A Puzzling Pair: Flail Leg Syndrome with Myokymia and Avascular Hip Necrosis—Case Study and Systematic Literature Review
Abstract
1. Introduction
2. Case Presentation
3. Materials and Methods
4. Results
4.1. Study Selection
4.2. Quality Assessment of Included Case Reports
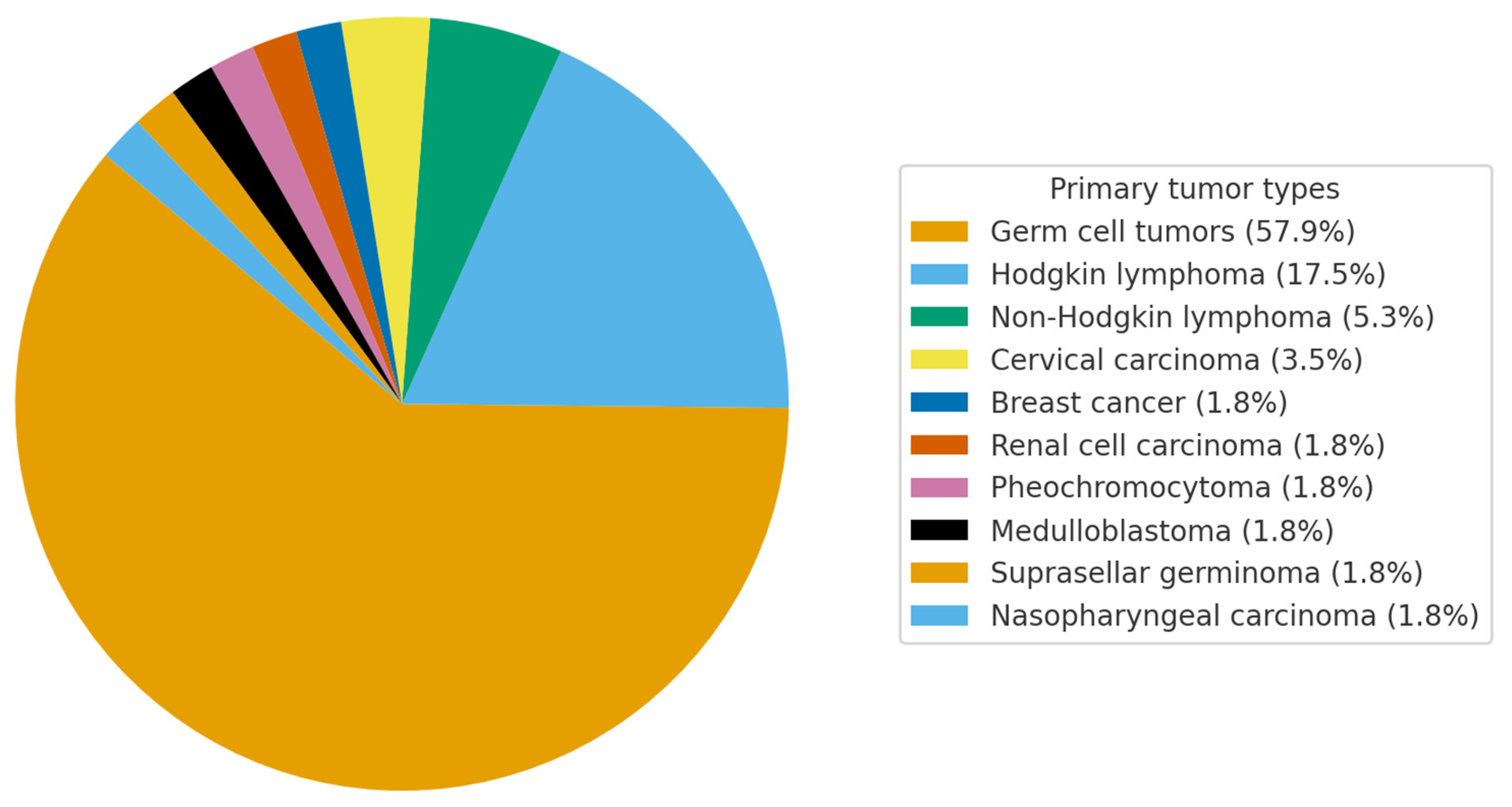
4.3. Clinical, Demographic, and Radiological Characteristics of the Cohort
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LMNS | Lower Motor Neuron Syndrome |
| CSF | Cerebrospinal Fluid |
| NCSs | Nerve Conduction Studies |
| SNAPs | Sensory Nerve Action Potentials |
| EMG | Electromyography |
| EBRT | External beam radiotherapy |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| JBI | Joanna Briggs Institute |
References
- Kosmin, M.; Rees, J. Radiation and the Nervous System. Pract. Neurol. 2022, 22, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Oishi, T.; Ryan, C.S.; Vazquez Do Campo, R.; Laughlin, R.S.; Rubin, D.I. Quantitative Analysis of Myokymic Discharges in Radiation versus Nonradiation Cases. Muscle Nerve 2021, 63, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.H.; Tang, J.S.; Shen, X.Y.; Niu, Z.X.; Xiao, J.L. Osteoradionecrosis of the Hip, a Troublesome Complication of Radiation Therapy: Case Series and Systematic Review. Front. Med. 2022, 9, 858929. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Matsuda, N.; Kobayashi, S.; Matsumoto, H.; Machii, M.; Soeda, T.; Ugawa, Y. Cauda Equina Involvement in Post-Radiation Lower Motor Neuron Syndrome. Intern. Med. 2015, 54, 1415–1419. [Google Scholar] [CrossRef][Green Version]
- Hsia, A.W.; Katz, J.S.; Hancock, S.L.; Peterson, K. Post-Irradiation Polyradiculopathy Mimics Leptomeningeal Tumor on MRI. Neurology 2003, 60, 1694–1696. [Google Scholar] [CrossRef]
- Van Der Sluis, R.W.; Wolfe, G.I.; Nations, S.P.; Bryan, W.W.; Krampitz, D.E.; Kissel, J.T.; Barohn, R.J. Post-Radiation Lower Motor Neuron Syndrome. J. Clin. Neuromuscul. Dis. 2000, 2, 10–17. [Google Scholar] [CrossRef]
- Anezaki, T.; Harada, T.; Kawachi, I.; Sanpei, K.; Soma, Y.; Tsuji, S. A case of post-irradiation lumbosacral radiculopathy successfully treated with corticosteroid and warfarin. Rinsho Shinkeigaku 1999, 39, 825–829. [Google Scholar]
