Impact of Colchicine Therapy on Ventriculoarterial Coupling in Familial Mediterranean Fever: A Cross-Sectional Study
Abstract
1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Blood Pressure and Biochemical Measurements
2.3. Echocardiographic Evaluation and VAC Calculation
2.4. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Romano, M.; Piskin, D.; Cinar, O.K.; Sag, E. Familial Mediterranean Fever; Recent Advances, Future Prospectives. Diagnostics 2025, 15, 813. [Google Scholar] [CrossRef] [PubMed]
- Sgouropoulou, V.; Stabouli, S.; Trachana, M. Arterial stiffness in Familial Mediterranean Fever: Correlations with disease-related parameters and colchicine treatment. Clin. Rheumatol. 2019, 38, 2577–2584. [Google Scholar] [CrossRef] [PubMed]
- Sethuramalingam, S.; Maiti, R.; Hota, D.; Srinivasan, A. Effect of Colchicine in Reducing Inflammatory Biomarkers and Cardiovascular Risk in Coronary Artery Disease: A Meta-analysis of Clinical Trials. Am. J. Ther. 2023, 30, e197–e208. [Google Scholar] [CrossRef] [PubMed]
- Leventoğlu, E.; Büyükkaragöz, B.; Yayla, E.N.S.; Şenol, P.E.; Bakkaloğlu, S.A. Arterial Stiffness and Ambulatory Blood Pressure Measurements in Children with Familial Mediterranean Fever. Clin. Pediatr. 2024, 63, 1198–1207. [Google Scholar] [CrossRef]
- Imazio, M.; Nidorf, M. Colchicine and the heart. Eur. Heart J. 2021, 42, 2745–2760. [Google Scholar] [CrossRef]
- Andreis, A.; Imazio, M.; De Ferrari, G.M. Colchicine for the treatment of cardiovascular diseases: Old drug, new targets. J. Cardiovasc. Med. 2021, 22, 1–8. [Google Scholar] [CrossRef]
- Vitali, A.; Zouein, F.A.; Booz, G.W.; Altara, R. Prognostic utility of assessing ventricular-arterial coupling in arterial hypertension and cardiovascular diseases. Minerva Cardioangiol. 2025; Online ahead of print. [Google Scholar] [CrossRef]
