Impact of the COVID-19 Pandemic on Odontogenic Abscess Clinical Patterns and Predictive Factors: A Retrospective Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. Statistical Analysis
3. Results
3.1. Characteristics of the Study Group
3.2. Manifestations of Odontogenic Abscesses Across Periods Relative to the COVID-19 Pandemic
3.3. Prognostic and Predictive Factors for Odontogenic Abscesses and Their Treatment
4. Discussion
- Multi-centre prospective studies with standardised data collection protocols;
- Investigations incorporating patient-reported outcomes and behavioural factors;
- Longer-term follow-up to assess post-pandemic recovery of dental care systems;
- Economic analyses of the cost implications of delayed odontogenic infection treatment.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Altıntaş, E. Complications of Dental Infections Due to Diagnostic Delay during COVID-19 Pandemic. BMJ Case Rep. CP 2022, 15, e247553. [Google Scholar] [CrossRef]
- Hoerter, J.E.; Malkin, B.D. Odontogenic Orofacial Space Infections. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Peng, X.; Xu, X.; Li, Y.; Cheng, L.; Zhou, X.; Ren, B. Transmission Routes of 2019-nCoV and Controls in Dental Practice. Int. J. Oral Sci. 2020, 12, 9. [Google Scholar] [CrossRef]
- Polskie Towarzystwo Stomatologiczne. COVID-19 a Praca Lekarza Dentysty: Wytyczne PTS Uaktualnione; Polskie Towarzystwo Stomatologiczne: Wrocław, Poland, 2020. [Google Scholar]
- Łaganowski, K.; Ortarzewska, M.; Czajka-Jakubowska, A.; Surdacka, A.; Nijakowski, K. Long-Term Impact of the COVID-19 Pandemic on Dental Care Delivery in Poland: A Single-Center Retrospective Analysis. Int. Dent. J. 2025, 75, 1544–1553. [Google Scholar] [CrossRef] [PubMed]
- Nijakowski, K.; Cieślik, K.; Łaganowski, K.; Gruszczyński, D.; Surdacka, A. The Impact of the COVID-19 Pandemic on the Spectrum of Performed Dental Procedures. Int. J. Environ. Res. Public Health 2021, 18, 3421. [Google Scholar] [CrossRef]
- Dickson-Swift, V.; Kangutkar, T.; Knevel, R.; Down, S. The Impact of COVID-19 on Individual Oral Health: A Scoping Review. BMC Oral Health 2022, 22, 422. [Google Scholar] [CrossRef]
- Drew, S.; Lazar, A.; Amin, D.; Abramowicz, S. Odontogenic Infections Are More Frequent and More Severe during COVID-19 Pandemic. J. Oral Maxillofac. Surg. 2021, 79, e88–e89. [Google Scholar] [CrossRef]
- Dudde, F.; Schuck, O.; Duda, S.; Giese, M. Impact of the COVID-19 Pandemic on Odontogenic Infections in Maxillofacial Surgery. Head Face Med. 2025, 21, 11. [Google Scholar] [CrossRef] [PubMed]
