Endoscopic Mitral Surgery in Noonan Syndrome—Case Report and Considerations
Abstract
1. Introduction
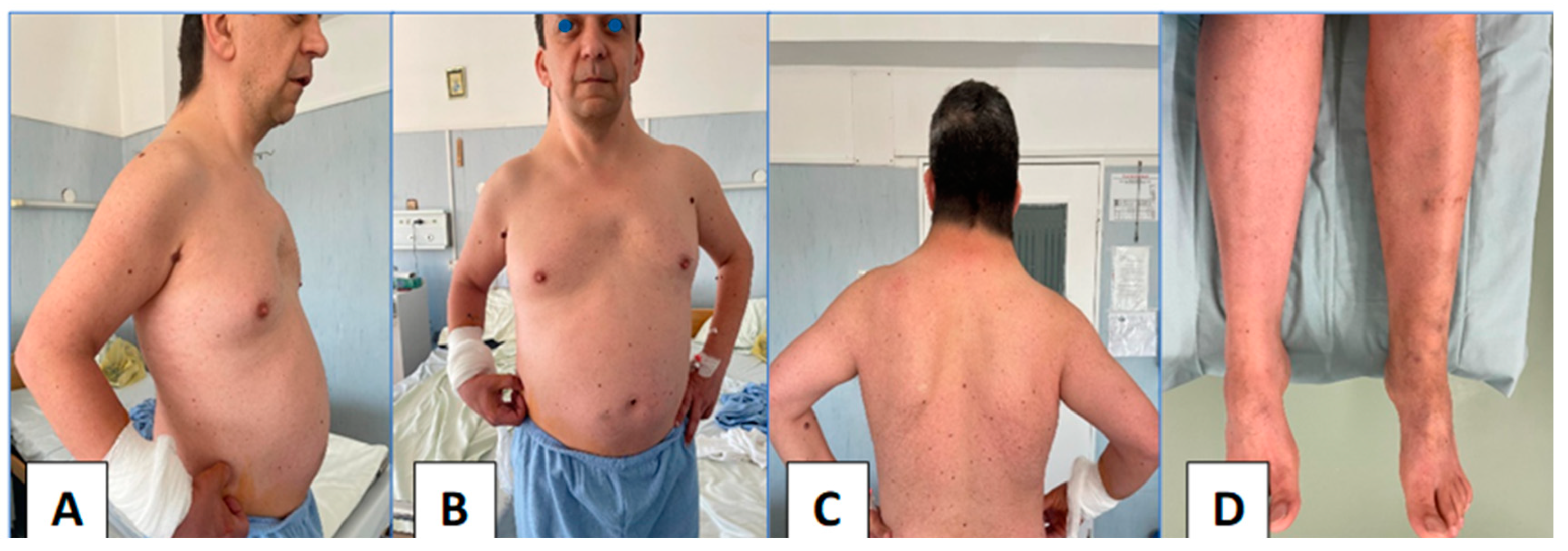
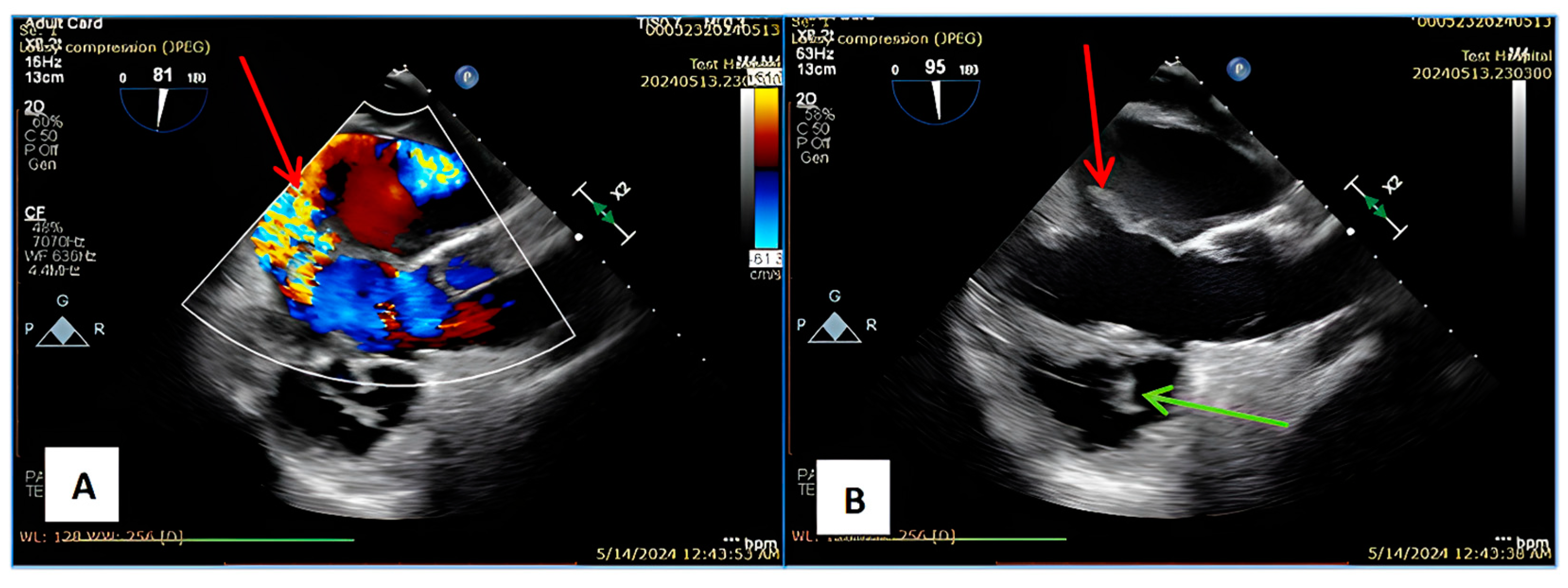
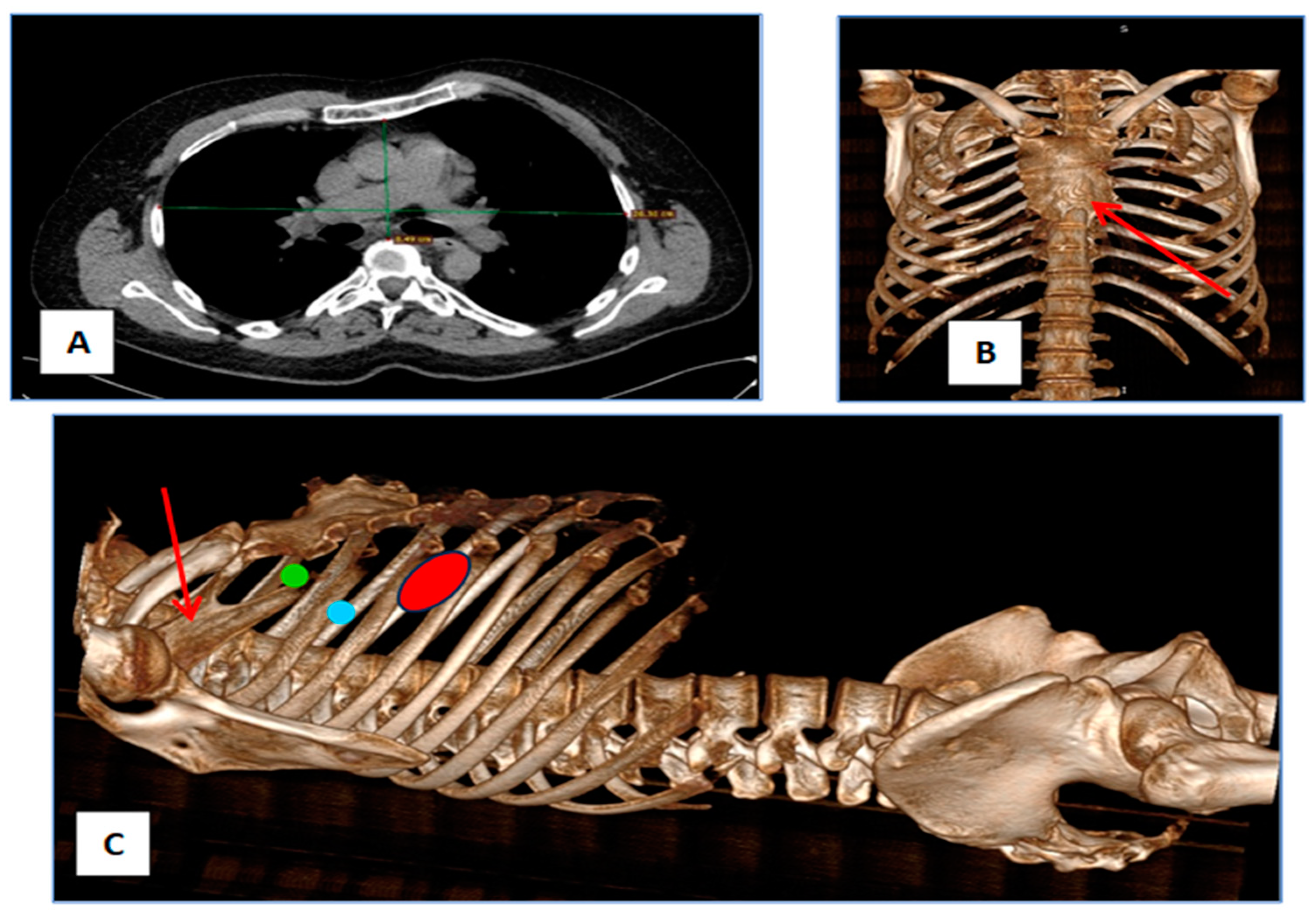
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hussain, S.; Swystun, A.G.; Caputo, M.; Angelini, G.D.; Vohra, H.A. A Review and Meta-Analysis of Conventional Sternotomy versus Minimally Invasive Mitral Valve Surgery for Degenerative Mitral Valve Disease Focused on the Last Decade of Evidence. Perfusion 2024, 39, 988–997. [Google Scholar] [CrossRef] [PubMed]
- Casselman, F.P.; Van Slycke, S.; Wellens, F.; De Geest, R.; Degrieck, I.; Van Praet, F.; Vermeulen, Y.; Vanermen, H. Mitral Valve Surgery Can Now Routinely Be Performed Endoscopically. Circulation 2003, 108, 10. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.E.; Allanson, J.E.; Tartaglia, M.; Gelb, B.D. Noonan Syndrome. Lancet 2013, 381, 333–342. [Google Scholar] [CrossRef]
- Mendez, H.M.M.; Opitz, J.M.; Reynolds, J.F. Noonan Syndrome: A Review. Am. J. Med. Genet. 1985, 21, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Prendiville, T.W.; Gauvreau, K.; Tworog-Dube, E.; Patkin, L.; Kucherlapati, R.S.; Roberts, A.E.; Lacro, R.V. Cardiovascular Disease in Noonan Syndrome. Arch. Dis. Child. 2014, 99, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Xie, Y.; Wang, S.; Zhang, Z. Cardiovascular Abnormalities and Gene Mutations in Children With Noonan Syndrome. Front. Genet. 2022, 13, 915129. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.E.; Basson, C.T.; Goldmuntz, E.; Magoulas, P.L.; McDermott, D.A.; McDonald-McGinn, D.M.; McPherson, E.; Morris, C.A.; Noonan, J.; Nowak, C.; et al. Adults with Genetic Syndromes and Cardiovascular Abnormalities: Clinical History and Management. Genet. Med. 2008, 10, 469–494. [Google Scholar] [CrossRef] [PubMed]