- Monge García, M.I.; Santos, A. Understanding ventriculo-arterial coupling. Ann. Transl. Med. 2020, 8, 795. [Google Scholar] [CrossRef]
- Gamarra, A.; Díez-Villanueva, P.; Salamanca, J.; Aguilar, R.; Mahía, P.; Alfonso, F. Development and Clinical Application of Left Ventricular–Arterial Coupling Non-Invasive Assessment Methods. J. Cardiovasc. Dev. Dis. 2024, 11, 141. [Google Scholar] [CrossRef]
- Gaczoł, M.; Rajzer, M.; Wojciechowska, W. Ventricular-arterial coupling: Changes with ageing and implications across cardiovascular conditions. Blood Press. 2025, 34, 2457698. [Google Scholar] [CrossRef]
- Ben-Shabat, N.; Gendelman, O.; Fisher, L.; Shani, U.; Patt, Y.S.; Watad, A.; Skuja, V.; McGonagle, D.; Amital, H. Increased risk for stroke in patients with familial Mediterranean fever: Results from a large population-based study. Rheumatology 2023, 62, 3940–3946. [Google Scholar] [CrossRef]
- Andreis, A.; Imazio, M.; Casula, M.; Avondo, S.; De Ferrari, G.M. Colchicine efficacy and safety for the treatment of cardiovascular diseases. Intern. Emerg. Med. 2021, 16, 1691–1700. [Google Scholar] [CrossRef]
- Matteucci, A.; Bonanni, M.; Versaci, F.; Frati, G.; Peruzzi, M.; Sangiorgi, G.; Biondi-Zoccai, G.; Massaro, G. Cardiovascular medicine: A year in review. Minerva Cardioangiol. 2022, 70, 40–55. [Google Scholar] [CrossRef]
- Chen, C.H.; Nakayama, M.; Nevo, E.; Fetics, B.J.; Maughan, W.L.; Kass, D.A. Coupled systolic-ventricular and vascular stiffening with age: Implications for pressure regulation and cardiac reserve in the elderly. J. Am. Coll. Cardiol. 