The Surgical Management of Chronic Thromboembolic Pulmonary Hypertension
Abstract
1. Introduction
2. Pathophysiology and Genetics
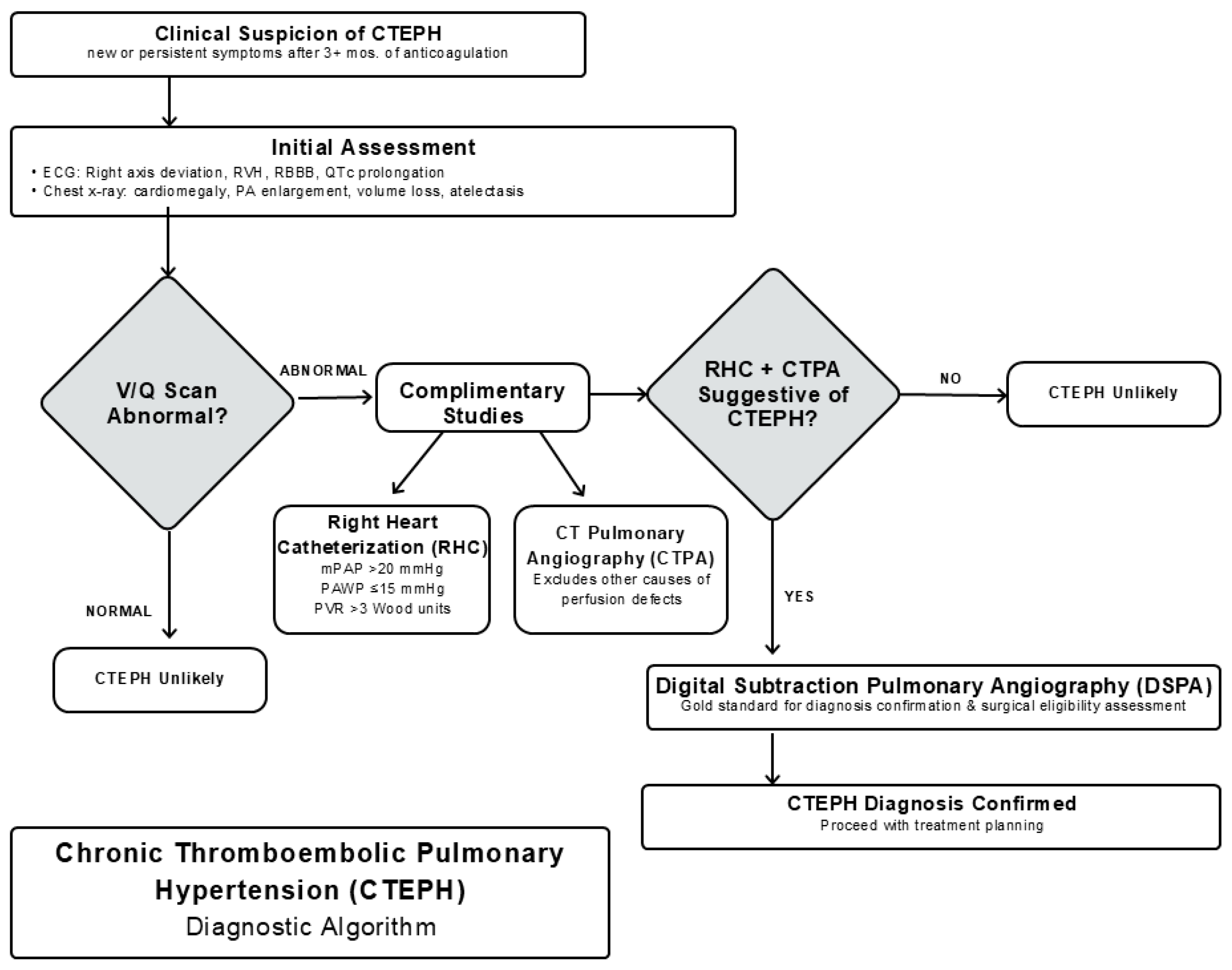
3. Clinical Presentation and Diagnosis
4. Multidisciplinary Chronic Thromboembolic Pulmonary Hypertension Teams
5. Basics of Medical Management
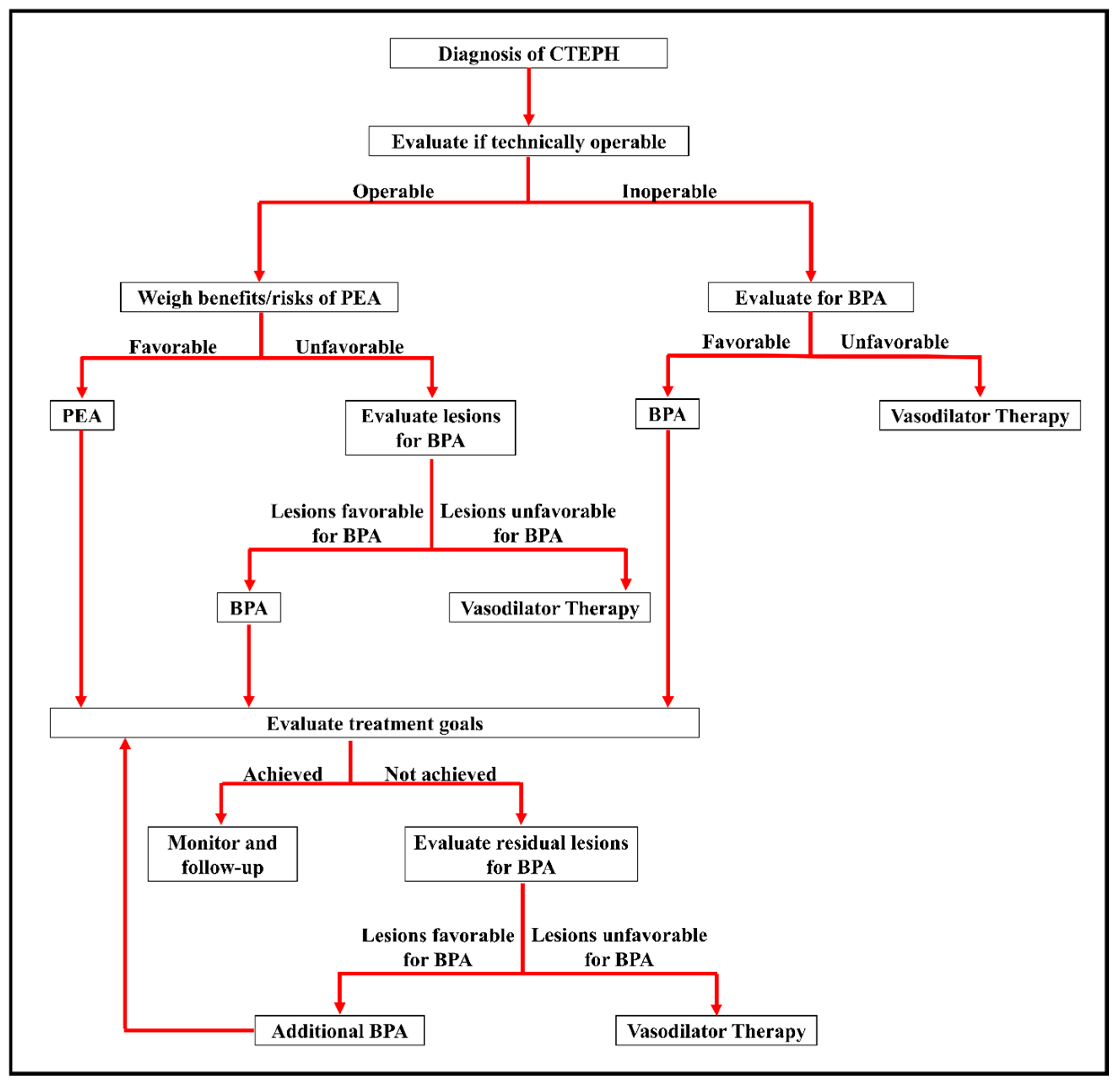
6. Considerations in Patient Selection for Surgery