- Karangelis, D.; Loggos, S.; Mitropoulos, F. Minimally Invasive versus Conventional Mitral Valve Surgery. A Clinical Equipoise or Not Really? J. Investig. Surg. 2021, 34, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Billar, R.J.; Manoubi, W.; Kant, S.G.; Wijnen, R.M.H.; Demirdas, S.; Schnater, J.M. Association between Pectus Excavatum and Congenital Genetic Disorders: A Systematic Review and Practical Guide for the Treating Physician. J. Pediatr. Surg. 2021, 56, 2239–2252. [Google Scholar] [CrossRef]
- Baldo, F.; Fachin, A.; Da Re, B.; Rubinato, E.; Bobbo, M.; Barbi, E. New Insights on Noonan Syndrome’s Clinical Phenotype: A Single Center Retrospective Study. BMC Pediatr. 2022, 22, 734. [Google Scholar] [CrossRef]
- Noonan, J.A. Hypertelorism with Turner phenotype. A new syndrome with associated congenital heart disease. Am. J. Dis. Child. 1968, 116, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Linglart, L.; Gelb, B.D. Congenital Heart Defects in Noonan Syndrome: Diagnosis, Management, and Treatment. Am. J. Med. Genet. Part C 2020, 184, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Stephens, E.H.; Dearani, J.A.; Jaroszewski, D.E. Pectus Excavatum in Cardiac Surgery Patients. Ann. Thorac. Surg. 2023, 115, 1312–1321. [Google Scholar] [CrossRef]