- Wohlgemuth, W.A.; Rottach, K.; Jaenke, G.; Stöhr, M. Radiogenic Amyotrophy. Cauda Equina Lesion as a Late Radiation Sequela. Nervenarzt 1998, 69, 1061–1065. [Google Scholar] [CrossRef]
- Tallaksen, C.M.E.; Jetne, V.; Fosså, S. Acta Oncologica Postradiation Lower Motor Neuron Syndrome: A Case Report and Brief Literature Review. Acta Oncol. 2009, 36, 345–347. [Google Scholar] [CrossRef]
- Bowen, J.; Gregory, R.; Squier, M.; Donaghy, M. The Post-Irradiation Lower Motor Neuron Syndrome Neuronopathy or Radiculopathy? Brain 1996, 119 Pt 5, 1429–1439. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, R.A.; Chroni, E.; Panayiotopoulos, C.P.; Enevoldson, T.P. Late Onset Radiation-Induced Motor Neuron Syndrome. J. Neurol. Neurosurg. Psychiatry 1992, 55, 741–742. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M. Post Radiation Monomelic Amyotrophy. J. Neurol. Neurosurg. Psychiatry 1992, 55, 629. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lamy, C.; Mas, J.L.; Varet, B.; Ziegler, M.; de Recondo, J. Postradiation Lower Motor Neuron Syndrome Presenting as Monomelic Amyotrophy. J. Neurol. Neurosurg. Psychiatry 1991, 54, 648–649. [Google Scholar] [CrossRef]
- Feistner, H.; Weissenborn, K.; Münte, T.F.; Heinze, H.-J.; Malin, J.P. Post-Irradiation Lesions of the Caudal Roots. Acta Neurol. Scand. 1989, 80, 277–281. [Google Scholar] [CrossRef]
- Gállego, J.; Delgado, G.; Tuñón, T.; Villanueva, J.A. Delayed Postirradiation Lower Motor Neuron Syndrome. Ann. Neurol. 1986, 19, 308–309. [Google Scholar] [CrossRef]
- De Carolis, P.; Montagna, P.; Cipulli, M.; Baldrati, A.; D’Alessandro, R.; Sacquegna, T. Isolated Lower Motoneuron Involvement Following Radiotherapy. J. Neurol. Neurosurg. Psychiatry 1986, 49, 718–719. [Google Scholar] [CrossRef][Green Version]
- Lagueny, A.; Aupy, M.; Aupy, P.; Ferrer, X.; Henry, P.; Julien, J. Post-radiotherapy anterior horn cell syndrome. Rev. Neurol. 1985, 141, 222–227. [Google Scholar][Green Version]
- Horowitz, S.L.; Stewart, J.D. Lower Motor Neuron Syndrome Following Radiotherapy. Can. J. Neurol. Sci. 1983, 10, 56–58. [Google Scholar] [CrossRef][Green Version]
- Schiødt, A.V.; Kristensen, O. Neurologic Complications after Irradiation of Malignant Tumors of the Testis. Acta Radiol. Oncol. Radiat. Phys. Biol. 1978, 17, 369–378. [Google Scholar] [CrossRef]