- Grill, F.D.; Rothlauf, P.; Ritschl, L.M.; Deppe, H.; Stimmer, H.; Scheufele, F.; Schwarz, M.; Wolff, K.-D.; Fichter, A.M. The COVID-19 Pandemic and Its Possible Impact on the Treatment of Odontogenic and Intraoral Abscesses. Head Face Med. 2023, 19, 36. [Google Scholar] [CrossRef]
- Mahendran, K.; Patel, S.; Sproat, C. Psychosocial Effects of the COVID-19 Pandemic on Staff in a Dental Teaching Hospital. Br. Dent. J. 2020, 229, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Samara, E.; Paul, R.; Ko, Y.Y.; Ameerally, P. The Effect of COVID-19 Outbreak on Hospital Admissions for Dental Infections. Adv. Oral Maxillofac. Surg. 2021, 2, 100025. [Google Scholar] [CrossRef]
- Louizakis, A.; Tatsis, D.; Paraskevopoulos, K.; Antoniou, A.; Kyrgidis, A.; Vahtsevanos, K.; Louizakis, A.; Tatsis, D.; Paraskevopoulos, K.; Antoniou, A.; et al. The Effect of the COVID-19 Pandemic on Odontogenic Cervicofacial Infections in a Single Center in Greece. Cureus 2024, 16, e61333. [Google Scholar] [CrossRef] [PubMed]
- Vytla, S.; Gebauer, D. Clinical Guideline for the Management of Odontogenic Infections in the Tertiary Setting. Aust. Dent. J. 2017, 62, 464–470. [Google Scholar] [CrossRef]
- Dalewski, B.; Palka, L.; Kiczmer, P.; Sobolewska, E. The Impact of SARS-CoV-2 Outbreak on the Polish Dental Community’s Standards of Care—A Six-Month Retrospective Survey-Based Study. Int. J. Environ. Res. Public Health 2021, 18, 1281. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, J.; Iwata, E.; Huang, W.; Takata, N.; Tachibana, A.; Akashi, M. Hematologic and Inflammatory Parameters for Determining Severity of Odontogenic Infections at Admission: A Retrospective Study. BMC Infect. Dis. 2022, 22, 931. [Google Scholar] [CrossRef]
- Kim, J.-K.; Lee, J.-H. Clinical Utility of Procalcitonin in Severe Odontogenic Maxillofacial Infection. Maxillofac. Plast. Reconstr. Surg. 2021, 43, 3. [Google Scholar] [CrossRef] [PubMed]
- Hoti, A.; Sutej, I.; Jakupi, A. Impact of the COVID-19 Pandemic on Antibiotic Prescriptions at the University Clinical Dentistry Center of Kosovo. Antibiotics 2025, 14, 405. [Google Scholar] [CrossRef]
- Bernatek, M.; Sommermeyer, H.; Pituch, H.; Wultańska, D.; Kopczyński, Z.; Piątek, J.; Wojtyła-Buciora, P. Clindamycin-Resistant Clostridioides Difficile: A Challenge in Dentistry. J. Health Inequalities 2022, 8, 82–88. [Google Scholar] [CrossRef]
