1. Introduction
Joint-preserving arthroscopic and open surgical operations are one of the most frequently performed orthopaedic surgical procedures [
1]. Nevertheless, there are no uniform post-treatment recommendations regarding the rehabilitation process [
2,
3]. These vary almost from institution to institution. Recently, there have been attempts to improve this through scientific studies. However, most such studies relate to ACL-reconstruction interventions and only a few to other pathologies [
2,
4,
5]. However, the studies all agree that good rehabilitation plays a decisive role in the patient’s outcome [
6].
ACL injuries are among the most common sports- and trauma-related knee injuries, with an estimated incidence of 68.6 per 100,000 person-years in young athletes [
7]. Non-contact mechanisms such as sudden pivoting, deceleration, or valgus stress ac-count for the majority of ACL ruptures, while contact injuries occur less frequently [
8]. Surgical reconstruction is typically performed using autografts (e.g., hamstring or patellar tendon) or allografts, with arthroscopic techniques being the current standard of care [
9]. Despite high surgical success rates, postoperative outcomes strongly depend on rehabilitation quality [
2]. These considerations highlight the need for standardized rehabilitation pathways and justify the evaluation of digital monitoring systems like Orthelligent as potential tools to improve recovery and adherence.
Over the last few years, a trend has emerged in the study situation away from the classic rehabilitation approach with physiotherapy alone towards supplementary interventions using digital medical devices [
10]. Devices such as the Orthelligent tool from OPED (Valley, Germany) are usually not used on their own but are designed to supplement rehabilitation sports and physiotherapy for use at home [
10]. In the course of this development, a number of studies were also carried out to evaluate whether measurement using individual sensors is adequate and reliable [
11].
Recent studies have increasingly investigated the role of digital and virtual rehabilitation modalities after ACL reconstruction. A recent meta-analysis demonstrated that virtual reality-based rehabilitation significantly improves knee function, strength, and balance compared to standard care [
12]. Another systematic review confirmed that VR-based interventions reduce pain and enhance dynamic stability [
13]. Moreover, mobile health–based home rehabilitation programs have been shown in randomized trials to improve early outcomes and patient compliance [
14]. These data underline the contemporary relevance of integrating digital solutions such as Orthelligent into standardized ACL rehabilitation pathways.
Some studies have also been able to demonstrate effectiveness in terms of an improved rehabilitation process. This was shown by increased adherence to the rehabilitation protocols and an increased willingness to exercise compared to patients without the support of such tools [
10,
15,
16,
17].
Although there are data for “return to play” periods from professional football, it remains unclear how long normal patients need on average to be able to move without pain after knee surgery [
18]. It is also unclear to what extent recommendations for postoperative weight-bearing and exercise as well as exercises for the knee can be applied [
2].
For this reason, this study aimed to further evaluate how an average rehabilitation process can be structured and in which rehabilitation phases the most functional progress is achieved. The secondary aim is to identify which parameters show particular influence on the rehabilitation progress, such as anthropometric properties or pain, which is known to increase the risk of postoperative arthrofibrosis. Finally, we aimed to determine which rehabilitation exercises or groups of exercises show poorer results and should therefore be adapted in the rehabilitation process.
2. Materials and Methods
2.1. Study Design and Population
In a retrospective cohort analysis, routine data of patients following anterior cruciate ligament reconstruction (ACLR) were analyzed, which were documented using the digital medical product “Orthelligent”. The data corresponds to the routine data of the sensor and were collected anonymously in the period from August 2022 to December 2024. The study population therefore includes all patients who used the “Orthelligent home” version in the defined period. As the Orthelligent system is distributed throughout Germany, included patients underwent ACL reconstruction at different orthopedic centers across the country. Surgeries were not limited to a single institution, and postoperative rehabilitation was carried out either at local physiotherapy practices or at home using the Orthelligent home system. After knee surgery, patients are prescribed the app by their orthopaedic surgeon as an additional tool to normal rehabilitation. It was also possible to acquire the app independently and without a prescription by borrowing the sensor for a fee. Baseline demographic data (sex, age, BMI, height, weight) were collected and are summarized in
Table 1. Information on concomitant pharmacological treatments (e.g., analgesics and thromboprophylaxis) and perioperative complications was not systematically available in the dataset and is therefore acknowledged as a limitation.
The study was also registered with the research management of Paracelsus Medical University (FMS_IF_006.25-I-2; Regeneration) and it was evaluated by the Institutional Review Board (IRB-2025-08) that no ethics vote is necessary.
AI Statement: Artificial intelligence (AI) tools were used to support the drafting and editing of this text. All final decisions on content, wording, and interpretation were made by the authors.
2.2. Inclusion and Exclusion Criteria
Inclusion criteria: Time of surgery within this period and at least one test. The indication in the sense of an isolated or combined injury to the anterior cruciate ligament was also an inclusion criterion. The indication in terms of an isolated or combined injury to the anterior cruciate ligament was also an inclusion criterion. This resulted in 4 injury patterns that could be included: (1) Isolated ACL rupture; (2) ACL rupture and meniscus injury; (3) ACL rupture and collateral ligament injury; (4) ACL rupture with collateral ligament and meniscus injury. Aborted tests were also documented by the sensor, and the app subsequently asked why the test was aborted so that these tests could also be included in the evaluation and an assessment of the difficulty of the exercises could be carried out.
Exclusion criteria: All those who did not have a corresponding indication or surgery in the defined time were excluded. All Patients older than 65 years were also excluded.