- Poston, P.M.; Patel, S.S.; Rajput, M.; Rossi, N.O.; Ghanamah, M.S.; Davis, J.E.; Turek, J.W. The Correction Index: Setting the Standard for Recommending Operative Repair of Pectus Excavatum. Ann. Thorac. Surg. 2014, 97, 1176–1180. [Google Scholar] [CrossRef] [PubMed]
- Gillinov, M.; Burns, D.J.P.; Wierup, P. The 10 Commandments for Mitral Valve Repair. Innovations 2020, 15, 4–10. [Google Scholar] [CrossRef]
- Kastengren, M.; Svenarud, P.; Ahlsson, A.; Dalén, M. Minimally Invasive Mitral Valve Surgery Is Associated with a Low Rate of Complications. J. Intern. Med. 2019, 286, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Hage, A.; Ghoneim, A.; Chu, M.W.A. Minimally Invasive Endoscopic Mitral Repair: How I Teach It. Ann. Thorac. Surg. 2022, 114, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Van Der Merwe, J.; Casselman, F.; Stockman, B.; Vermeulen, Y.; Degrieck, I.; Van Praet, F. Endoscopic Atrioventricular Valve Surgery in Adults with Difficult-to-Access Uncorrected Congenital Chest Wall Deformities. Interdiscip. Cardiovasc. Thorac. Surg. 2016, 23, 851–855. [Google Scholar] [CrossRef]
- Müller, L.; Höfer, D.; Holfeld, J.; Hangler, H.; Bonaros, N.; Grimm, M. Indications and Contra-Indications for Minimally Invasive Mitral Valve Surgery. J. Vis. Surg. 2018, 4, 255. [Google Scholar] [CrossRef]
- Hemmati, P.; Dearani, J.A.; Daly, R.C.; King, K.S.; Ammash, N.M.; Cetta, F.; Schaff, H.V. Early Outcomes of Cardiac Surgery in Patients with Noonan Syndrome. Semin. Thorac. Cardiovasc. Surg. 2019, 31, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Bastidas, J.G.; Razzouk, A.J.; Hasaniya, N.W.; Bailey, L.L. Left Thoracotomy: An Ideal Approach for Mitral Valve Replacement in Patient With Severe Chest Wall Deformity. Ann. Thorac. Surg. 2013, 96, e63–e64. [Google Scholar] [CrossRef]