1998, 32, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, I.; Aboyans, V.; Blacher, J.; Brodmann, M.; Brutsaert, D.L.; Chirinos, J.A.; De Carlo, M.; Delgado, V.; Lancellotti, P.; Lekakis, J.; et al. The role of ventricular–arterial coupling in cardiac disease and heart failure: Assessment, clinical implications and therapeutic interventions. A consensus document of the European Society of Cardiology Working Group on Aorta & Peripheral Vascular Diseases, European Association of Cardiovascular Imaging, and Heart Failure Association. Eur. J. Heart Fail. 2019, 21, 402–424. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, I.; Pavlidis, G.; Vlastos, D. Chapter 32—Clinical Implications of Ventricular-Arterial Coupling and the Role of Therapeutic Interventions. In Early Vascular Aging (EVA), 2nd ed.; Cunha, P.G., Nilsson, P.M., Olsen, M.H., Boutouyrie, P., Laurent, S., Eds.; Academic Press: Boston, MA, USA, 2024; pp. 401–416. [Google Scholar]
- Deftereos, S.G.; Beerkens, F.J.; Shah, B.; Giannopoulos, G.; Vrachatis, D.A.; Giotaki, S.G.; Siasos, G.; Nicolas, J.; Arnott, C.; Patel, S.; et al. Colchicine in Cardiovascular Disease: In-Depth Review. Circulation 2022, 145, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Taki, J.; Wakabayashi, H.; Hiromasa, T.; Inaki, A.; Ogawa, K.; Shiba, K.; Kinuya, S. Colchicine treatment early after infarction attenuates myocardial inflammatory response demonstrated by 14C-methionine imaging and subsequent ventricular remodeling by quantitative gated SPECT. Ann. Nucl. Med. 2021, 35, 253–259. [Google Scholar] [CrossRef]
- Sari, I.; Yuksel, A.; Kozaci, D.; Selcuk, S.; Gokce, G.; Yildiz, Y.; Demirel, H.; Sop, G.; Alacacioglu, A.; Gunay, N.; et al. The effect of regular colchicine treatment on biomarkers related with vascular injury in newly diagnosed patients with familial Mediterranean fever. Inflammation 2012, 35, 1191–1197. [Google Scholar] [CrossRef]