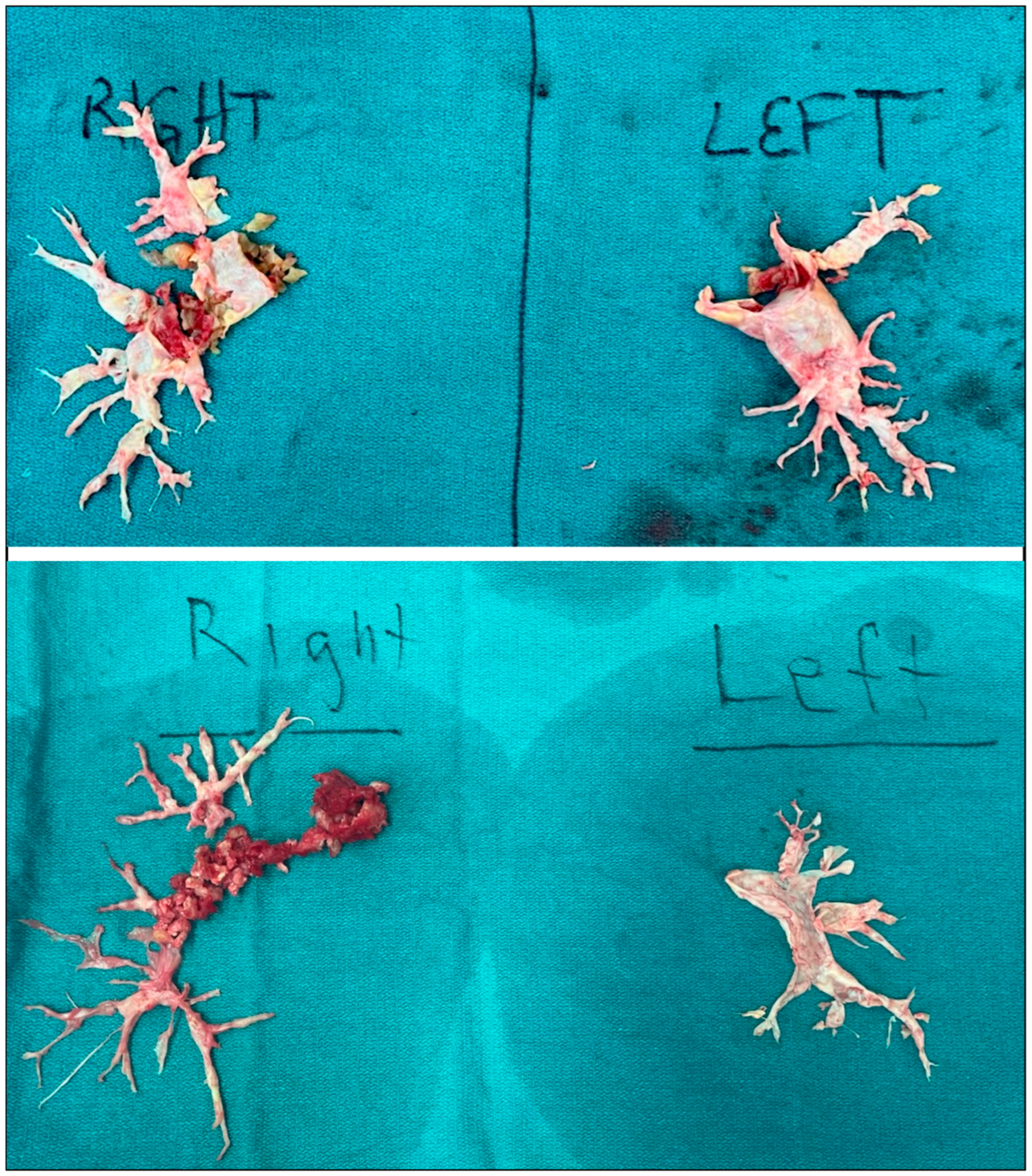
7. Surgical Technique
- Median sternotomy and pericardiotomy;
- Establishment of cardiopulmonary bypass;
- Systemic cooling to 18–20 °C;
- Right pulmonary thromboendarterectomy with short-interval (<20 min) circulatory arrest;
- Left pulmonary thromboendarterectomy with short-interval (<20 min) circulatory arrest;
- Rewarming and closure of patent foramen ovale (if present).