- Segura-Egea, J.J.; Gould, K.; Şen, B.H.; Jonasson, P.; Cotti, E.; Mazzoni, A.; Sunay, H.; Tjäderhane, L.; Dummer, P.M.H. European Society of Endodontology Position Statement: The Use of Antibiotics in Endodontics. Int. Endod. J. 2018, 51, 20–25. [Google Scholar] [CrossRef]
- GUS Healthcare Expenditure in 2020–2022. Available online: https://stat.gov.pl/en/topics/health/health/healthcare-expenditure-in-20202022,18,3.html (accessed on 9 August 2025).
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and Inflammation: The Linking Mechanism and the Complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef]
- Pugliese, G.; Liccardi, A.; Graziadio, C.; Barrea, L.; Muscogiuri, G.; Colao, A. Obesity and Infectious Diseases: Pathophysiology and Epidemiology of a Double Pandemic Condition. Int. J. Obes. 2022, 46, 449–465. [Google Scholar] [CrossRef]
- Daltaban, Ö.; Aytekin, Z. Fear and Anxiety of COVID-19 in Dental Patients during the COVID-19 Pandemic: A Cross-Sectional Survey in Turkey. Dent. Med. Probl. 2022, 59, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Boyd, L.D.; Oh, U.; Vineyard, J. Patients’ Fear, Stress, and Anxiety Toward Attending Dental Visits During the COVID-19 Pandemic. J. Dent. Hyg. 2022, 96, 15–23. [Google Scholar] [PubMed]
- Alpan, A.L.; Ozdede, M. Evaluation of Access and Attitudes of Patients to Dental Treatments during COVID-19 Pandemic. J. Stomatol. 2022, 75, 231–237. [Google Scholar] [CrossRef]
| Median/n | IQR/% | |
|---|---|---|
| Age | 33 | 26–47 |
| Gender: | ||
| Female | 41 | 40.59 |
| Male | 60 | 59.41 |
| BMI | 24.39 | 21.36–27.41 |
| Location: | ||
| Country | 31 | 30.69 |
| City | 70 | 69.31 |
| Period: | ||
| Pre-pandemic | 38 | 37.62 |
| Pandemic | 20 | 19.8 |
| Post-pandemic | 43 | 42.57 |
| Comorbidities: | 32 | 31.68 |
| Diabetes | 6 | 5.94 |
| Hypertension | 16 | 15.84 |
| Hypothyroidism | 5 | 4.95 |
| Respiratory | 4 | 3.96 |
| Cardiovascular | 10 | 9.9 |
| Haematologic | 2 | 1.98 |
| Mental | 4 | 3.96 |
| Stimulants: | 38 | 37.62 |
| Alcohol | 5 | 4.95 |
| Drugs | 1 | 0.99 |
| Tobacco | 36 | 35.64 |
| Median/n | IQR/% | |
|---|---|---|
| Hospitalisation length, days | 5 | 4–6 |
| Abscess volume, cm3 | 18.01 | 6.63–42.77 |
| Affected area number | 1 | 1–2 |
| Admission biochemical parameters: | ||
| WBC, ×103/μL | 14.25 | 11.60–17.03 |
| CRP, mg/Ll | 138.3 | 82.5–210.7 |
| Procalcitonin, ng/mL | 0.13 | 0.08–0.38 |
| Main symptoms: | ||
| Pain | 94 | 93.07 |
| Swelling | 83 | 82.18 |
| Trismus | 61 | 60.40 |
| Swallowing difficulties | 39 | 38.61 |