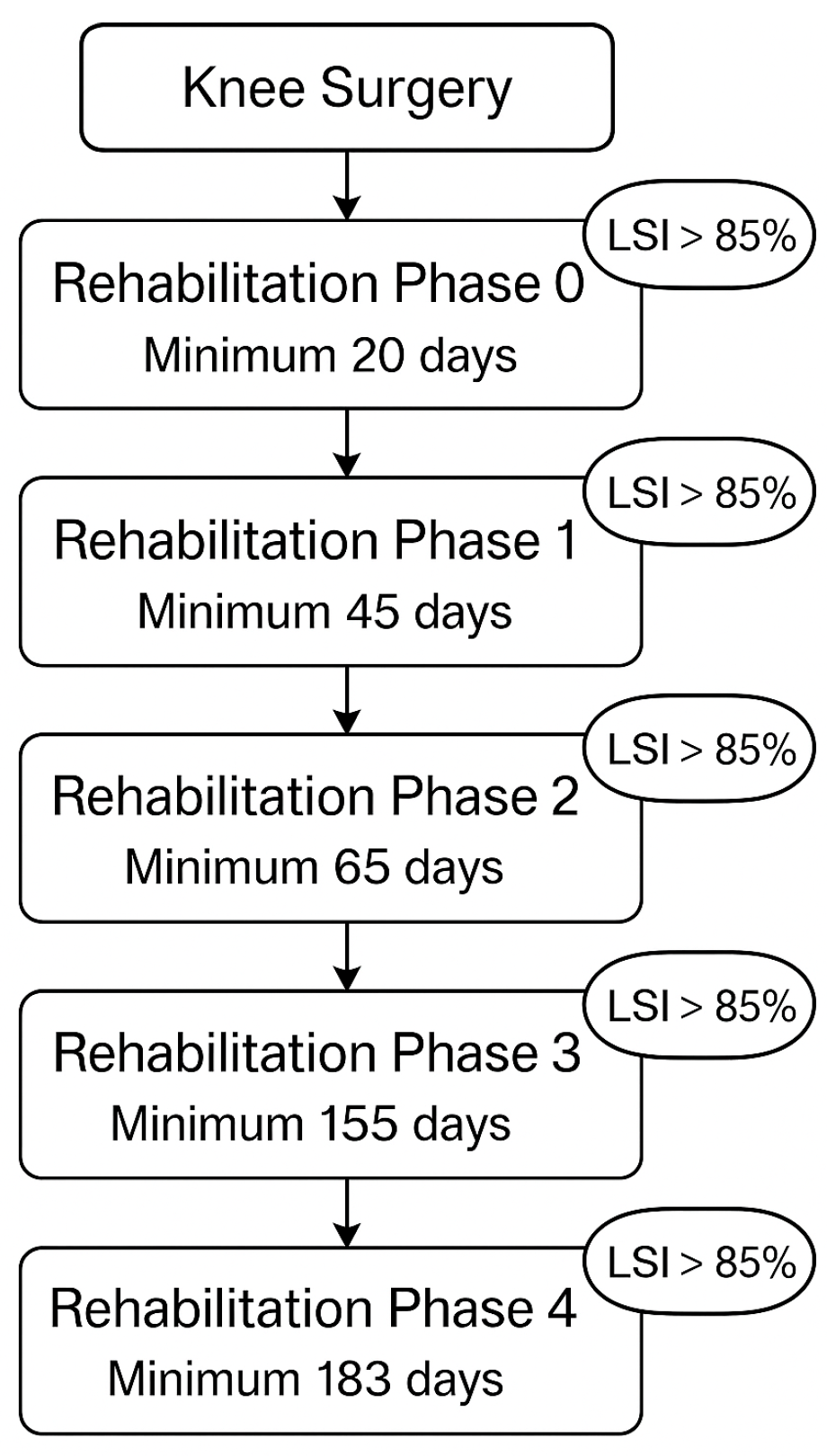
2.3. Structure of the Rehab Program
The rehabilitation program, which forms the basis for the algorithm, consists of five phases based on established ACL clinical practice guidelines [
2,
3]. At the beginning, each patient was asked to state the type of injury, the treatment that had taken place or was about to take place, as well as anthropometric data such as height and weight. Each patient could choose training frequency per week and change this setting during progress. The patient was asked to indicate whether they are in a pre- or post-operative phase and provide a surgery date. The age was recorded in age groups from 0 to 65 years. From 0 to 15 and from the age of 15 in steps of five years.
An initial evaluation test was then carried out on the healthy leg and the injured leg, in which the range of motion, flexion and extension, and stability in the single-leg stance were tested. These tests calculated the flexion in degrees and the extension deficit in millimetres, as well as the limb symmetry index from the comparative data with the healthy leg.
Each patient started in rehabilitation phase 0. After the evaluation test, the patient was assigned exercises, depending on the preset exercise frequency, which they had to complete with the sensor attached and then indicate the difficulty and potential pain in the app. The exercises were assigned to the rehabilitation phases and evaluated by previous studies and a panel of OPED experts so that patients are not given exercises that are too difficult at too early a stage [
10].
Exercise content ranged from basic range-of-motion and weight-bearing activities in early phases to strength, neuromuscular control, and plyometric training in later phases. Patients reported training frequency (number of sessions per week) individually, as mentioned, and the Orthelligent app adapted exercise difficulty accordingly. This ensured gradual, guideline-based progression while allowing individualized adjustments.
After completing an exercise phase for a defined period, a new evaluation test was carried out followed by a new assignment to the rehabilitation phases. Progress can only be made if the LSI is greater than 85% and a minimum number of days have been completed. Phase 0 had to be completed after at least the 20 postoperative day, phase 1 by day 45, phase 2 by day 65, phase 3 by day 155, and phase 4 by day 183 (
Figure 1). This prevents too rapid progression and possible overloading and damage.