8. Surgical Outcomes and Complications
9. The Role of ECMO After PTE
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CTEPH | chronic thromboembolic pulmonary thromboembolism |
| PH | pulmonary hypertension |
| PE | pulmonary embolism |
| PTE | pulmonary thromboendarterectomy |
| CTED | chronic thromboembolic disease |
| RHC | right heart catheterization |
| CTPA | CT pulmonary arteriography |
| mPAP | mean pulmonary artery pressure |
| PVR | pulmonary vascular resistance |
| DSPA | digital subtraction pulmonary arteriography |
| CPET | cardiopulmonary exercise testing |
| MRI | magnetic resonance imaging |
| BPA | balloon pulmonary angioplasty |
| DOAC | direct oral anticoagulant |
| NIRS | near-infrared spectroscopy |
| BIS | bispectral index |
| CPB | cardiopulmonary bypass |
| SVC | superior vena cava |
| IVC | inferior vena cava |
| RPA | right pulmonary artery |
| ECMO | extracorporeal membrane oxygenation |
| VA-ECMO | veno-arterial extracorporeal membrane oxygenation |
| VV-ECMO | veno-venous extracorporeal membrane oxygenation |
References
- Papamatheakis, D.G.; Poch, D.S.; Fernandes, T.M.; Kerr, K.M.; Kim, N.H.; Fedullo, P.F. Chronic Thromboembolic Pulmonary Hypertension. J. Am. Coll. Cardiol. 2020, 76, 2155–2169. [Google Scholar] [CrossRef]
- Benotti, J.R.; Ockene, I.S.; Alpert, J.S.; Dalen, J.E. The Clinical Profile of Unresolved Pulmonary Embolism. Chest 1983, 84, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Guérin, L.; Couturaud, F.; Parent, F.; Revel, M.-P.; Gillaizeau, F.; Planquette, B.; Pontal, D.; Guégan, M.; Simonneau, G.; Meyer, G.; et al. Prevalence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: Prevalence of CTEPH after pulmonary embolism. Thromb. Haemost. 2014, 112, 598–605. [Google Scholar] [CrossRef]
- Pengo, V.; Lensing, A.W.A.; Prins, M.H.; Marchiori, A.; Davidson, B.L.; Tiozzo, F.; Albanese, P.; Biasiolo, A.; Pegoraro, C.; Iliceto, S.; et al. Incidence of Chronic Thromboembolic Pulmonary Hypertension after Pulmonary Embolism. N. Engl. J. Med. 2004, 350, 2257–2264. [Google Scholar] [CrossRef]
- Ende-Verhaar, Y.M.; Cannegieter, S.C.; Vonk Noordegraaf, A.; Delcroix, M.; Pruszczyk, P.; Mairuhu, A.T.A.; Huisman, M.V.; Klok, F.A. Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: A contemporary view of the published literature. Eur. Respir. J. 2017, 49, 1601792. [Google Scholar] [CrossRef]
- Fauché, A.; Presles, E.; Sanchez, O.; Jaïs, X.; Le Mao, R.; Robin, P.; Pernod, G.; Bertoletti, L.; Jego, P.; Parent, F.; et al. Frequency and predictors for chronic thromboembolic pulmonary hypertension after a first unprovoked pulmonary embolism: Results from PADIS studies. J. Thromb. Haemost. 2022, 20, 2850–2861. [Google Scholar] [CrossRef]
- Bonderman, D.; Jakowitsch, J.; Adlbrecht, C.; Schemper, M.; Kyrle, P.A.; Schönauer, V.; Exner, M.; Klepetko, W.; Kneussl, M.P.; Maurer, G.; et al. Medical conditions increasing the risk of chronic thromboembolic pulmonary hypertension. Thromb. Haemost. 2005, 93, 512–516. [Google Scholar] [CrossRef]
- Lang, I.M. Chronic thromboembolic pulmonary hypertension—Not so rare after all. N. Engl. J. Med. 2004, 350, 2236–2238. [Google Scholar] [CrossRef]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef] [PubMed]
- Becattini, C.; Agnelli, G.; Pesavento, R.; Silingardi, M.; Poggio, R.; Taliani, M.R.; Ageno, W. Incidence of Chronic Thromboembolic Pulmonary Hypertension After a First Episode of Pulmonary Embolism. Chest 2006, 130, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Condliffe, R.; Kiely, D.G.; Gibbs, J.S.R.; Corris, P.A.; Peacock, A.J.; Jenkins, D.P.; Hodgkins, D.; Goldsmith, K.; Hughes, R.J.; Sheares, K.; et al. Improved Outcomes in Medically and Surgically Treated Chronic Thromboembolic Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2008, 177, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.; Jenkins, D.P. Surgical management of chronic thromboembolic pulmonary hypertension. Br. J. Hosp. Med. 2013, 74, 31–35. [Google Scholar] [CrossRef]
- Kunieda, T.; Nakanishi, N.; Satoh, T.; Kyotani, S.; Okano, Y.; Nagaya, N. Prognoses of Primary Pulmonary Hypertension and Chronic Majorvessel Thromboembolic Pulmonary Hypertension Determined from Cumulative Survival Curves. Intern. Med. 1999, 38, 543–546. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Riedel, M.; Stanek, V.; Widimsky, J.; Prerovsky, I. Longterm Follow-up of Patients with Pulmonary Thromboembolism. Chest 1982, 81, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, Y.; Jaïs, X.; Jevnikar, M.; Boucly, A.; Weatherald, J.; Brenot, P.; Planche, O.; Parent, F.; Savale, L.; Fadel, E.; et al. Predictors of survival in patients with not-operated chronic thromboembolic pulmonary hypertension. J. Heart Lung Transplant. 2019, 38, 833–842. [Google Scholar] [CrossRef]
- Task Force for Diagnosis and Treatment of Pulmonary Hypertension of European Society of Cardiology (ESC); European Respiratory Society (ERS); International Society of Heart and Lung Transplantation (ISHLT); Galiè, N.; Hoeper, M.M.; Humbert, M.; Torbicki, A.; Vachiery, J.L.; Barbera, J.A.; Beghetti, M.; et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Respir. J. 2009, 34, 1219–1263. [Google Scholar] [CrossRef]
- Freed, D.H.; Thomson, B.M.; Tsui, S.S.L.; Dunning, J.J.; Sheares, K.K.; Pepke-Zaba, J.; Jenkins, D.P. Functional and haemodynamic outcome 1 year after pulmonary thromboendarterectomy. Eur. J. Cardiothorac. Surg. 2008, 34, 525–530. [Google Scholar] [CrossRef]
- Ishida, K.; Masuda, M.; Tanabe, N.; Matsumiya, G.; Tatsumi, K.; Nakajima, N. Long-term outcome after pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. J. Thorac. Cardiovasc. Surg. 2012, 144, 321–326. [Google Scholar] [CrossRef]