- van der Merwe, J.; Casselman, F.; Degrieck, I.; Van Praet, F. Minimally Invasive Pectus Excavatum Correction and Endoscopic Port Access Mitral Valve Surgery. Rambam Maimonides Med. J. 2024, 15, e0003. [Google Scholar] [CrossRef]
- Takaki, H.; Okamoto, K.; Kudo, M.; Yozu, R.; Shimizu, H. Mitral Valve Repair via a Minithoracotomy in a Patient With Pectus Excavatum. Innovations 2016, 11, 67–69. [Google Scholar]
- Sacco-Casamassima, M.G.; Goldstein, S.D.; Birdsong, M.; McIltrot, K.H.; Abdullah, F.; Colombani, P.M. Z-Type Pattern Pectus Excavatum/Carinatum in A Case of Noonan Syndrome. Ann. Thorac. Surg. 2015, 99, 1835–1837. [Google Scholar] [CrossRef] [PubMed]
- Aerts, L.; Sardari Nia, P. Mastering the Learning Curve of Endoscopic Mitral Valve Surgery. Front. Cardiovasc. Med. 2023, 10, 1162330. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef] [PubMed]
- Barg, A.A.; Yeshayahu, Y.; Avishai, E.; Budnik, I.; Cohen, O.; Brutman-Barazani, T.; Dardik, R.; Raas-Rothschild, A.; Levy-Mendelovich, S.; Livnat, T.; et al. Bleeding Phenotype and Hemostatic Evaluation by Thrombin Generation in Children with Noonan Syndrome: A Prospective Study. Pediatr. Blood Cancer 2024, 71, E30761. [Google Scholar] [CrossRef] [PubMed]
- Harpa, M.M.; Oltean, S.F.; Al Hussein, H.; Anitei, D.E.; Puscas, I.A.; Bănceu, C.M.; Veres, M.; Opriș, D.R.; Balau, R.A.; Suciu, H. Successful Treatment of Unilateral Pulmonary Edema as Minimally Invasive Mitral Valve Surgery Complication—Case Presentation. J. Clin. Med. 2024, 13, 7654. [Google Scholar] [CrossRef] [PubMed]