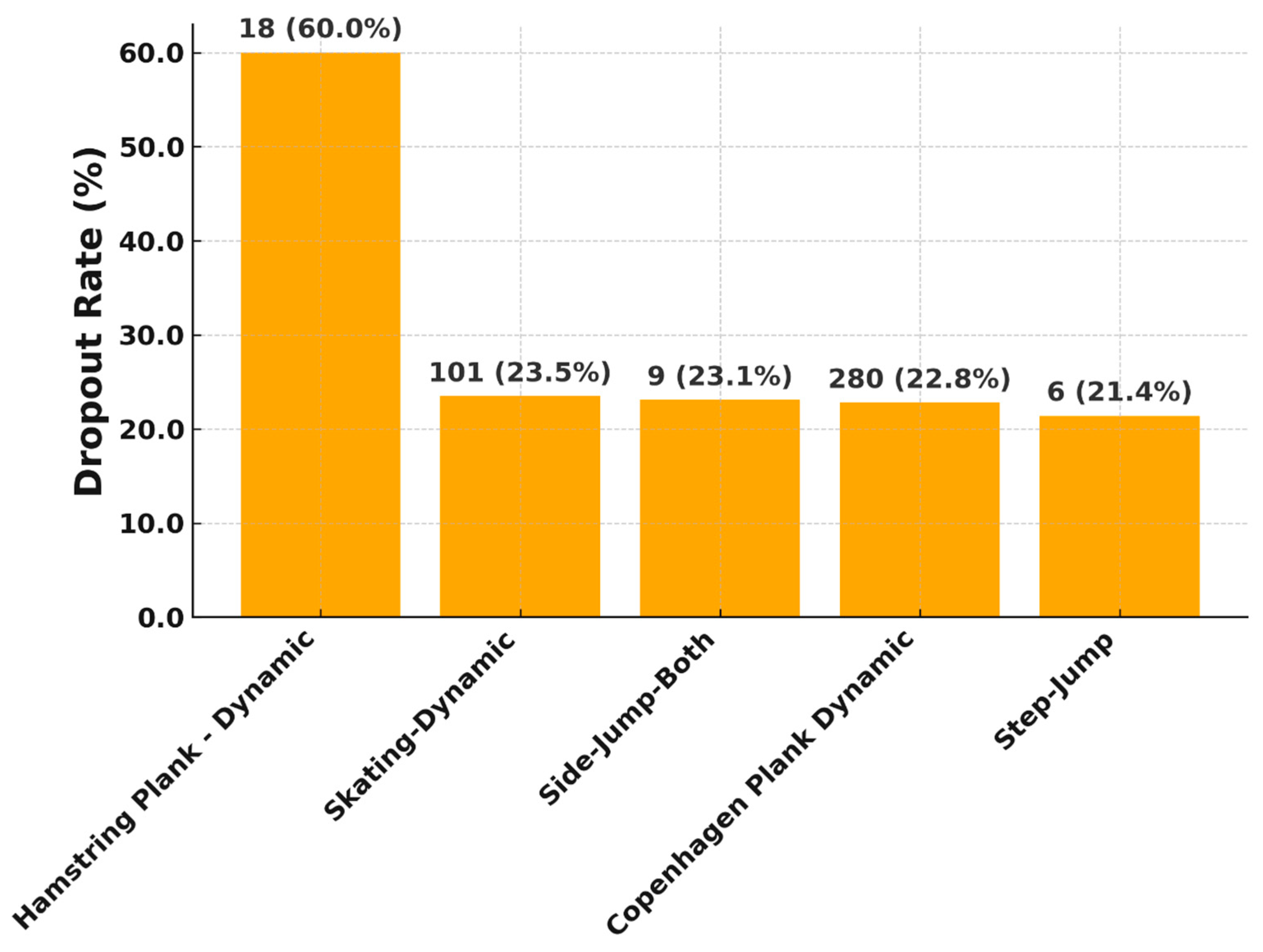
Depending on the progression of the rehabilitation process, the exercises became more difficult, coordinatively more complex and also require more strength (e.g., Phase 0: Seated Single Leg Quad Extension, Leg-lift-supported; Phase 1: Single Leg Balance, Mini-squat; Phase 2: Copenhagen Plank Dynamic, Side Lying Adductor Raise; Phase 3: Sumo Squat, Elevated Hamstring Plank–Dynamic; Phase 4: Lying-squat, Single Leg Glute Bridge on Wall). Different levels and units were measured for each test, so the exercises could be compared with each other. As the LSI was recorded for all exercises, a comparison of the rehabilitation progress over time was possible. A drop-out was defined as not achieving or breaking off the exercise goal set, in particular when a patient stopped the exercise, and the patient was asked directly by the app for the reason of abortion.
2.4. Statistical Analysis
IBM SPSS Statistics Version 30.0, Microsoft Excel (Microsoft 365) and Cloud Software Group, Inc. (2023) Data Science Workbench, Version 14, were used for the statistical analysis. Generalized linear models (GLM) with generalized estimating equations (GEE) were used to quantify functional developments over the course of rehabilitation. This method allows a robust estimation of correlated longitudinal data, as they are generated in the context of repetitive testing over different rehabilitation phases.
The dependent variable was the limb symmetry index (LSI), measured in percent, with a normally distributed error structure and identity link function. The independent variables included elapsed time since surgery and covariates such as pain score, body weight and height. The rehabilitation phase was included as a categorical influencing factor. The Wald-Chi2 test was used to test the significance of individual influencing variables.
Supplementary analyses were performed, including pairwise comparisons of estimated marginal means between rehabilitation phases, as well as the influence of categorical predictors on pain data using Chi2 tests. In total, the analysis comprised 5675 individual measurements in 335 patients. The focus was on identifying the rehabilitation phases with the greatest functional progress and determining the factors that significantly influence these developments. The results provided differentiated findings on the effectiveness of digitally supported rehabilitation processes and their controllability through relevant clinical and subjective parameters.
4. Discussion
The most important finding of this study was that the use of an app-structured rehabilitation program showed significant functional improvement during early rehabilitation phases. Time since surgery was a significant positive predictor while pain showed a strong negative impact on performance.