- Mayer, E.; Jenkins, D.; Lindner, J.; D’Armini, A.; Kloek, J.; Meyns, B.; Ilkjaer, L.B.; Klepetko, W.; Delcroix, M.; Lang, I.; et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: Results from an international prospective registry. J. Thorac. Cardiovasc. Surg. 2011, 141, 702–710. [Google Scholar] [CrossRef]
- Madani, M.M.; Auger, W.R.; Pretorius, V.; Sakakibara, N.; Kerr, K.M.; Kim, N.H.; Fedullo, P.F.; Jamieson, S.W. Pulmonary endarterectomy: Recent changes in a single institution’s experience of more than 2,700 patients. Ann. Thorac. Surg. 2012, 94, 97–103; discussion 103. [Google Scholar] [CrossRef]
- Peacock, A. Controversies, Uncertainties and Future Research on the Treatment of Chronic Thromboembolic Pulmonary Hypertension. Proc. Am. Thorac. Soc. 2006, 3, 608–614. [Google Scholar] [CrossRef]
- Morris, T.A.; Marsh, J.J.; Chiles, P.G.; Auger, W.R.; Fedullo, P.F.; Woods, V.L. Fibrin Derived from Patients with Chronic Thromboembolic Pulmonary Hypertension Is Resistant to Lysis. Am. J. Respir. Crit. Care Med. 2006, 173, 1270–1275. [Google Scholar] [CrossRef] [PubMed]
- Hoeper, M.M.; Mayer, E.; Simonneau, G.; Rubin, L.J. Chronic Thromboembolic Pulmonary Hypertension. Circulation 2006, 113, 2011–2020. [Google Scholar] [CrossRef]
- Condliffe, R.; Kiely, D.G.; Gibbs, J.S.R.; Corris, P.A.; Peacock, A.J.; Jenkins, D.P.; Goldsmith, K.; Coghlan, J.G.; Pepke-Zaba, J. Prognostic and aetiological factors in chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2009, 33, 332–338. [Google Scholar] [CrossRef]
- Azarian, R.; Wartski, M.; Collignon, M.A.; Parent, F.; Hervé, P.; Sors, H.; Simonneau, G. Lung perfusion scans and hemodynamics in acute and chronic pulmonary embolism. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 1997, 38, 980–983. [Google Scholar]
- Klok, F.A.; Dzikowska-Diduch, O.; Kostrubiec, M.; Vliegen, H.W.; Pruszczyk, P.; Hasenfuß, G.; Huisman, M.V.; Konstantinides, S.; Lankeit, M. Derivation of a clinical prediction score for chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. J. Thromb. Haemost. 2016, 14, 121–128. [Google Scholar] [CrossRef]
- Pepke-Zaba, J.; Delcroix, M.; Lang, I.; Mayer, E.; Jansa, P.; Ambroz, D.; Treacy, C.; D’Armini, A.M.; Morsolini, M.; Snijder, R.; et al. Chronic Thromboembolic Pulmonary Hypertension (CTEPH): Results from an International Prospective Registry. Circulation 2011, 124, 1973–1981. [Google Scholar] [CrossRef] [PubMed]
- Bonderman, D.; Wilkens, H.; Wakounig, S.; Schäfers, H.-J.; Jansa, P.; Lindner, J.; Simkova, I.; Martischnig, A.M.; Dudczak, J.; Sadushi, R.; et al. Risk factors for chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2009, 33, 325–331. [Google Scholar] [CrossRef]
- Rosen, K.; Raanani, E.; Kogan, A.; Kenet, G.; Misgav, M.; Lubetsky, A.; Niznik, S.; Schäfers, H.-J.; Segel, M.J.; Agmon-Levin, N. Chronic thromboembolic pulmonary hypertension in patients with antiphospholipid syndrome: Risk factors and management. J. Heart Lung Transplant. 2022, 41, 208–216. [Google Scholar] [CrossRef]
- Lutsey, P.L.; Evensen, L.H.; Thenappan, T.; Prins, K.W.; Walker, R.F.; Farley, J.F.; MacLehose, R.F.; Alonso, A.; Zakai, N.A. Incidence and Risk Factors of Pulmonary Hypertension After Venous Thromboembolism: An Analysis of a Large Health Care Database. J. Am. Heart Assoc. 2022, 11, e024358. [Google Scholar] [CrossRef] [PubMed]
- Bonderman, D.; Turecek, P.; Jakowitsch, J.; Weltermann, A.; Adlbrecht, C.; Schneider, B.; Kneussl, M.; Rubin, L.; Kyrle, P.; Klepetko, W.; et al. High prevalence of elevated clotting factor VIII in chronic thromboembolic pulmonary hypertension. Thromb. Haemost. 2003, 90, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Kyrle, P.A.; Minar, E.; Hirschl, M.; Bialonczyk, C.; Stain, M.; Schneider, B.; Weltermann, A.; Speiser, W.; Lechner, K.; Eichinger, S. High Plasma Levels of Factor VIII and the Risk of Recurrent Venous Thromboembolism. N. Engl. J. Med. 2000, 343, 457–462. [Google Scholar] [CrossRef]
- Wolf, M.; Boyer-Neumann, C.; Parent, F.; Eschwege, V.; Jaillet, H.; Meyer, D.; Simonneau, G. Thrombotic risk factors in pulmonary hypertension. Eur. Respir. J. 2000, 15, 395. [Google Scholar] [CrossRef] [PubMed]
- Bonderman, D.; Jakowitsch, J.; Redwan, B.; Bergmeister, H.; Renner, M.-K.; Panzenböck, H.; Adlbrecht, C.; Georgopoulos, A.; Klepetko, W.; Kneussl, M.; et al. Role for staphylococci in misguided thrombus resolution of chronic thromboembolic pulmonary hypertension. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 678–684. [Google Scholar] [CrossRef]
- Lang, I.M.; Marsh, J.J.; Olman, M.A.; Moser, K.M.; Schleef, R.R. Parallel analysis of tissue-type plasminogen activator and type 1 plasminogen activator inhibitor in plasma and endothelial cells derived from patients with chronic pulmonary thromboemboli. Circulation 1994, 90, 706–712. [Google Scholar] [CrossRef]
- Satoh, T.; Satoh, K.; Yaoita, N.; Kikuchi, N.; Omura, J.; Kurosawa, R.; Numano, K.; Al-Mamun, E.; Siddique, M.A.H.; Sunamura, S.; et al. Activated TAFI Promotes the Development of Chronic Thromboembolic Pulmonary Hypertension: A Possible Novel Therapeutic Target. Circ. Res. 2017, 120, 1246–1262. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Delcroix, M.; Jais, X.; Madani, M.M.; Matsubara, H.; Mayer, E.; Ogo, T.; Tapson, V.F.; Ghofrani, H.-A.; Jenkins, D.P. Chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801915. [Google Scholar] [CrossRef]
- Moser, K.M.; LeMoine, J.R. Is Embolic Risk Conditioned by Location of Deep Venous Thrombosis? Ann. Intern. Med. 1981, 94, 439–444. [Google Scholar] [CrossRef]
- Sabbula, B.R.; Sankari, A.; Akella, J. Chronic Thromboembolic Pulmonary Hypertension. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK549836/ (accessed on 28 July 2025).