- Deftereos, S.; Giannopoulos, G.; Papoutsidakis, N.; Panagopoulou, V.; Kossyvakis, C.; Raisakis, K.; Cleman, M.W.; Stefanadis, C. Colchicine and the heart: Pushing the envelope. J. Am. Coll. Cardiol. 2013, 62, 1817–1825. [Google Scholar] [CrossRef]
- Yüksel, S.; Ayvazyan, L.; Gasparyan, A.Y. Familial mediterranean Fever as an emerging clinical model of atherogenesis associated with low-grade inflammation. Open Cardiovasc. Med. J. 2010, 4, 51–56. [Google Scholar] [CrossRef]
- Dekleva, M.; Lazic, J.S.; Soldatovic, I.; Inkrot, S.; Arandjelovic, A.; Waagstein, F.; Gelbrich, G.; Cvijanovic, D.; Dungen, H.D. Improvement of Ventricular-Arterial Coupling in Elderly Patients with Heart Failure After Beta Blocker Therapy: Results from the CIBIS-ELD Trial. Cardiovasc. Drugs Ther. 2015, 29, 287–294. [Google Scholar] [CrossRef]
- DuPont, J.J.; Kenney, R.M.; Patel, A.R.; Jaffe, I.Z. Sex differences in mechanisms of arterial stiffness. Br. J. Pharmacol. 2019, 176, 4208–4225. [Google Scholar] [CrossRef]
| Variable | FMF (−) (n = 81) | FMF (+) (n = 97) | p-Value |
|---|---|---|---|
| Age, years | 43.6 ± 11.3 | 35.1 ± 12.3 | <0.001 |
| Female sex, n (%) | 38 (46.9) | 45 (46.4) | 0.532 |
| Body mass index, kg/m2 | 28.3 ± 5.5 | 26.2 ± 4.8 | 0.005 |
| Disease duration, years | 0 (0) | 13.9 ± 10.1 | <0.001 |
| Diabetes mellitus, n (%) | 16 (19.8) | 2 (2.1) | <0.001 |
| Hypertension, n (%) | 17 (21.0) | 6 (6.2) | 0.003 |
| Hyperlipidemia, n (%) | 45 (55.6) | 26 (26.8) | <0.001 |
| Coronary artery disease, n (%) | 11 (13.8) | 0 (0.0) | <0.001 |
| Smoking, n (%) | 29 (36.7) | 20 (20.6) | 0.014 |
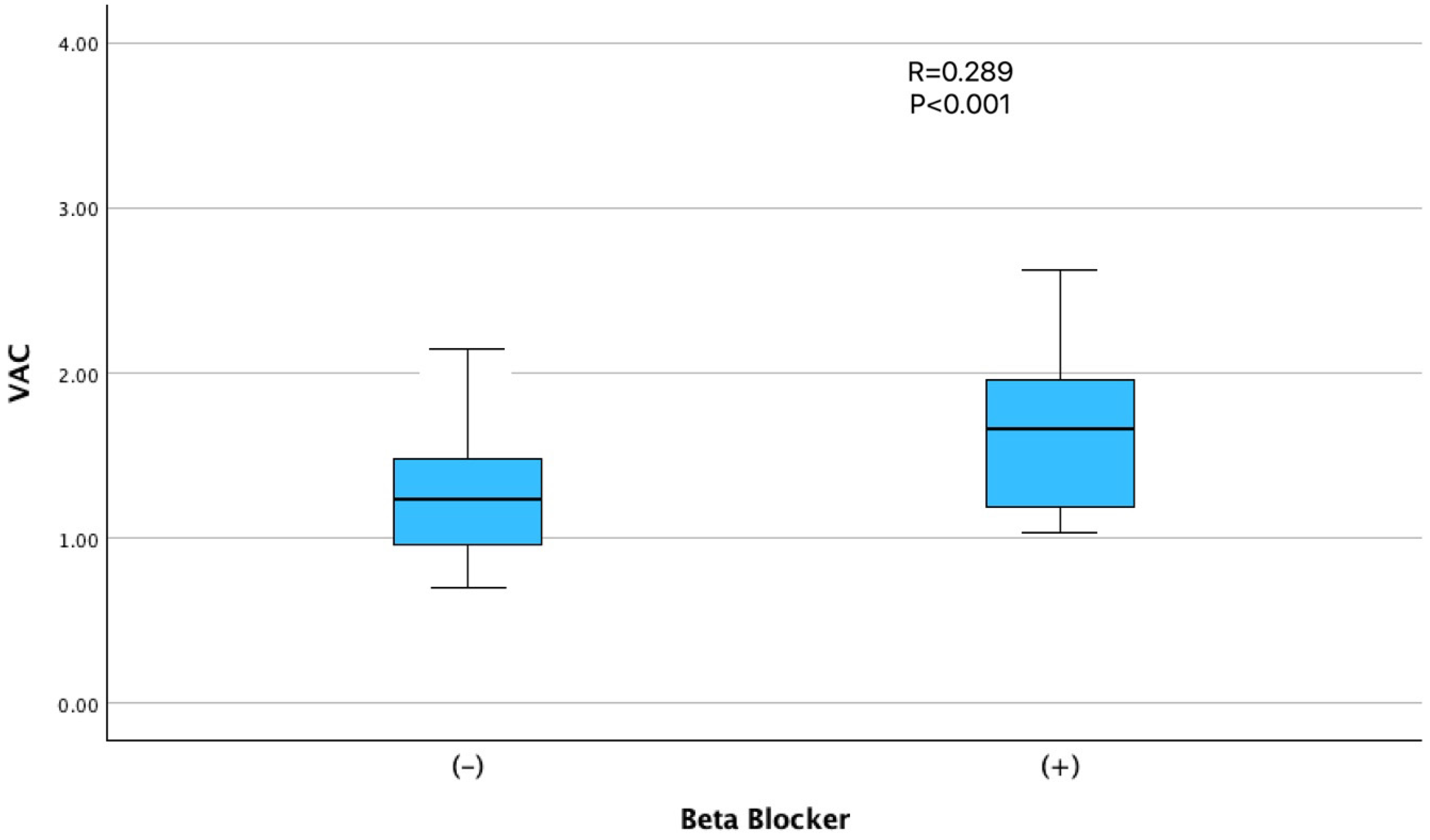
| β-blocker use, n (%) | 19 (23.5) | 0 (0.0) | <0.001 |
| CCB use, n (%) | 9 (11.1) | 4 (4.1) | 0.067 |
| ACE inhibitor or ARB, n (%) | 14 (17.3) | 4 (4.1) | 0.004 |
| Diuretic use, n (%) | 8 (9.9) | 0 (0.0) | 0.002 |
| Statin use, n (%) | 6 (7.4) | 0 (0.0) | 0.008 |