A common problem in rehabilitation research to date—particularly in the orthopaedic–surgical context—is that there are hardly any robust datasets available that map the time course of functional recovery in detail. The vast majority of studies are limited to selective measurements (e.g., 6 or 12 weeks postoperatively) or only record final outcome values (e.g., return to sport after 6 months) [
4,
18]. In this respect, our present data collection with thousands of feedback on a variety of exercises and classification according to the rehabilitation phases (0–5) could be useful for further development of artificial intelligence (AI)-based rehabilitation programs. For the first time, it allows a phase-related analysis for increased pain or problems during exercising. Exercises that lead to dropouts in certain rehabilitation phases were identified.
Systematic longitudinal reported data on exercise adherence, resilience, and pain changes over the course of time are urgently needed for shaping a personalized rehabilitation approach for each individual patient. This study based on routine data is unique to date and fills a key gap in the literature.
Nevertheless, classification and interpretation of the data must be assessed carefully. By using routine data from a system that potentially improves the rehabilitation process, it is unclear whether these results can be transferred to the general population. Studies such as that by Höher et al. 2023, Essery et al. 2017 or Dunphy et al. 2020 showed increased adherence and patient satisfaction when using digital systems such as Orthelligent [
10,
16,
17]. The functional added value compared to standard procedures remains controversial. Randomized controlled trials with robust functional endpoints are lacking.
Digital monitoring can create valuable data, which can be used to investigate the microdynamic patterns of the rehabilitation process and thus still provide reliable results due to the large heterogeneous population. The documented reduction in pain and drop-outs, combined with increasing exercise quality (e.g., more “perfect” feedback), allows valid conclusions to be drawn about typical recovery patterns.
4.1. Functional Recovery Patterns
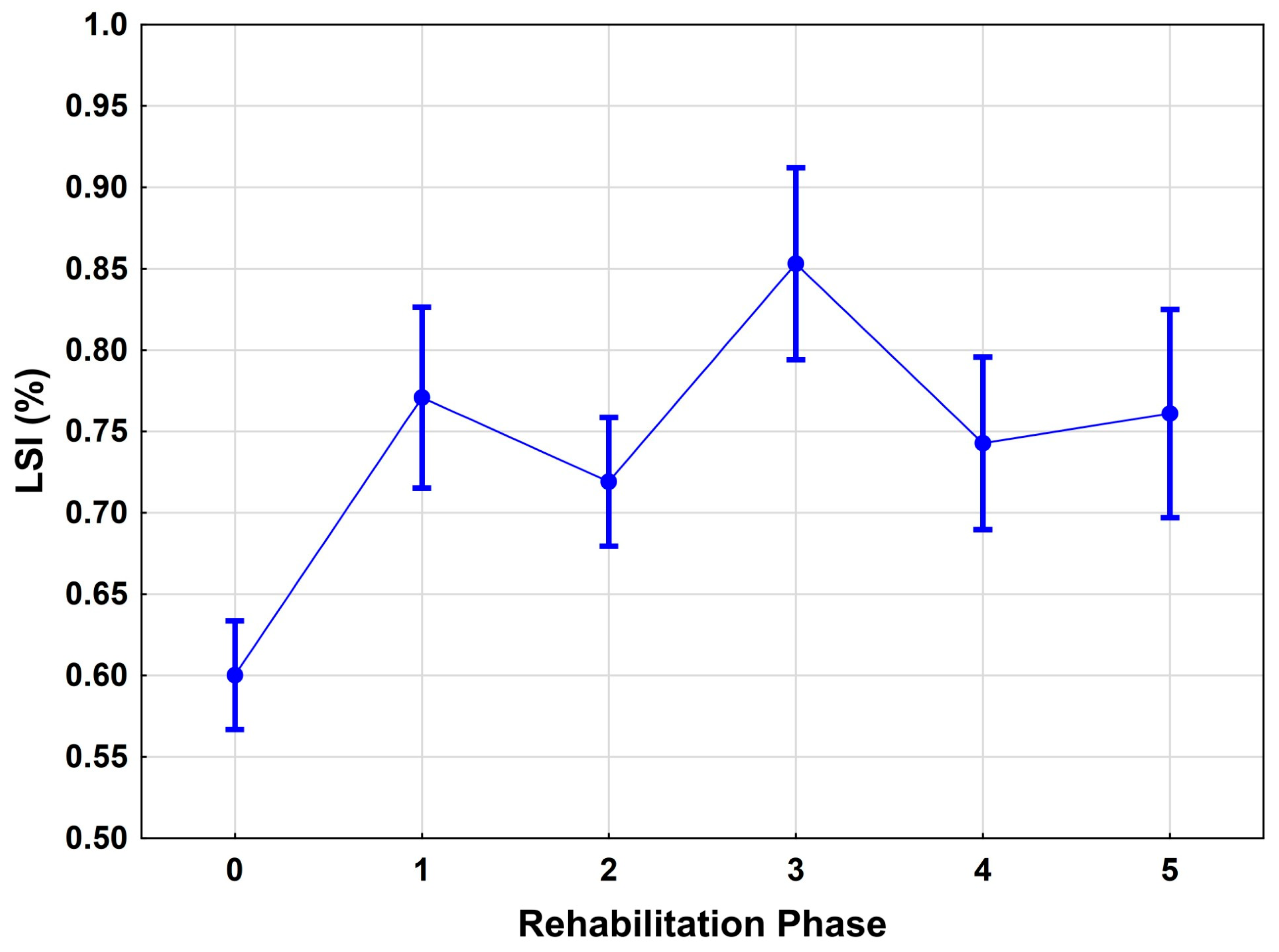
The present analysis demonstrates a clear, statistically significant improvement in functional capacity as measured by the Limb Symmetry Index (LSI) over the course of rehabilitation. Specifically, the LSI increased from a mean of 0.64 in phase 0 to 0.81 in phase 2, reflecting an early and marked gain in lower extremity function (
p < 0.001). These results confirm previous findings that the most substantial functional improvements typically occur in the early post-operative period, often within the first 3 to 6 months after anterior cruciate ligament reconstruction (ACLR) [
4,
19].