- Auger, W.R.; Kerr, K.M.; Kim, N.H.; Fedullo, P.F. Evaluation of Patients with Chronic Thromboembolic Pulmonary Hypertension for Pulmonary Endarterectomy. Pulm. Circ. 2012, 2, 155–162. [Google Scholar] [CrossRef]
- Kapitän, K.S.; Buchbinder, M.; Wagner, P.D.; Moser, K.M. Mechanisms of Hypoxemia in Chronic Thromboembolic Pulmonary Hypertension. Am. Rev. Respir. Dis. 1989, 139, 1149–1154. [Google Scholar] [CrossRef]
- Tapson, V.F.; Platt, D.M.; Xia, F.; Teal, S.A.; de la Orden, M.; Divers, C.H.; Satler, C.A.; Joish, V.N.; Channick, R.N. Monitoring for Pulmonary Hypertension Following Pulmonary Embolism: The INFORM Study. Am. J. Med. 2016, 129, 978–985.e2. [Google Scholar] [CrossRef]
- Dzikowska-Diduch, O.; Kostrubiec, M.; Kurnicka, K.; Lichodziejewska, B.; Pacho, S.; Miroszewska, A.; Bródka, K.; Skowrońska, M.; Łabyk, A.; Roik, M.; et al. The post-pulmonary syndrome—Results of echocardiographic driven follow up after acute pulmonary embolism. Thromb. Res. 2020, 186, 30–35. [Google Scholar] [CrossRef]
- Tunariu, N.; Gibbs, S.J.R.; Win, Z.; Gin-Sing, W.; Graham, A.; Gishen, P.; AL-Nahhas, A. Ventilation-Perfusion Scintigraphy Is More Sensitive than Multidetector CTPA in Detecting Chronic Thromboembolic Pulmonary Disease as a Treatable Cause of Pulmonary Hypertension. J. Nucl. Med. 2007, 48, 680–684. [Google Scholar] [CrossRef]
- Galiè, N.; Humbert, M.; Vachiery, J.-L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Noordegraaf, A.V.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension. Rev. Esp. Cardiol. Engl. Ed. 2016, 69, 177. [Google Scholar]
- Sirajuddin, A.; Donnelly, E.F.; Crabtree, T.P.; Henry, T.S.; Iannettoni, M.D.; Johnson, G.B.; Kazerooni, E.A.; Maldonado, F.; Olsen, K.M.; Wu, C.C.; et al. ACR Appropriateness Criteria® Suspected Pulmonary Hypertension. J. Am. Coll. Radiol. 2017, 14, S350–S361. [Google Scholar] [CrossRef][Green Version]
- Ryan, K.L.; Fedullo, P.F.; Davis, G.B.; Vasquez, T.E.; Moser, K.M. Perfusion Scan Findings Understate the Severity of Angiographic and Hemodynamic Compromise in Chronic Thromboembolic Pulmonary Hypertension. Chest 1988, 93, 1180–1185. [Google Scholar] [CrossRef]
- Gopalan, D.; Delcroix, M.; Held, M. Diagnosis of chronic thromboembolic pulmonary hypertension. Eur. Respir. Rev. Off. J. Eur. Respir. Soc. 2017, 26, 160108. [Google Scholar] [CrossRef] [PubMed]
- Lambert, L.; Michalek, P.; Burgetova, A. The diagnostic performance of CT pulmonary angiography in the detection of chronic thromboembolic pulmonary hypertension—Systematic review and meta-analysis. Eur. Radiol. 2022, 32, 7927–7935. [Google Scholar] [CrossRef]
- D’Armini, A.M. Diagnostic advances and opportunities in chronic thromboembolic pulmonary hypertension. Eur. Respir. Rev. 2015, 24, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Nicod, P.; Peterson, K.; Levine, M.; Dittrich, H.; Buchbinder, M.; Chappuis, F.; Moser, K. Pulmonary Angiography in Severe Chronic Pulmonary Hypertension. Ann. Intern. Med. 1987, 107, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Gerges, M.; Yacoub, M. Chronic thromboembolic pulmonary hypertension—Still evolving. Glob. Cardiol. Sci. Pract. 2020, 2020, e202011. Available online: https://globalcardiologyscienceandpractice.com/index.php/gcsp/article/view/427 (accessed on 28 July 2025). [CrossRef]
- Yang, J.; Madani, M.M.; Mahmud, E.; Kim, N.H. Evaluation and Management of Chronic Thromboembolic Pulmonary Hypertension. Chest 2023, 164, 490–502. [Google Scholar] [CrossRef]
- Held, M.; Grün, M.; Holl, R.; Hübner, G.; Kaiser, R.; Karl, S.; Kolb, M.; Schäfers, H.J.; Wilkens, H.; Jany, B. Cardiopulmonary exercise testing to detect chronic thromboembolic pulmonary hypertension in patients with normal echocardiography. Respir. Int. Rev. Thorac. Dis. 2014, 87, 379–387. [Google Scholar]
- Zhu, H.; Sun, X.; Cao, Y.; Pudasaini, B.; Yang, W.; Liu, J.; Guo, J. Cardiopulmonary exercise testing and pulmonary function testing for predicting the severity of CTEPH. BMC Pulm. Med. 2021, 21, 324. [Google Scholar] [CrossRef] [PubMed]
- Weatherald, J.; Farina, S.; Bruno, N.; Laveneziana, P. Cardiopulmonary Exercise Testing in Pulmonary Hypertension. Ann. Am. Thorac. Soc. 2017, 14 (Suppl. S1), S84–S92. [Google Scholar] [CrossRef]
- Mahmud, E.; Madani, M.M.; Kim, N.H.; Poch, D.; Ang, L.; Behnamfar, O.; Patel, M.P.; Auger, W.R. Chronic Thromboembolic Pulmonary Hypertension. J. Am. Coll. Cardiol. 2018, 71, 2468–2486. [Google Scholar] [CrossRef]
- Czerner, C.P.; Schoenfeld, C.; Cebotari, S.; Renne, J.; Kaireit, T.F.; Winther, H.B.; Pöhler, G.H.; Olsson, K.M.; Hoeper, M.M.; Wacker, F.; et al. Perioperative CTEPH patient monitoring with 2D phase-contrast MRI reflects clinical, cardiac and pulmonary perfusion changes after pulmonary endarterectomy. PLoS ONE 2020, 15, e0238171. [Google Scholar] [CrossRef] [PubMed]