| Oral antidiabetic use, n (%) | 6 (7.4) | 0 (0.0) | 0.008 |
| WBC (×103/µL) | 7.5 ± 1.9 | 7.3 ± 2.0 | 0.538 |
| Hemoglobin, g/dL | 13.9 ± 1.8 | 13.4 ± 1.7 | 0.046 |
| eGFR, mL/min/1.73 m2 | 102.2 ± 17.5 | 109.4 ± 17.9 | 0.003 |
| Serum creatinine, mg/dL | 0.79 ± 0.18 | 0.73 ± 0.17 | 0.053 |
| Fasting glucose, mg/dL | 102.4 ± 32.4 | 90.3 ± 9.3 | <0.001 |
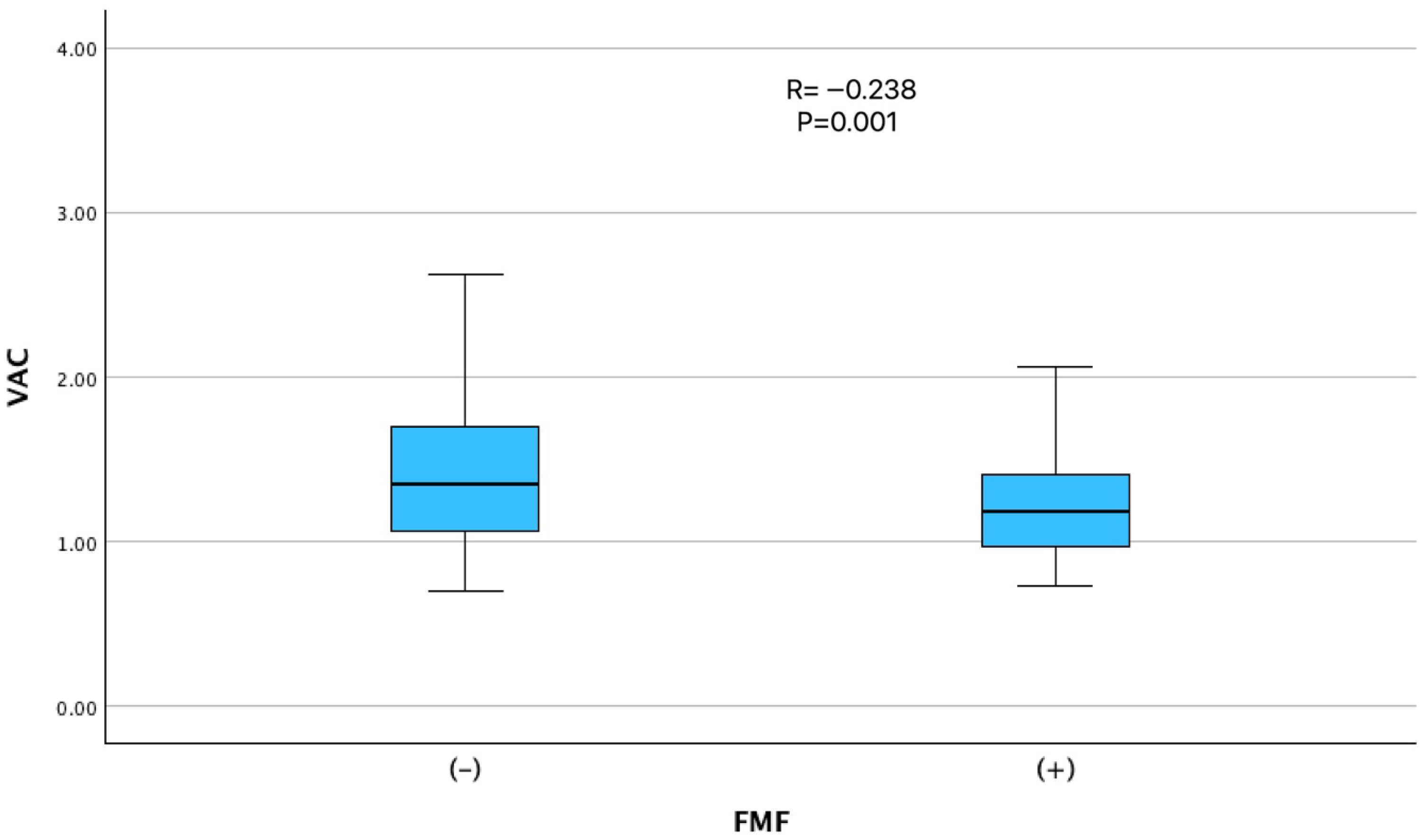
| VAC (Ea/Es ratio) | 1.40 ± 0.57 | 1.23 ± 0.34 | 0.001 |
| LVEF, % | 64.9 ± 12.3 | 60.2 ± 5.6 | <0.001 |
| Variable | r | p-Value |
|---|---|---|
| Age, years | 0.196 | 0.009 |
| Female sex | −0.162 | 0.031 |
| FMF diagnosis | −0.238 | 0.001 |
| FMF severity/activity scores | NS | NS |
| Disease duration, years | NS | NS |
| Body mass index, kg/m2 | NS | NS |
| Diabetes mellitus | NS | NS |
| Hypertension | 0.162 | 0.032 |
| Hyperlipidemia | 0.167 | 0.025 |
| Coronary artery disease | NS | NS |
| Smoking | NS | NS |
| β-blocker use | 0.289 | <0.001 |
| CCB use | NS | NS |
| ACE inhibitor/ARB use | 0.217 | 0.004 |
| Diuretic use | 0.188 | 0.012 |
| Statin use | NS | NS |
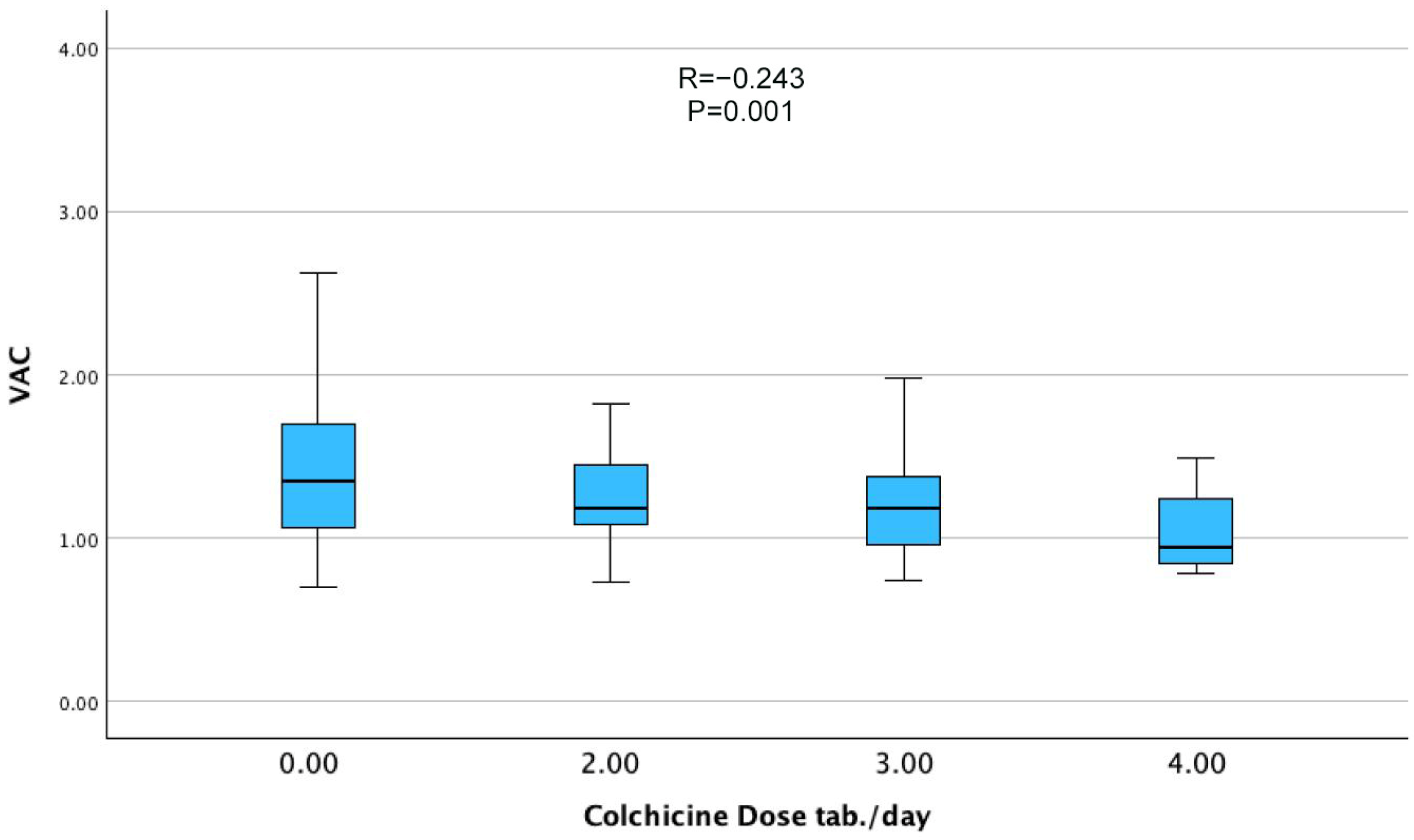
| Colchicine dose, mg/day | −0.243 | 0.001 |
| WBC (×103/µL) | NS | NS |
| Lymphocyte count (×103/µL) | 0.188 | 0.012 |
| Total protein, g/dL | NS | NS |
| Albumin, g/dL | NS | NS |
| Hemoglobin, g/dL | NS | NS |
| Fasting glucose, mg/dL | NS | NS |
| Serum creatinine, mg/dL | NS | NS |
| eGFR, mL/min/1.73 m2 | −0.142 | 0.063 |
| C-reactive protein, mg/L | NS | NS |
| LVEF, % | NS | NS |
| IVS thickness, mm | NS | NS |
| PW thickness, mm | NS | NS |
| E/A ratio | NS | NS |
| E/e′ ratio | NS | NS |
| Model 1 | ||||
|---|---|---|---|---|
| Variable | Unstandardized B | Standardized β | 95% CI | p-Value |
| FMF diagnosis | −0.171 | −0.176 | −0.322 to −0.020 | 0.027 |