Our results align with recent evidence on digital rehabilitation approaches. Virtual reality interventions have been reported to enhance functional outcomes and reduce pain in ACL patients [
12,
13]. Similarly, mobile health applications have been shown to improve early adherence and recovery [
14]. In contrast to these studies, our work provides a longitudinal analysis of over 5000 test events, capturing recovery trajectories across five rehabilitation phases. This design highlights the originality of our contribution and complements existing data from smaller, highly controlled trials.
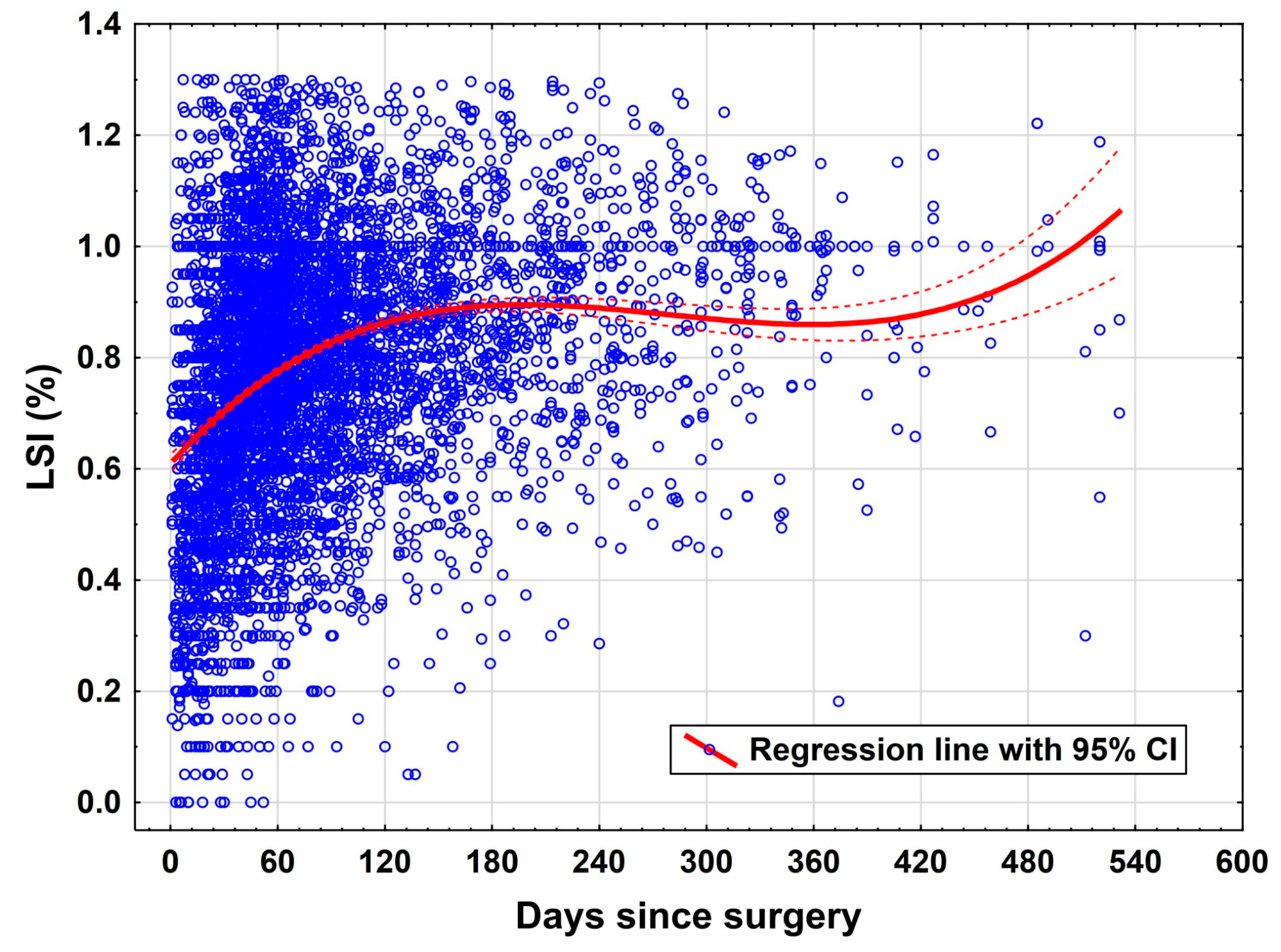
The most prominent predictor of functional improvement in this study was time elapsed since surgery. The variable “days since surgery” was associated with a statistically significant positive effect on LSI (
p = 0.034). This aligns with longitudinal rehabilitation studies that indicate a gradual, time-dependent recovery of neuromuscular control and muscular symmetry post ACLR [
4,
19,
20]. However, it is important to recognize that this temporal association does not necessarily reflect a uniform progression across individuals, as adherence to rehabilitation protocols and individual recovery rates vary widely.