- Kristensen, O.; Melgård, B.; Schiødt, A.V. Radiation Myelopathy of the Lumbo-Sacral Spinal Cord. Acta Neurol. Scand. 1977, 56, 217–222. [Google Scholar] [CrossRef]
- Sadowsky, C.H.; Sachs, E.; Ochoa, J. Postradiation Motor Neuron Syndrome. Arch. Neurol. 1976, 33, 786–787. [Google Scholar] [CrossRef]
- Abraham, A.; Drory, V.E. Postradiation Lower Motor Neuron Syndrome: Case Series and Literature Review. J. Neurol. 2013, 260, 1802–1806. [Google Scholar] [CrossRef] [PubMed]
- Delanian, S.; Pradat, P.F. Posteriori Conformal Radiotherapy Using Three-Dimensional Dosimetric Reconstitution in a Survivor of Adult-Onset Hodgkin’s Disease for Definitive Diagnosis of Lower Motor Neuron Disease. J. Clin. Oncol. 2010, 28, e599–e601. [Google Scholar] [CrossRef] [PubMed]
- Ésik, O.; Lengyel, Z.; Sáfrány, G.; Vönöczky, K.; Ágoston, P.; Székely, J.; Lengyel, E.; Márián, T.; Trón, L.; Bodrogi, I. A PET Study on the Characterization of Partially Reversible Radiogenic Lower Motor Neurone Disease. Spinal Cord. 2002, 40, 468–473. [Google Scholar] [CrossRef] [PubMed]
- De Greve, J.L.P.; Bruyland, M.; De Keyser, J.; Storme, G.; Ebinger, G. Lower Motor Neuron Disease in a Patient with Hodgkin’s Disease Treated with Radiotherapy. Clin. Neurol. Neurosurg. 1984, 86, 43–46. [Google Scholar] [CrossRef]
- Shapiro, B.E.; Rordorf, G.; Schwamm, L.; Preston, D.C. Delayed Radiation-Induced Bulbar Palsy. Neurology 1996, 46, 1604–1606. [Google Scholar] [CrossRef]
- Giray, E.; Karayigit, M.; Senocak, K.C.; Illeez, O.G.; Ozkan, F.U.; Aktas, I.; Gozke, E. Delayed Radiation-Induced Motor Neuron Syndrome: A Case Report. J. Back. Musculoskelet. Rehabil. 2023, 36, 1469–1475. [Google Scholar] [CrossRef]
- Gagnier, J.J.; Kienle, G.; Altman, D.G.; Moher, D.; Sox, H.; Riley, D.; Allaire, A.; Aronson, J.; Carpenter, J.; Gagnier, J.; et al. The CARE Guidelines: Consensus-Based Clinical Case Reporting Guideline Development. BMJ Case Rep. 2013, 2013, 38–43. [Google Scholar] [CrossRef]
- Knap, M.M.; Bentzen, S.M.; Overgaard, J. Late Neurological Complications after Irradiation of Malignant Tumors of the Testis. Acta Oncol. 2007, 46, 497–503. [Google Scholar] [CrossRef]
- Raheem, O.A.; Hickey, D.P. Postirradiation Lumbosacral Radiculopathy Following Seminoma Treatment Presenting as Flaccid Neuropathic Bladder: A Case Report. J. Med. Case Rep. 2011, 5, 148. [Google Scholar] [CrossRef]
