal Count with ECD2 < 2000 cells/mm2 (%) | Chi-Square Test Compared with G1 |
|---|---|---|---|
| G1 | 201 | 19 (9%) | - |
| G2 | 6 | 1 (17%) | 0.556 |
| G3 | 31 | 6 (19%) | 0.097 |
| G4 | 4 | 2 (50%) | 0.008 |
| G5 | 13 | 5 (38%) | 0.001 |
| G6 | 13 | 0 (0%) | 0.245 |
| Groups | Total Cornea Count | ECD1 < 2000 cells/mm2 | ECL = ECD2 − ECD1 cells/mm2 | ECD2 < 2000 cells/mm2 | |
|---|---|---|---|---|---|
| G1 | 50 mL Böhnke corneal holder | 283 | 29% | −1% | 9% |
| G2 | 100 mL no holder | 10 | 40% | −9% | 17% |
| G3 * | 100 mL Cornea-Claw® | 44 | 30% | −8% | 19% |
| G4 * | 15 mL no holder | 16 | 75% | −14% | 50% |
| G5 * | 60 mL no holder | 16 | 19% | −7% | 38% |
| G6 | 60 mL Cornea-Claw® | 14 | 7% | +2% | 0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safi, T.; Kolb-Wetterau, C.M.; Grabitz, S.D.; Buonfiglio, F.; Apel, M.; Wasielica-Poslednik, J. Impact of the Different Corneal Storage Flasks on Endothelial Cell Loss During Cultivation—A Retrospective Analysis. J. Clin. Med. 2025, 14, 7165. https://doi.org/10.3390/jcm14207165
Safi T, Kolb-Wetterau CM, Grabitz SD, Buonfiglio F, Apel M, Wasielica-Poslednik J. Impact of the Different Corneal Storage Flasks on Endothelial Cell Loss During Cultivation—A Retrospective Analysis. Journal of Clinical Medicine. 2025; 14(20):7165. https://doi.org/10.3390/jcm14207165
Chicago/Turabian StyleSafi, Tarek, Carolin Marion Kolb-Wetterau, Stephanie D. Grabitz, Francesco Buonfiglio, Melissa Apel, and Joanna Wasielica-Poslednik. 2025. "Impact of the Different Corneal Storage Flasks on Endothelial Cell Loss During Cultivation—A Retrospective Analysis" Journal of Clinical Medicine 14, no. 20: 7165. https://doi.org/10.3390/jcm14207165
APA StyleSafi, T., Kolb-Wetterau, C. M., Grabitz, S. D., Buonfiglio, F., Apel, M., & Wasielica-Poslednik, J. (2025). Impact of the Different Corneal Storage Flasks on Endothelial Cell Loss During Cultivation—A Retrospective Analysis. Journal of Clinical Medicine, 14(20), 7165. https://doi.org/10.3390/jcm14207165