| Fever | 22 | 21.78 |
| Breathing difficulties | 7 | 6.93 |
| Headache | 4 | 3.96 |
| Abscess area: | ||
| Submandibular | 67 | 66.34 |
| Submental | 12 | 11.88 |
| Neck | 11 | 10.89 |
| Pterygomandibular | 9 | 8.91 |
| Submasseteric | 9 | 8.91 |
| Mouth floor | 6 | 5.94 |
| Canine fossa | 5 | 4.95 |
| Perimandibular | 4 | 3.96 |
| Parapharyngeal | 3 | 2.97 |
| Intraoral | 2 | 1.98 |
| Buccal | 2 | 1.98 |
| Preauricular | 2 | 1.98 |
| Subtemporal | 2 | 1.98 |
| Peritonsillar | 1 | 0.99 |
| Maxillary sinus | 1 | 0.99 |
| Orbit | 0 | 0.00 |
| Pterygopalatine | 0 | 0.00 |
| Sublingual | 0 | 0.00 |
| Phlegmon | 25 | 24.75 |
| Empyema | 1 | 0.99 |
| Affected side: | ||
| Maxilla | 7 | 6.93 |
| Mandible | 81 | 80.20 |
| Both/undetermined | 13 | 12.87 |
| Causative tooth: | ||
| Incisor | 2 | 1.98 |
| Canine | 1 | 0.99 |
| Premolar | 5 | 4.95 |
| Molar | 30 | 29.70 |
| Wisdom | 48 | 47.52 |
| Various/undetermined | 15 | 14.85 |
| Anaesthesia: | ||
| General | 86 | 85.15 |
| Local | 13 | 12.87 |
| Reoperation | 6 | 5.94 |
| Antibiotics (prior to): | 51 | 50.50 |
| Amoxicillin + clavulanic acid | 10 | 9.90 |
| Clindamycin | 31 | 30.69 |
| Metronidazole | 6 | 5.94 |
| Others | 4 | 3.96 |
| Antibiotics (hospital): | 98 | 97.03 |
| Amoxicillin + clavulanic acid | 32 | 31.68 |
| Clindamycin | 25 | 24.75 |
| Metronidazole | 69 | 68.32 |
| Cefuroxim | 20 | 19.80 |
| Ceftriaxone | 17 | 16.83 |
| Others | 1 | 0.99 |
| Pre-Pandemic | Pandemic | Post-Pandemic | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | ||
| Age | 39 | 30–52 | 33.5 | 23–39.5 | 30 | 25–45 | 0.265 |
| BMI | 24.77 | 22.31–27.24 | 24.52 | 19.44–27.77 | 23.57 | 21.60–27.15 | 0.723 |
| Hospitalisation length, days | 5 | 3–6 | 4.5 | 3–8.5 | 5 | 4–5 | 0.883 |
| Abscess volume, cm3 | 22.36 | 6.63–42.77 | 10.05 | 5.55–57.41 | 17.93 | 7.50–37.06 | 0.857 |
| Affected area number | 1 | 1–2 | 1 | 1–2 | 1 | 1–2 | 0.982 |
| Admission biochemical parameters: | |||||||
| WBC, ×103/μL | 14.18 | 12.04–17.91 | 13.02 | 9.81–15.79 | 14.57 | 11.60–17.03 | 0.543 |
| CRP, mg/L | 122.6 | 78.5–223.7 | 134.95 | 80.2–192.8 | 143.2 | 84.1–210.1 | 0.863 |
| Procalcitonin, ng/mL | 0.14 | 0.07–0.36 | 0.17 | 0.09–1.06 | 0.10 | 0.05–0.20 | 0.176 |
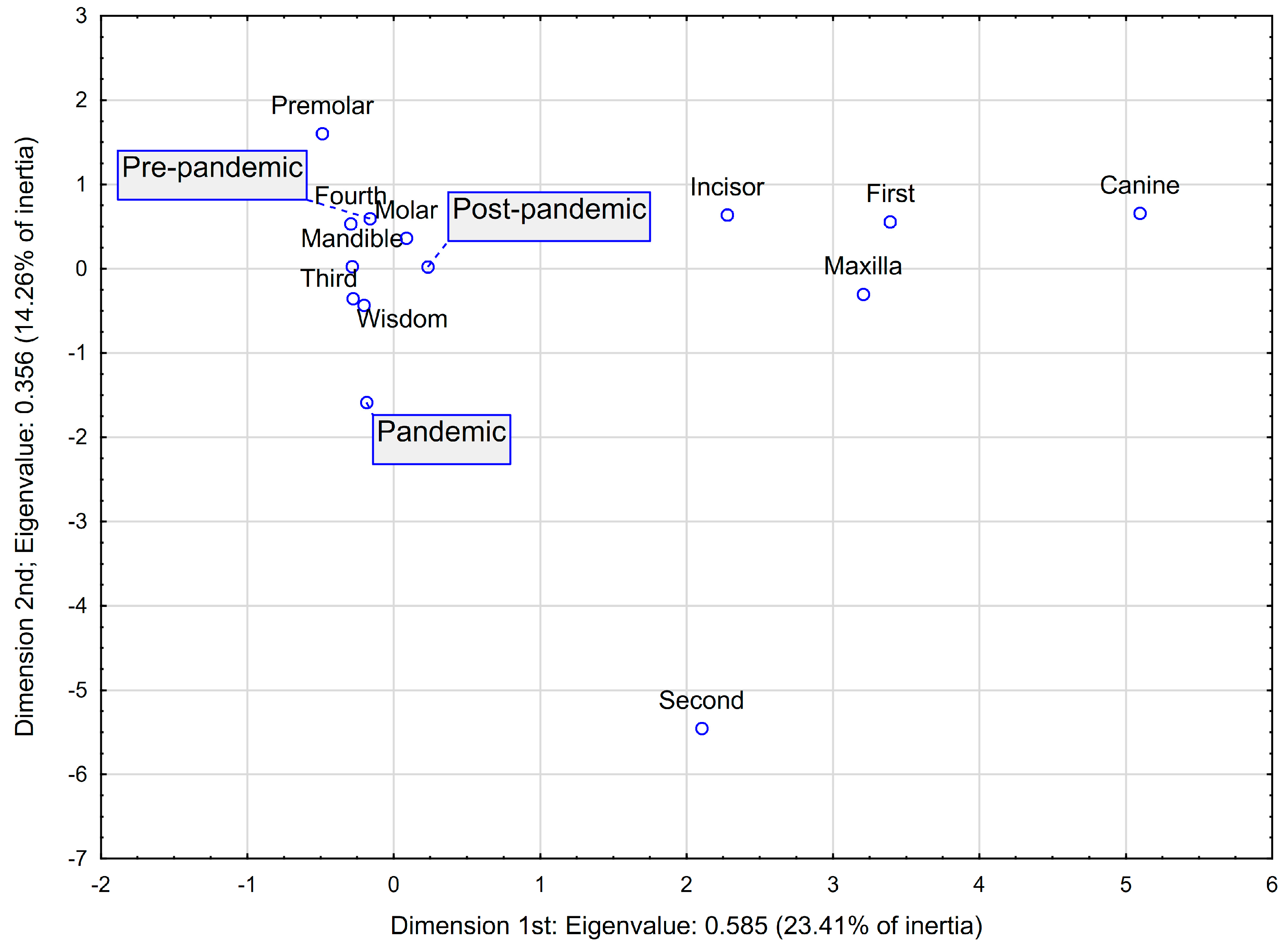
| x | y | Quality | Relative Inertia | x Inertia | x cos2 | y Inertia | y cos2 | |
|---|---|---|---|---|---|---|---|---|
| Period: pre-pandemic | −0.1616 | 0.5931 | 0.5686 | 0.0581 | 0.0047 | 0.0188 | 0.1033 | 0.2533 |