- Tan, S.V.; Pye, I.F. Postradiation Motor Neuron Syndrome of the Upper Cervical Region—A Manifestation of the Combined Effect of Cranial Irradiation and Intrathecal Chemotherapy? J. Neurol. Neurosurg. Psychiatry 1991, 54, 469–470. [Google Scholar] [CrossRef][Green Version]
- Memon, A.B.; Playfoot, K.A. Radiation—induced Tongue Myokymia with Hypoglossal Nerve Damage, Mimicker of Motor Neuron Disease. Clin. Case Rep. 2017, 5, 1056. [Google Scholar] [CrossRef]
- Bhatia, S.; Miller, R.C.; Lachance, D.L. Neck Extensor Muscle Weakness (Dropped Head Syndrome) Following Radiotherapy. Radiol. Oncol. 2006, 40, 29–33+60. [Google Scholar]
- Greenfield, M.M.; Stark, F.M. Post-Irradiation Neuropathy. Am. J. Roentgenol. Radium Ther. 1948, 60, 617–622. [Google Scholar]
- Umeda, M.; Naruse, S.; Ito, A.; Fujita, N. Gandolinium Enhancement of the Anterior Portion of the Lumbosacral Roots in a Case of Post-Irradiation Lumbosacral Radiculopathy. Rinsho Shinkeigaku 2005, 45, 758–761. [Google Scholar]

| Nerve/Recording Site/Stimulation Site | Latency (ms)/(Normal Values) | Amplitude (mV)/(Normal Values) | Duration (ms)/(Normal Values) | Velocity (m/s)/(Normal Values) |
|---|---|---|---|---|
| Left Median-APB | ||||
| Wrist | 3.59 (≤4.3) | 16.1 (≥10) | 6.25 (≤10) | |
| Elbow | 7.08 | 15.9 | 6.82 | 65.9 (≥50) |
| Left Ulnar-ADM | ||||
| Wrist | 2.71 (≤3.3) | 20.0 (≥10) | 6.09 (≤10) | |
| Bellow Elbow | 5.52 | 19.4 | 5.57 | 71.1 (≥50) |
| Left Peroneal-EDB | ||||
| Ankle | 4.58 (≤6.0) | 1.5 (≥2) | 4.38 (≤10) | |
| Bellow Fibular Head | 11.46 | 1.5 | 4.95 | 40.7 (≥43) |
| Right Peroneal | ||||
| Ankle | 2.71 | 12.6 (≥2) | 5.42 | |
| Bellow Fibular Head | 9.11 | 10.4 | 5.73 | 45.3 |
| Left Tibial-AH | ||||
| Ankle | 4.90 (≤6.0) | 0.8 (≥2) | 9.01 (≤10) | |
| Knee | 14.22 | 0.7 | 9.53 | 40.8 (≥43) |
| Right Tibial-AH | ||||
| Ankle | 2.92 | 13.0 (≥ 2) | 6.20 | |
| Nerve/Stimulation Site | Recording Site | Latency (ms)/(Normal Values) | Amplitude (μV)/(Normal Values) | Distance (cm)/(Normal Values) | Velocity (m/s)/(Normal Values) |
|---|---|---|---|---|---|
| Left Median | |||||
| Wrist | II finger | 2.45 (≤3.0) | 17.1(≥10) | 14 | 57.2 (≥50) |
| Left Ulnar | |||||