- Nugent, D.J.; Romano, A.A.; Sabharwal, S.; Cooper, D.L. Evaluation of Bleeding Disorders in Patients with Noonan Syndrome: A Systematic Review. JBM 2018, 9, 185–192. [Google Scholar] [CrossRef]
- Kleimeier, L.E.R.; Van Schaik, C.; Leenders, E.; Itkin, M.; Klein, W.M.; Draaisma, J.M.T. Lymphatic Phenotype of Noonan Syndrome: Innovative Diagnosis and Possible Implications for Therapy. JCM 2022, 11, 3128. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harpa, M.M.; Anitei, E.-D.; Ghiragosian, C.; Calburean, P.; Opris, D.R.; Banceu, M.C.; Arbanasi, E.M.; Suciu, H.; Al Hussein, H. Endoscopic Mitral Surgery in Noonan Syndrome—Case Report and Considerations. J. Clin. Med. 2025, 14, 583. https://doi.org/10.3390/jcm14020583
Harpa MM, Anitei E-D, Ghiragosian C, Calburean P, Opris DR, Banceu MC, Arbanasi EM, Suciu H, Al Hussein H. Endoscopic Mitral Surgery in Noonan Syndrome—Case Report and Considerations. Journal of Clinical Medicine. 2025; 14(2):583. https://doi.org/10.3390/jcm14020583
Chicago/Turabian StyleHarpa, Marius Mihai, Emanuel-David Anitei, Claudiu Ghiragosian, Paul Calburean, Diana Roxana Opris, Marian Cosmin Banceu, Emil Marian Arbanasi, Horatiu Suciu, and Hussam Al Hussein. 2025. "Endoscopic Mitral Surgery in Noonan Syndrome—Case Report and Considerations" Journal of Clinical Medicine 14, no. 2: 583. https://doi.org/10.3390/jcm14020583
APA StyleHarpa, M. M., Anitei, E.-D., Ghiragosian, C., Calburean, P., Opris, D. R., Banceu, M. C., Arbanasi, E. M., Suciu, H., & Al Hussein, H. (2025). Endoscopic Mitral Surgery in Noonan Syndrome—Case Report and Considerations. Journal of Clinical Medicine, 14(2), 583. https://doi.org/10.3390/jcm14020583