- Ley, S.; Kauczor, H.-U.; Heussel, C.P.; Kramm, T.; Mayer, E.; Thelen, M.; Kreitner, K.-F. Value of contrast-enhanced MR angiography and helical CT angiography in chronic thromboembolic pulmonary hypertension. Eur. Radiol. 2003, 13, 2365–2371. [Google Scholar] [CrossRef] [PubMed]
- Ley, S.; Ley-Zaporozhan, J.; Pitton, M.B.; Schneider, J.; Wirth, G.M.; Mayer, E.; Düber, C.; Kreitner, K.-F. Diagnostic performance of state-of-the-art imaging techniques for morphological assessment of vascular abnormalities in patients with chronic thromboembolic pulmonary hypertension (CTEPH). Eur. Radiol. 2012, 22, 607–616. [Google Scholar] [CrossRef]
- Johns, C.S.; Swift, A.J.; Rajaram, S.; Hughes, P.J.C.; Capener, D.J.; Kiely, D.G.; Wild, J.M. Lung perfusion: MRI vs. SPECT for screening in suspected chronic thromboembolic pulmonary hypertension. J. Magn. Reson. Imaging 2017, 46, 1693–1697. [Google Scholar] [CrossRef]
- Araszkiewicz, A.; Jankiewicz, S.; Sławek-Szmyt, S.; Klotzka, A.; Grygier, M.; Mularek-Kubzdela, T.; Lesiak, M. Rapid clinical and haemodynamic improvement in a patient with intermediate-high risk pulmonary embolism treated with transcatheter aspiration thrombectomy. Postępy Kardiologii Interwencyjnej/Adv. Interv. Cardiol. 2019, 15, 497–498. [Google Scholar] [CrossRef]
- Christopher Malaisrie, S.; Chiu, S.; Schimmel, D.; Samant, M.; Avery, R.; Rahsepar, A.; Allen, B.; Raza, Y.; Freed, B.; Mylvaganam, R.; et al. Outcomes of Multidisciplinary Care at a Chronic Thromboembolic Pulmonary Hypertension Center. Pulm. Circ. 2025, 15, e70085. [Google Scholar] [CrossRef]
- Yang, B.; Zaki, A.; Oh, N.; Umana-Pizano, J.; Haddadin, I.; Goyanes, A.; Smedira, N.; Elgharably, H.; Zhen-Yu Tong, M.; Heresi, G.A. Role of a multidisciplinary team approach in the management of chronic thromboembolic pulmonary hypertension. JTCVS Open 2025, 24, 147–155. [Google Scholar] [CrossRef]
- Kawakami, T.; Matsubara, H.; Shinke, T.; Abe, K.; Kohsaka, S.; Hosokawa, K.; Taniguchi, Y.; Shimokawahara, H.; Yamada, Y.; Kataoka, M.; et al. Balloon pulmonary angioplasty versus riociguat in inoperable chronic thromboembolic pulmonary hypertension (MR BPA): An open-label, randomised controlled trial. Lancet Respir. Med. 2022, 10, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Shimokawahara, H.; Nishizaki, M.; Inami, T.; Kubota, K.; Taniguchi, Y.; Miyagi, A.; Kikuchi, H.; Goda, A.; Miyanaga, S.; Hashimoto, H.; et al. Effect of riociguat on exercise following balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension in Japan (THERAPY-HYBRID-BPA): A multicentre, double-blind, randomised, controlled, phase 4 trial. Lancet Respir. Med. 2025, 13, 789–799. [Google Scholar] [CrossRef]
- Piazza, G.; Goldhaber, S.Z. Chronic Thromboembolic Pulmonary Hypertension. N. Engl. J. Med. 2011, 364, 351–360. [Google Scholar] [CrossRef]
- Hosokawa, K.; Watanabe, H.; Taniguchi, Y.; Ikeda, N.; Inami, T.; Yasuda, S.; Murohara, T.; Hatano, M.; Tamura, Y.; Yamashita, J.; et al. A Multicenter, Single-Blind, Randomized, Warfarin-Controlled Trial of Edoxaban in Patients with Chronic Thromboembolic Pulmonary Hypertension: KABUKI Trial. Circulation 2024, 149, 406–409. [Google Scholar] [CrossRef]
- Sena, S.; Bulent, M.; Derya, K.; Deniz, K.; Halil, A.; Okan, E.; Bedrettin, Y. Real-life data of direct anticoagulant use, bleeding risk and venous thromboembolism recurrence in chronic thromboembolic pulmonary hypertension patients: An observational retrospective study. Pulm. Circ. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Humbert, M.; Simonneau, G.; Pittrow, D.; Delcroix, M.; Pepke-Zaba, J.; Langleben, D.; Mielniczuk, L.M.; Escribano Subias, P.; Snijder, R.J.; Barberà, J.A.; et al. Oral anticoagulants (NOAC and VKA) in chronic thromboembolic pulmonary hypertension. J. Heart Lung Transplant. 2022, 41, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.; Keshishian, A.; Hines, D.M.; Dina, O.; Le, H.; Rosenblatt, L.; Liu, X.; Zhang, Q.; Vo, L. Risk of stroke/systemic embolism, major bleeding, and associated costs in non-valvular atrial fibrillation patients who initiated apixaban, dabigatran, or rivaroxaban compared with warfarin in the United States medicare population: Updated analysis. Curr. Med. Res. Opin. 2022, 38, 2131–2140. [Google Scholar] [CrossRef] [PubMed]
- Olschewski, H.; Simonneau, G.; Galiè, N.; Higenbottam, T.; Naeije, R.; Rubin, L.J.; Nikkho, S.; Speich, R.; Hoeper, M.M.; Behr, J.; et al. Inhaled Iloprost for Severe Pulmonary Hypertension. N. Engl. J. Med. 2002, 347, 322–329. [Google Scholar] [CrossRef]
- Sadushi-Kolici, R.; Jansa, P.; Kopec, G.; Torbicki, A.; Skoro-Sajer, N.; Campean, I.-A.; Halank, M.; Simkova, I.; Karlocai, K.; Steringer-Mascherbauer, R.; et al. Subcutaneous treprostinil for the treatment of severe non-operable chronic thromboembolic pulmonary hypertension (CTREPH): A double-blind, phase 3, randomised controlled trial. Lancet Respir. Med. 2019, 7, 239–248. [Google Scholar] [CrossRef]