| Period: pandemic | −0.1836 | −1.5861 | 0.5879 | 0.0837 | 0.0023 | 0.0066 | 0.2872 | 0.4892 |
| Period: post-pandemic | 0.2330 | 0.0237 | 0.6350 | 0.0581 | 0.0097 | 0.0391 | 0.0002 | 0.0004 |
| Mandible | −0.2842 | 0.0267 | 0.9454 | 0.0081 | 0.0317 | 0.9114 | 0.0005 | 0.0080 |
| Maxilla | 3.2072 | −0.3012 | 0.9454 | 0.0919 | 0.3577 | 0.9114 | 0.0052 | 0.0080 |
| Quadrant: third | −0.2781 | −0.3554 | 0.2372 | 0.0477 | 0.0173 | 0.0849 | 0.0464 | 0.1387 |
| Quadrant: fourth | −0.2923 | 0.5325 | 0.2421 | 0.0605 | 0.0144 | 0.0559 | 0.0786 | 0.1854 |
| Quadrant: first | 3.3911 | 0.5572 | 0.8861 | 0.0930 | 0.3428 | 0.8625 | 0.0152 | 0.0233 |
| Quadrant: second | 2.1036 | −5.4516 | 0.6063 | 0.0988 | 0.0220 | 0.0521 | 0.2424 | 0.3496 |
| Wisdom | −0.2034 | −0.4343 | 0.5199 | 0.0442 | 0.0099 | 0.0523 | 0.0738 | 0.2382 |
| Molar | 0.0850 | 0.3630 | 0.2207 | 0.0651 | 0.0011 | 0.0039 | 0.0322 | 0.0706 |
| Premolar | −0.4874 | 1.6043 | 0.2864 | 0.0942 | 0.0059 | 0.0147 | 0.1050 | 0.1589 |
| Incisor | 2.2779 | 0.6387 | 0.1337 | 0.0977 | 0.0516 | 0.1235 | 0.0067 | 0.0097 |
| Canine | 5.0957 | 0.6566 | 0.3696 | 0.0988 | 0.1290 | 0.3055 | 0.0035 | 0.0051 |
| Hospitalisation Length | Abscess Volume | Affected Area Number | ||||
|---|---|---|---|---|---|---|
| Rs | p-Value | Rs | p-Value | Rs | p-Value | |
| Age | 0.059 | 0.560 | 0.118 | 0.430 | 0.153 | 0.127 |
| BMI | 0.253 | 0.011 * | −0.079 | 0.596 | 0.144 | 0.151 |
| Comorbidities | 0.195 | 0.051 | −0.050 | 0.736 | 0.256 | 0.010 * |
| Stimulants | −0.044 | 0.659 | 0.055 | 0.715 | 0.099 | 0.325 |
| Hospitalisation length | n/a | 0.239 | 0.106 | 0.495 | <0.001 * | |
| Affected area number | 0.495 | <0.001 * | 0.260 | 0.078 | n/a | |
| Abscess volume | 0.239 | 0.106 | n/a | 0.260 | 0.078 | |
| Antibiotics (prior to) | −0.186 | 0.063 | 0.188 | 0.205 | −0.119 | 0.235 |
| Antibiotics (hospital) | −0.222 | 0.026 * | −0.033 | 0.828 | −0.167 | 0.096 |
| Admission WBC | 0.183 | 0.066 | 0.342 | 0.018 * | 0.301 | 0.002 * |
| Admission CRP | 0.385 | <0.001 * | 0.104 | 0.485 | 0.393 | <0.001 * |
| Admission procalcitonin | 0.289 | 0.005 * | 0.046 | 0.765 | 0.258 | 0.013 * |
| Standardised Beta | Standard Error | p-Value | |
|---|---|---|---|
| Hospitalisation length: R2 = 0.815, p-value < 0.001 | |||
| Intercept | <0.001 * | ||
| Admission procalcitonin | 0.315 | 0.098 | 0.003 * |
| Admission CRP | 0.240 | 0.088 | 0.010 * |
| Abscess volume | 0.308 | 0.090 | 0.002 * |
| Reoperation: 1 | 0.299 | 0.092 | 0.003 * |
| Stimulants: 1 | −0.154 | 0.077 | 0.054 |