4.2. Pain as a Limiting Factor
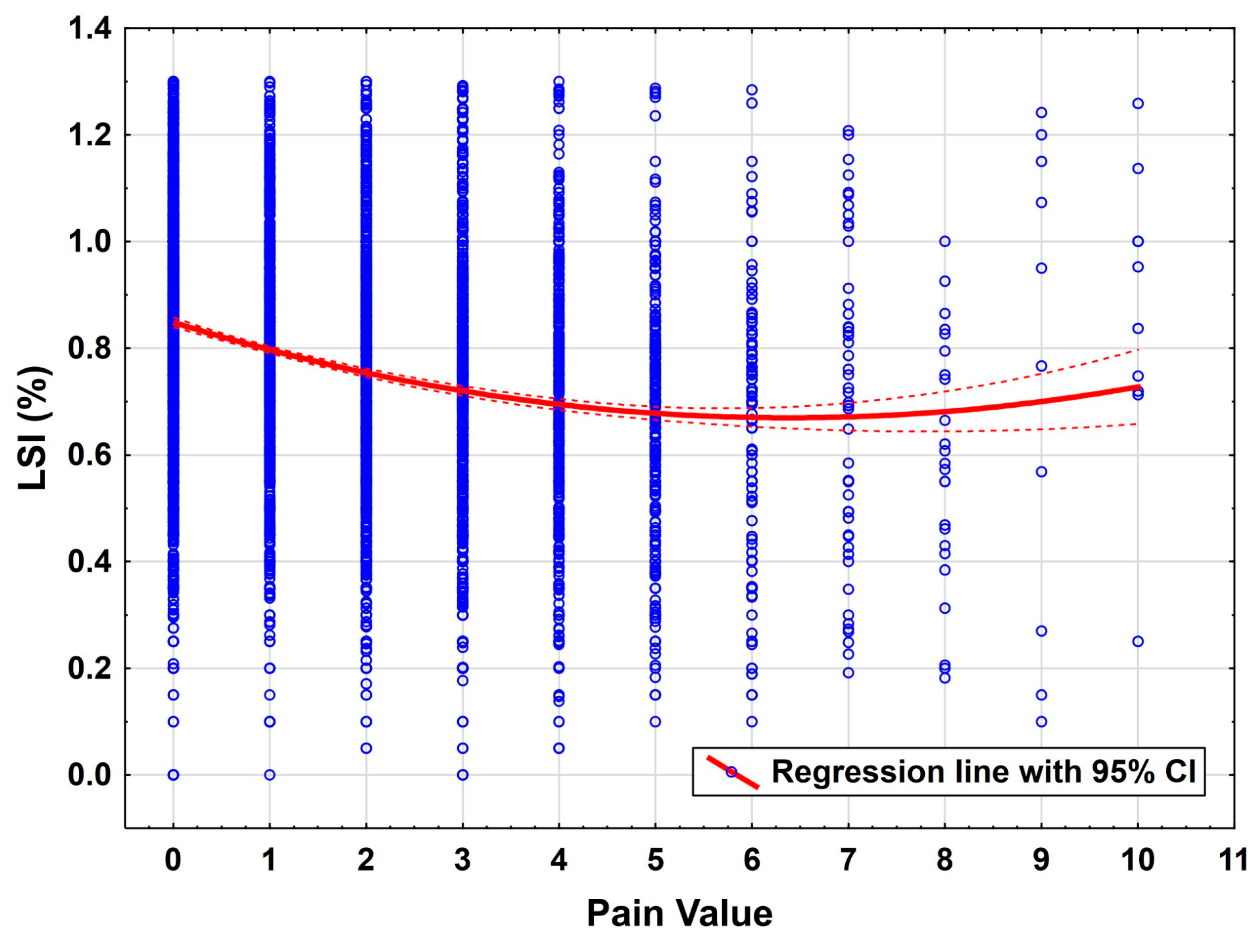
Pain as assessed by the Visual Analogue Scale (VAS) was found to have a strong negative influence on performance (
p < 0.001). This emphasizes the central role of pain management in functional rehabilitation. Pain can act as both a mechanical and psychological inhibitor of motor performance, which in turn may suppress engagement in critical neuromuscular training components and is known as a risk factor for arthrofibrosis [
21,
22]. Therefore, early and sustained pain control should be considered a priority within structured rehabilitation programs.
4.3. Anthropometric Factors
Contrary to some previous assumptions, anthropometric parameters such as body height and body weight showed no significant effect on functional outcomes in this cohort. While several biomechanical models suggest that increased body mass may impose greater joint loading and potentially delay recovery [
23,
24,
25], this was not supported in the present data. The lack of effect may reflect the fact that standardized load-adapted rehabilitation strategies (such as those implemented by “Orthelligent”) are able to compensate for individual anthropometric differences.
4.4. Exercise-Specific Challenges
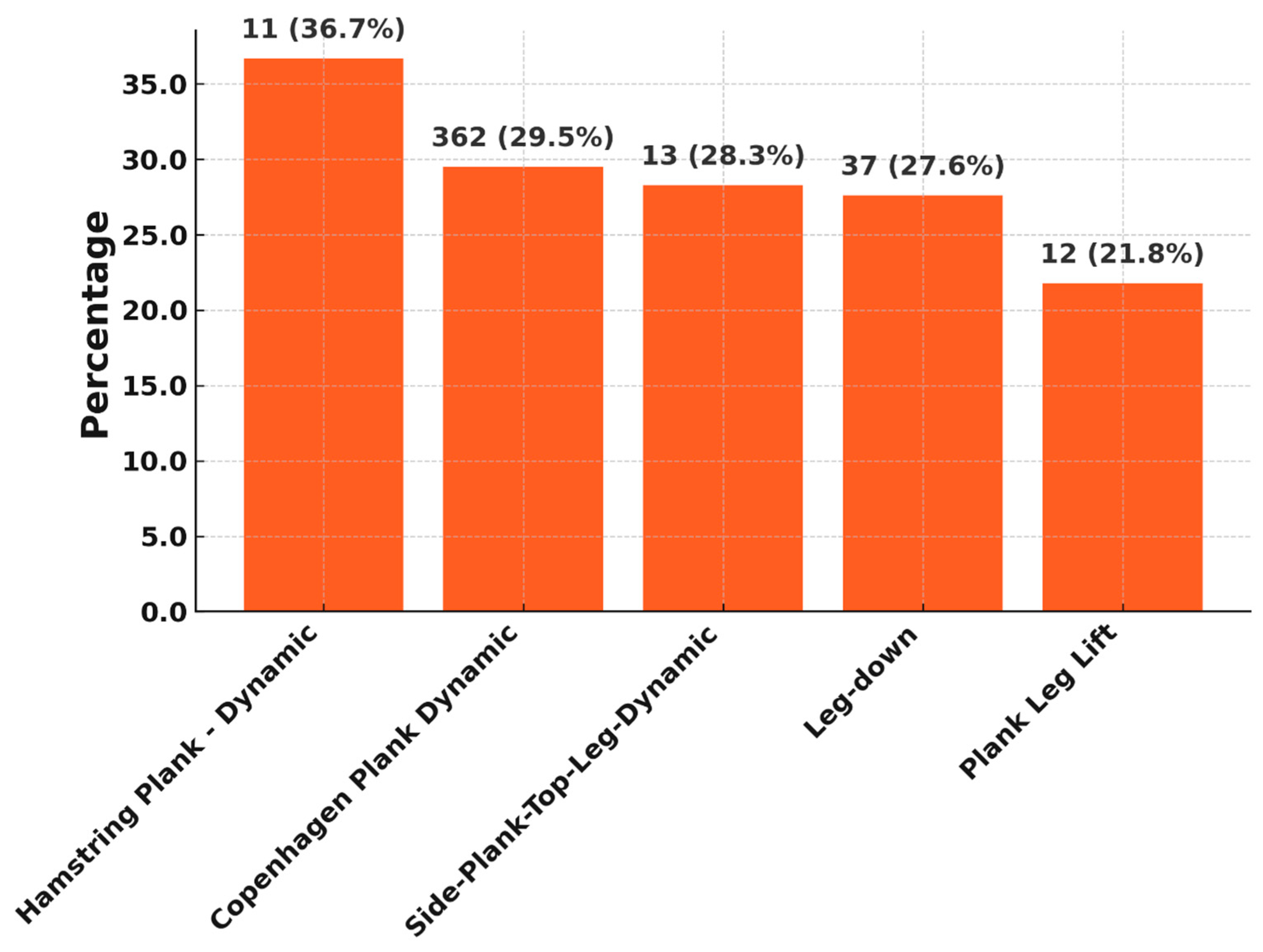
Certain exercises showed a high proportion of “too hard” ratings from patients. This indicates a perceived high subjective load, which must be viewed critically in the early phase of rehabilitation. The Hamstring Plank-Dynamic exercise, with over 36% “too hard” feedback, is the most stressful. This is in line with findings from the literature, which show that hamstring-dominant exercises are biomechanically and proprioceptively challenging in early postoperative phases and require a high level of muscular control [
2].
Copenhagen planks are also considered high intensity in the sports physiotherapy literature and are typically used in prevention and late rehabilitation. Their early use can lead to excessive demands, especially without sufficient supervision [
15]. The fact that these exercises in the algorithm-controlled sensor are already used in late rehabilitation phases and that the total number of exercises that are too difficult decreases significantly over the rehabilitation phases indicates that there tends to be a small group of patients who need a little longer for such difficult exercises.
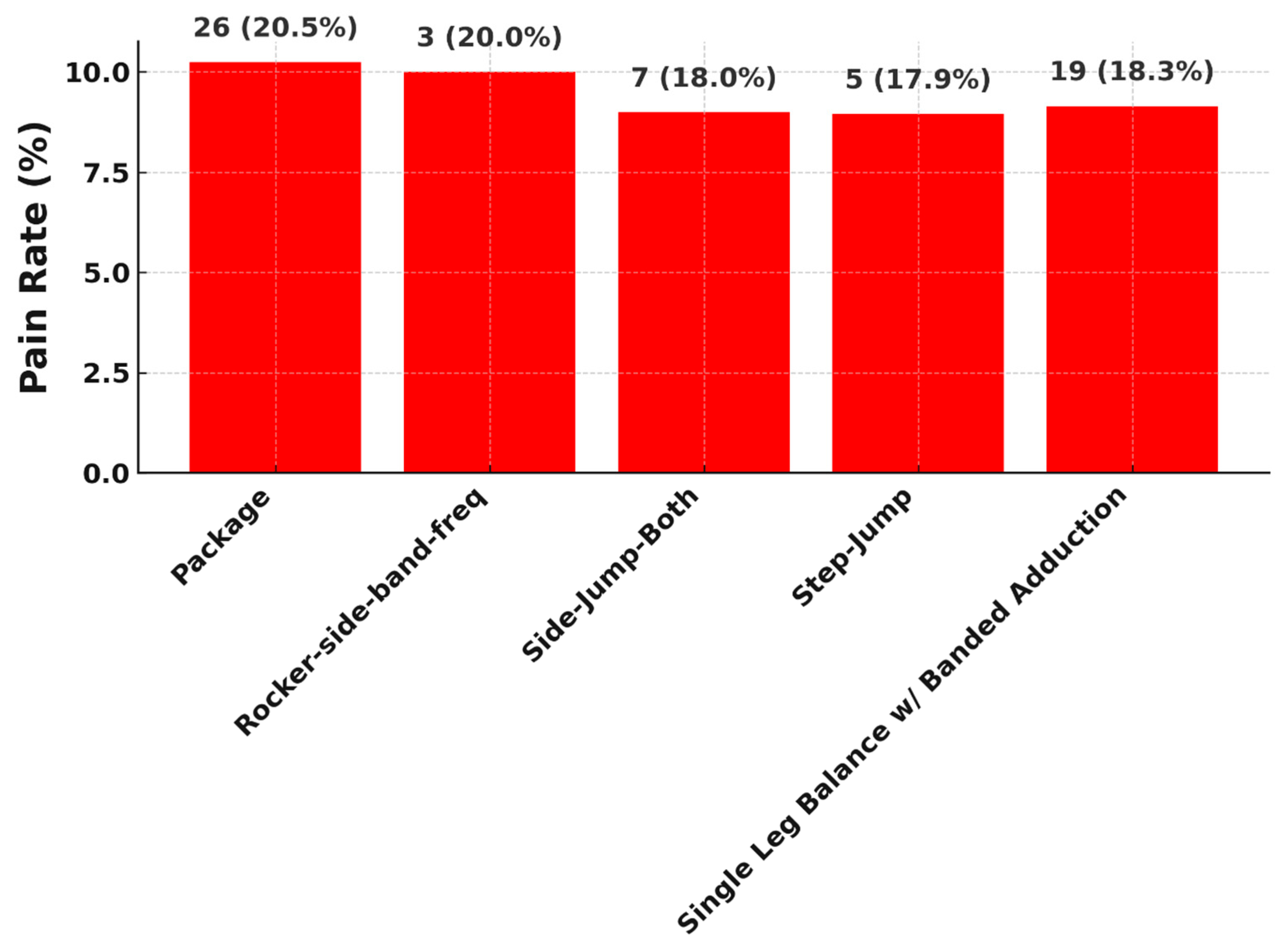
All of the listed exercises involve multi-joint, load-bearing movements with either dynamic instability or unilateral loading, which are known to provoke higher neuromuscular demand. Exercises like the Step Jump and Side Jump Both involve rapid plyometric motion and sudden load deceleration, which can aggravate discomfort particularly in patients recovering from anterior cruciate ligament reconstruction (ACLR) or other post-surgical knee states. Prior studies indicate that jump-landing tasks are frequently associated with elevated joint forces and increased muscle co-contraction around the knee, both of which contribute to higher pain perception during rehabilitation [