- Jaïs, X.; D’Armini, A.M.; Jansa, P.; Torbicki, A.; Delcroix, M.; Ghofrani, H.A.; Hoeper, M.M.; Lang, I.M.; Mayer, E.; Pepke-Zaba, J.; et al. Bosentan for Treatment of Inoperable Chronic Thromboembolic Pulmonary Hypertension. J. Am. Coll. Cardiol. 2008, 52, 2127–2134. [Google Scholar] [CrossRef]
- Ghofrani, H.-A.; D’Armini, A.M.; Grimminger, F.; Hoeper, M.M.; Jansa, P.; Kim, N.H.; Mayer, E.; Simonneau, G.; Wilkins, M.R.; Fritsch, A.; et al. Riociguat for the Treatment of Chronic Thromboembolic Pulmonary Hypertension. N. Engl. J. Med. 2013, 369, 319–329. [Google Scholar] [CrossRef]
- Hoeper, M.M. Pharmacological therapy for patients with chronic thromboembolic pulmonary hypertension. Eur. Respir. Rev. 2015, 24, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Marra, A.M.; Halank, M.; Benjamin, N.; Bossone, E.; Cittadini, A.; Eichstaedt, C.A.; Egenlauf, B.; Harutyunova, S.; Fischer, C.; Gall, H.; et al. Right ventricular size and function under riociguat in pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension (the RIVER study). Respir. Res. 2018, 19, 258. [Google Scholar] [CrossRef] [PubMed]
- Madani, M.M. Surgical Treatment of Chronic Thromboembolic Pulmonary Hypertension: Pulmonary Thromboendarterectomy. Methodist DeBakey Cardiovasc. J. 2016, 12, 213. [Google Scholar] [CrossRef]
- Jenkins, D.P.; Madani, M.; Mayer, E.; Kerr, K.; Kim, N.; Klepetko, W.; Morsolini, M.; Dartevelle, P. Surgical treatment of chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2013, 41, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Wiedenroth, C.B.; Jenkins, D.; Brenot, P.; Lang, I.M.; Matsubara, H.; Pepke-Zaba, J.; Channick, R.; Jais, X.; Simonneau, G.; Delcroix, M.; et al. Management of chronic thromboembolic pulmonary hypertension. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2025, 44, S8–S14. [Google Scholar] [CrossRef]
- Boulate, D.; Perros, F.; Dorfmuller, P.; Arthur-Ataam, J.; Guihaire, J.; Lamrani, L.; Decante, B.; Humbert, M.; Eddahibi, S.; Dartevelle, P.; et al. Pulmonary microvascular lesions regress in reperfused chronic thromboembolic pulmonary hypertension. J. Heart Lung Transplant. 2015, 34, 457–467. [Google Scholar] [CrossRef]
- de Perrot, M. Operability assessment in chronic thromboembolic pulmonary hypertension (CTEPH): Don’t miss the chance of a second opinion! J. Thorac. Cardiovasc. Surg. 2016, 152, 656–657. [Google Scholar] [CrossRef][Green Version]
- D’Armini, A.M.; Morsolini, M.; Mattiucci, G.; Grazioli, V.; Pin, M.; Valentini, A.; Silvaggio, G.; Klersy, C.; Dore, R. Pulmonary endarterectomy for distal chronic thromboembolic pulmonary hypertension. J. Thorac. Cardiovasc. Surg. 2014, 148, 1005–1011, 1012.e1–e2; discussion 1011–1012. [Google Scholar] [CrossRef]
- Aggarwal, V.; Giri, J.; Visovatti, S.H.; Mahmud, E.; Matsubara, H.; Madani, M.; Rogers, F.; Gopalan, D.; Rosenfield, K.; McLaughlin, V.V.; et al. Status and Future Directions for Balloon Pulmonary Angioplasty in Chronic Thromboembolic Pulmonary Disease with and Without Pulmonary Hypertension: A Scientific Statement from the American Heart Association. Circulation 2024, 149, e1090–e1107. [Google Scholar] [CrossRef]
- Dardi, F.; Rotunno, M.; Guarino, D.; Suarez, S.M.; Niro, F.; Loforte, A.; Taglieri, N.; Ballerini, A.; Magnani, I.; Bertozzi, R.; et al. Comparison of different treatment strategies in patients with chronic thromboembolic pulmonary hypertension: A single centre real-world experience. Int. J. Cardiol. 2023, 391, 131333. [Google Scholar] [CrossRef]
- Kataoka, M.; Inami, T.; Kawakami, T.; Fukuda, K.; Satoh, T. Balloon Pulmonary Angioplasty (Percutaneous Transluminal Pulmonary Angioplasty) for Chronic Thromboembolic Pulmonary Hypertension: A Japanese Perspective. JACC Cardiovasc. Interv. 2019, 12, 1382–1388. [Google Scholar] [CrossRef]
- Thistlethwaite, P.A.; Kaneko, K.; Madani, M.M.; Jamieson, S.W. Technique and outcomes of pulmonary endarterectomy surgery. Ann. Thorac. Cardiovasc. Surg. Off. J. Assoc. Thorac. Cardiovasc. Surg. Asia 2008, 14, 274–282. [Google Scholar]
- Jamieson, S.W.; Kapelanski, D.P.; Sakakibara, N.; Manecke, G.R.; Thistlethwaite, P.A.; Kerr, K.M.; Channick, R.N.; Fedullo, P.F.; Auger, W.R. Pulmonary endarterectomy: Experience and lessons learned in 1,500 cases. Ann. Thorac. Surg. 2003, 76, 1457–1464. [Google Scholar] [CrossRef]
- Hayashi, H.; Ning, Y.; Kurlansky, P.; Vaynrub, A.; Bacchetta, M.; Rosenzweig, E.B.; Takeda, K. Characteristics and prognostic significance of right heart remodeling and tricuspid regurgitation after pulmonary endarterectomy. J. Thorac. Cardiovasc. Surg. 2024, 167, 658–667.e7. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, C.A.; Waziri, F.; Ringgaard, S.; Mellemkjær, S.; Clemmensen, T.S.; Hjortdal, V.E.; Nielsen, S.L.; Poulsen, S.H. Reverse remodeling of tricuspid valve morphology and function in chronic thromboembolic pulmonary hypertension patients following pulmonary thromboendarterectomy: A cardiac magnetic resonance imaging and invasive hemodynamic study. BMC Cardiovasc. Disord. 2021, 21, 450. [Google Scholar] [CrossRef] [PubMed]