| β-blocker use | 0.304 | 0.197 | 0.060 to 0.548 | 0.015 |
| Female sex | −0.123 | −0.127 | −0.264 to 0.018 | 0.088 |
| Model 2 | ||||
| Variable | Unstandardized B | Standardized β | 95% CI | p-Value |
| Colchicine dose | −0.063 | −0.186 | −0.115 to −0.011 | 0.018 |
| β-blocker use | 0.301 | 0.194 | 0.058 to 0.543 | 0.014 |
| Female sex | −0.216 | −0.130 | −0.267 to 0.015 | 0.079 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duman, H.; Durak, H.; Cüre, O.; Çetin, M.; Özyıldız, A.G.; Ergül, E.; Şahin, M.A.; Özsipahi, A.; Tuncer, A.Y.; Dindar, B.; et al. Impact of Colchicine Therapy on Ventriculoarterial Coupling in Familial Mediterranean Fever: A Cross-Sectional Study. J. Clin. Med. 2025, 14, 6902. https://doi.org/10.3390/jcm14196902
Duman H, Durak H, Cüre O, Çetin M, Özyıldız AG, Ergül E, Şahin MA, Özsipahi A, Tuncer AY, Dindar B, et al. Impact of Colchicine Therapy on Ventriculoarterial Coupling in Familial Mediterranean Fever: A Cross-Sectional Study. Journal of Clinical Medicine. 2025; 14(19):6902. https://doi.org/10.3390/jcm14196902
Chicago/Turabian StyleDuman, Hakan, Hüseyin Durak, Osman Cüre, Mustafa Çetin, Ali Gökhan Özyıldız, Elif Ergül, Müjgan Ayşenur Şahin, Ahmet Özsipahi, Ahmet Yasin Tuncer, Barış Dindar, and et al. 2025. "Impact of Colchicine Therapy on Ventriculoarterial Coupling in Familial Mediterranean Fever: A Cross-Sectional Study" Journal of Clinical Medicine 14, no. 19: 6902. https://doi.org/10.3390/jcm14196902
APA StyleDuman, H., Durak, H., Cüre, O., Çetin, M., Özyıldız, A. G., Ergül, E., Şahin, M. A., Özsipahi, A., Tuncer, A. Y., Dindar, B., & Emlek, N. (2025). Impact of Colchicine Therapy on Ventriculoarterial Coupling in Familial Mediterranean Fever: A Cross-Sectional Study. Journal of Clinical Medicine, 14(19), 6902. https://doi.org/10.3390/jcm14196902