26,
27]. The Single Leg Balance with Banded Adduction, despite its apparent simplicity, requires deep hip and core activation while challenging proprioception and stability. The use of elastic resistance bands intensifies the adduction load and can strain recovering tissues, especially in patients with deficits in gluteal strength or neuromuscular control [
28]. Similarly, Rocker Side Band-Frequent is characterized by repetitive lateral movement with band resistance, stressing the lateral kinetic chain, which can provoke discomfort due to the constant tension and low-level isometric hold requirements. Exercises like Package-which may involve a compound or novel movement pattern-are often rated painful because they lack individualized progression schemes and may not be well aligned with patients’ current functional levels. Evidence suggests that poorly timed progression or lack of personalization in rehabilitation programming increases the likelihood of overexertion and pain flare-ups [
29].
4.5. Clinical Implications
The present data offer, for the first time, a differentiated view of functional development across defined rehabilitation phases based on a large volume of real-world data. This is particularly valuable for identifying time windows for targeted interventions, adapting rehab progressions, and personalizing therapy plans. While causal interpretations remain limited due to the observational nature of the data, the findings strongly suggest that routine DMD-generated data can validly reflect recovery progress and contributing factors, especially in early rehab stages. In clinical practice, digital rehabilitation monitoring may support therapists and surgeons by providing objective feedback on patient progress, enabling early detection of pain-related limitations, and facilitating individualized exercise progression. These insights can be readily implemented to optimize patient management in the early postoperative phase. However, barriers to widespread adoption include costs of digital systems, variability in acceptance among clinicians, and the need for integration into existing healthcare infrastructures. Addressing these issues will be essential for broader implementation.
4.6. Limitations and Strengths
This study presents unique insights into functional rehabilitation progress using routine data collected via a digital medical device (DMD). Nevertheless, several limitations must be acknowledged.