- Vuylsteke, A.; Sharples, L.; Charman, G.; Kneeshaw, J.; Tsui, S.; Dunning, J.; Wheaton, E.; Klein, A.; Arrowsmith, J.; Hall, R.; et al. Circulatory arrest versus cerebral perfusion during pulmonary endarterectomy surgery (PEACOG): A randomised controlled trial. Lancet 2011, 378, 1379–1387. [Google Scholar] [CrossRef]
- Madani, M.; Mayer, E.; Fadel, E.; Jenkins, D.P. Pulmonary Endarterectomy. Patient Selection, Technical Challenges, and Outcomes. Ann. Am. Thorac. Soc. 2016, 13 (Suppl. S3), S240–S247. [Google Scholar] [CrossRef]
- Miyahara, S.; Schröder, T.A.; Wilkens, H.; Karliova, I.; Langer, F.; Kunihara, T.; Schäfers, H.-J. Long-term Outcomes After Pulmonary Endarterectomy in 499 Patients Over a 20-Year Period. Ann. Thorac. Surg. 2021, 111, 1585–1592. [Google Scholar] [CrossRef]
- Heuts, S.; Kawczynski, M.J.; Leus, A.; Godinas, L.; Belge, C.; van Empel, V.; Meyns, B.; Maessen, J.G.; Delcroix, M.; Verbelen, T. The volume-outcome relationship for pulmonary endarterectomy in chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2025, 65, 2401865. [Google Scholar] [CrossRef] [PubMed]
- Delcroix, M.; Pepke-Zaba, J.; D’Armini, A.M.; Fadel, E.; Guth, S.; Hoole, S.P.; Jenkins, D.P.; Kiely, D.G.; Kim, N.H.; Madani, M.M.; et al. Worldwide CTEPH Registry: Long-Term Outcomes with Pulmonary Endarterectomy, Balloon Pulmonary Angioplasty, and Medical Therapy. Circulation 2024, 150, 1354–1365. [Google Scholar] [CrossRef] [PubMed]
- Braams, N.J.; Ruigrok, D.; Schokker, M.G.M.; Padervinskiene, L.; De Man, F.S.; Marcus, J.T.; Lely, R.J.; Beijk, M.A.M.; Klok, F.A.; Huisman, M.V.; et al. Pulmonary vascular imaging characteristics after pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. J. Heart Lung Transplant. 2020, 39, 248–256. [Google Scholar] [CrossRef]
- Cannon, J.E.; Su, L.; Kiely, D.G.; Page, K.; Toshner, M.; Swietlik, E.; Treacy, C.; Ponnaberanam, A.; Condliffe, R.; Sheares, K.; et al. Dynamic Risk Stratification of Patient Long-Term Outcome After Pulmonary Endarterectomy: Results from the United Kingdom National Cohort. Circulation 2016, 133, 1761–1771. [Google Scholar] [CrossRef] [PubMed]
- Berman, M.; Hardman, G.; Sharples, L.; Pepke-Zaba, J.; Sheares, K.; Tsui, S.; Dunning, J.; Jenkins, D.P. Pulmonary endarterectomy: Outcomes in patients aged >70. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2012, 41, e154–e160. [Google Scholar] [CrossRef]
- Donahoe, L.; Granton, J.; McRae, K.; Thenganatt, J.; Moric, J.; Keshavjee, S.; de Perrot, M. Role of extracorporeal life support after pulmonary endarterectomy: A single-centre experience. Interact. Cardiovasc. Thorac. Surg. 2016, 23, 74–78. [Google Scholar] [CrossRef]
- Sugiyama, K.; Suzuki, S.; Fujiyoshi, T.; Koizumi, N.; Sato, M.; Ogino, H. Extracorporeal membrane oxygenation after pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. J. Card. Surg. 2019, 34, 428–434. [Google Scholar] [CrossRef]
| Risk | Clinical History | Comorbidities | Imaging Features | Hemodynamics |
|---|---|---|---|---|
| Lower Risk | +DVT/PE −RHF | None or Minimal NYHA Functional Class II or III | Clear disease, concordance on all studies Bilateral lower lobe distribution | PVR < 1000 dyn·s·cm−5 PVR is proportionate to distribution of obstruction on imaging Higher PA pulse pressure |
| Higher Risk | −DVT/PE +RHF | History of lung and/or left heart disease NYHA Functional Class IV | Inconsistent on imaging studies No lower lobe disease | PVR > 1200 dyn·s·cm−5 PVR out of proportion to obstruction on imaging Higher PA diastolic pressure |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McGann, K.C.; Wang, C.C.; Trahanas, J.M.; Bommareddi, S.; Lima, B.; Ahmad, A.; Chin, C.W.; Robbins, I.M.; Pugh, M.E.; Hemnes, A.R.; et al. The Surgical Management of Chronic Thromboembolic Pulmonary Hypertension. J. Clin. Med. 2025, 14, 6862. https://doi.org/10.3390/jcm14196862
McGann KC, Wang CC, Trahanas JM, Bommareddi S, Lima B, Ahmad A, Chin CW, Robbins IM, Pugh ME, Hemnes AR, et al. The Surgical Management of Chronic Thromboembolic Pulmonary Hypertension. Journal of Clinical Medicine. 2025; 14(19):6862. https://doi.org/10.3390/jcm14196862
Chicago/Turabian StyleMcGann, Kevin C., Chen Chia Wang, John M. Trahanas, Swaroop Bommareddi, Brian Lima, Awab Ahmad, Clifford W. Chin, Ivan M. Robbins, Meredith E. Pugh, Anna R. Hemnes, and et al. 2025. "The Surgical Management of Chronic Thromboembolic Pulmonary Hypertension" Journal of Clinical Medicine 14, no. 19: 6862. https://doi.org/10.3390/jcm14196862
APA StyleMcGann, K. C., Wang, C. C., Trahanas, J. M., Bommareddi, S., Lima, B., Ahmad, A., Chin, C. W., Robbins, I. M., Pugh, M. E., Hemnes, A. R., Funke, B., Shah, A. S., & Williams, A. M. (2025). The Surgical Management of Chronic Thromboembolic Pulmonary Hypertension. Journal of Clinical Medicine, 14(19), 6862. https://doi.org/10.3390/jcm14196862