| Wrist | V finger | 2.19 (≤3.0) | 16.6 (≥10) | 14 | 64.0 (≥50) |
| Left Sural | |||||
| Calf | Ankle | 2.45 | 11.6 (≥10) | 14 | 57.2 (≥50) |
| Nerve/Recording Site | Minimal F Wave Latency (ms)/(Normal Values) |
|---|---|
| Left ulnar—ADM | 23.6 (≤33) |
| Left peroneal—EDB | 62.2 (≤55) |
| Left tibial—AH | Absent |
| Right peroneal—EDB | 58.6 (≤55) |
| Right tibial—AH | 59.1 (≤55) |
| Needle EMG | Spontaneous Activity | MUAP | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Muscle | Fib. | PSW | Fasciculations | Amplitude (mV) | Duration | Polyphasicity | Pattern Reduction | CRD | Other |
| Left tibialis anterior | 2+ | 2+ | No | 7 | 1+ | 1+ | 3+ | No | No |
| Left gastrocnemius (Medial head) | 3+ | 3+ | No | 10 | 1+ | 1+ | 3+ | No | No |
| Left vastus lateralis | 3+ | 3+ | No | 8 | 1+ | N | 2–3+ | No | No |
| Right tibialis Anterior | No | No | No | 5 | 1+ | 1+ | 3+ | No | Prolonged insertional activity |
| Left first dorsal interosseous | No | No | No | 5 | 1+ | 1+ | N/1+ | No | No |
| Left deltoid | No | No | No | 2–5 | 1+ | 2+ | N/1+ | No | No |
| Left masseter | No | No | No | 2 | N | N | N | No | No |
| Left gluteus medius | No | No | No | 4 | 1+ | 2+ | 2+ | No | Prolonged insertional activity, myokymia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrijan, T.; Menih, M.; Gselman, S. A Puzzling Pair: Flail Leg Syndrome with Myokymia and Avascular Hip Necrosis—Case Study and Systematic Literature Review. J. Clin. Med. 2025, 14, 6955. https://doi.org/10.3390/jcm14196955
Petrijan T, Menih M, Gselman S. A Puzzling Pair: Flail Leg Syndrome with Myokymia and Avascular Hip Necrosis—Case Study and Systematic Literature Review. Journal of Clinical Medicine. 2025; 14(19):6955. https://doi.org/10.3390/jcm14196955
Chicago/Turabian StylePetrijan, Timotej, Marija Menih, and Saša Gselman. 2025. "A Puzzling Pair: Flail Leg Syndrome with Myokymia and Avascular Hip Necrosis—Case Study and Systematic Literature Review" Journal of Clinical Medicine 14, no. 19: 6955. https://doi.org/10.3390/jcm14196955
APA StylePetrijan, T., Menih, M., & Gselman, S. (2025). A Puzzling Pair: Flail Leg Syndrome with Myokymia and Avascular Hip Necrosis—Case Study and Systematic Literature Review. Journal of Clinical Medicine, 14(19), 6955. https://doi.org/10.3390/jcm14196955