Limitations: First, the retrospective, observational design inherently limits causal inferences. Although generalized estimating equations (GEE) were employed to account for intra-individual correlation across repeated measurements, unmeasured confounders and individual variability (e.g., in adherence, preoperative condition, psychological readiness) may still bias the results. Second, reliance on self-administered sensor-based exercises may introduce measurement variability. Although studies have validated the reliability of such systems, the consistency of execution without direct clinical supervision can vary, particularly in patients with low motor control or comprehension of instructions. Third, while the pain, “too hard,” and dropout rates offer novel insights, these remain subjective and may be influenced by individual thresholds, motivation, or environmental factors. Furthermore, a key limitation of our study is the absence of direct quadriceps morphology and strength assessment. Quadriceps recovery is a central determinant of functional outcome after ACL reconstruction and is strongly linked to return-to-sport rates and prevention of re-injury. Because the Orthelligent system does not include standardized force testing, quadriceps data could not be incorporated. Future studies should combine digital monitoring with objective dynamometric strength measurements to provide a more comprehensive picture of rehabilitation progression. Additionally, information on concomitant treatments (e.g., medications) and potential complications were not obtained by the application system and are therefore not taken into account. Furthermore, while the present analysis benefits from the large sample size and real-world character of the data, generalizability is limited. Patients were treated in diverse centers across Germany, and rehabilitation strategies were heterogeneous. Thus, the results may not fully translate to healthcare systems with different structures, reimbursement schemes, or cultural factors. At the same time, this heterogeneity mirrors actual routine care, which can be regarded as a strength, since it enhances the ecological validity of the findings.
Strengths: However, the study also possesses several notable strengths. With over 5.600 individual test events across 335 patients, the dataset represents one of the largest collections of rehabilitation progress post-ACL reconstruction. Unlike highly controlled RCTs that may lack generalizability, this dataset reflects routine care patterns across a heterogeneous population. Unlike most prior studies that rely on snapshots at fixed timepoints (e.g., 6 or 12 weeks post-op), this analysis captures functional change continuously across distinct rehabilitation phases.
This allows, for the first time, an evidence-based assessment of when peak functional gains occur and how patient-reported burden (pain, difficulty) evolves over time. By distinguishing between early (phases 0–2) and later rehabilitation stages, the study identifies a “therapeutic window” for maximal improvement, supporting prior findings of nonlinear recovery trajectories.