| Antibiotics (prior to): 1 | −0.084 | 0.084 | 0.323 |
| Abscess volume: R2 = 0.480, p-value = 0.001 | |||
| Intercept | 0.142 | ||
| Admission procalcitonin | 0.464 | 0.130 | 0.001 * |
| Affected area number | 0.122 | 0.137 | 0.381 |
| Period: post-pandemic | −0.274 | 0.127 | 0.039 * |
| Antibiotics (prior to): 1 | 0.281 | 0.130 | 0.038 * |
| Gender: males | 0.224 | 0.131 | 0.098 |
| Admission WBC | 0.159 | 0.140 | 0.264 |
| Affected area number: R2 = 0.283, p-value = 0.023 | |||
| Intercept | 0.267 | ||
| Admission WBC | 0.351 | 0.143 | 0.019 * |
| Location: city | 0.252 | 0.143 | 0.088 |
| Age | 0.190 | 0.141 | 0.186 |
| Side: mandible | 0.161 | 0.144 | 0.274 |
| Reoperation: R2 = 0.603, p-value < 0.001 | |||
| Intercept | 0.002 * | ||
| Hospitalisation length | 0.594 | 0.166 | 0.001 * |
| Comorbidities: 1 | 0.262 | 0.111 | 0.025 * |
| Abscess volume | −0.294 | 0.137 | 0.040 * |
| Admission procalcitonin | 0.301 | 0.163 | 0.074 |
| Affected area number | 0.173 | 0.113 | 0.138 |
| Stimulants: 1 | 0.143 | 0.110 | 0.205 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nijakowski, K.; Ksel, S.; Marushko, O.; Nowak, A.; Jankowski, J.; Kwiatkowski, J.; Marushko, O.; Słowik, Ł.; Okła, M. Impact of the COVID-19 Pandemic on Odontogenic Abscess Clinical Patterns and Predictive Factors: A Retrospective Cross-Sectional Study. J. Clin. Med. 2025, 14, 6953. https://doi.org/10.3390/jcm14196953
Nijakowski K, Ksel S, Marushko O, Nowak A, Jankowski J, Kwiatkowski J, Marushko O, Słowik Ł, Okła M. Impact of the COVID-19 Pandemic on Odontogenic Abscess Clinical Patterns and Predictive Factors: A Retrospective Cross-Sectional Study. Journal of Clinical Medicine. 2025; 14(19):6953. https://doi.org/10.3390/jcm14196953
Chicago/Turabian StyleNijakowski, Kacper, Stanisław Ksel, Olesya Marushko, Aleksy Nowak, Jakub Jankowski, Jacek Kwiatkowski, Olena Marushko, Łukasz Słowik, and Maciej Okła. 2025. "Impact of the COVID-19 Pandemic on Odontogenic Abscess Clinical Patterns and Predictive Factors: A Retrospective Cross-Sectional Study" Journal of Clinical Medicine 14, no. 19: 6953. https://doi.org/10.3390/jcm14196953
APA StyleNijakowski, K., Ksel, S., Marushko, O., Nowak, A., Jankowski, J., Kwiatkowski, J., Marushko, O., Słowik, Ł., & Okła, M. (2025). Impact of the COVID-19 Pandemic on Odontogenic Abscess Clinical Patterns and Predictive Factors: A Retrospective Cross-Sectional Study. Journal of Clinical Medicine, 14(19), 6953. https://doi.org/10.3390/jcm14196953








