Intermediate-Risk Pulmonary Embolism: Patients’ Stratification, Prognosis, and Therapeutic Options—Time to Pay Attention to the Middle Child
Abstract
1. Introduction
2. Definition and Epidemiology
3. The Pulmonary Embolism Severity Index (PESI) Clinical Scoring System
4. Lower Extremity Doppler
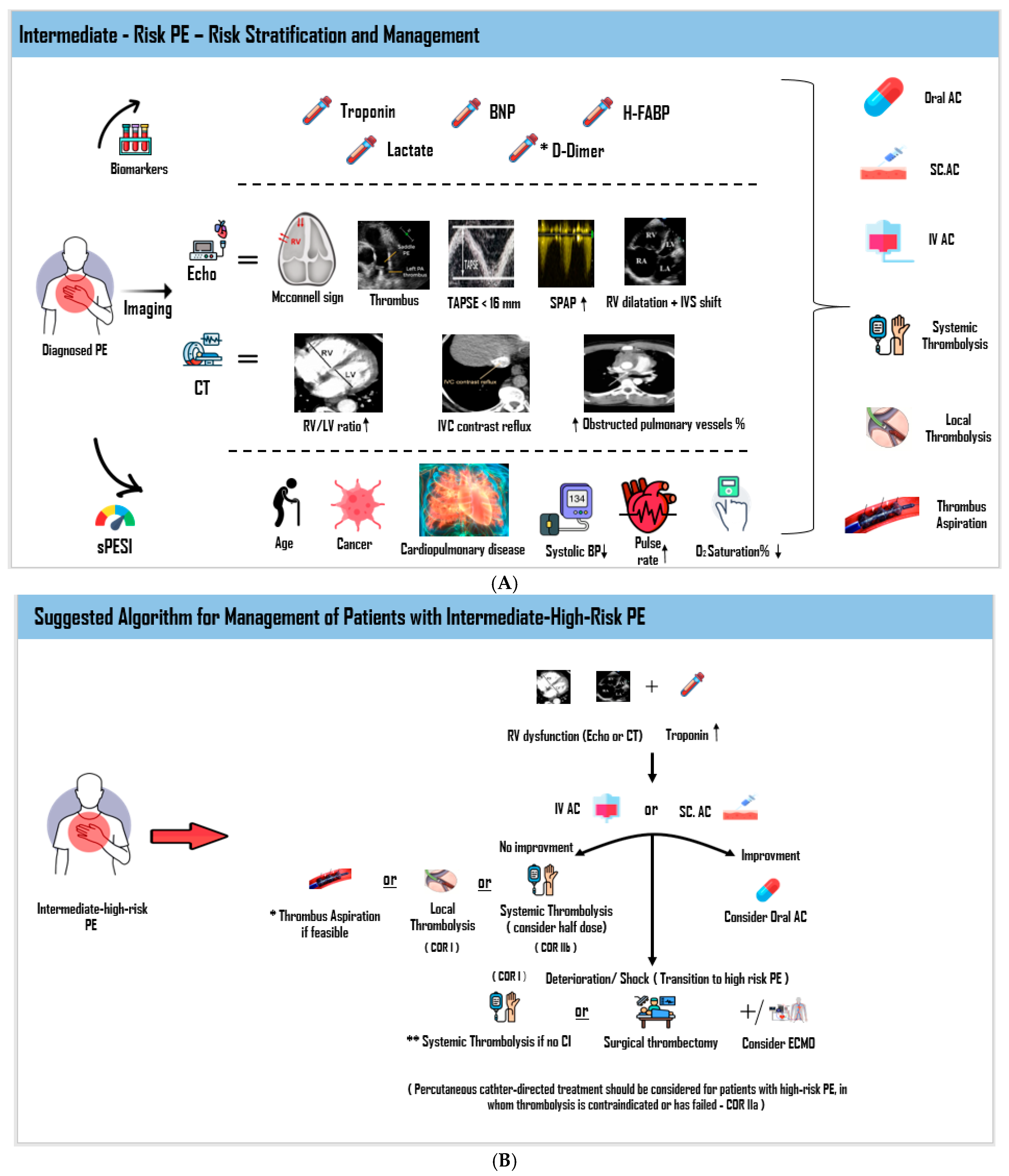
5. Non-Invasive Evaluation and Stratification of PE Patients
5.1. Echocardiography
- A normal RV function does not rule out the presence of a PE. The negative predictive value of echocardiography for PE is about 40–50% [1].
- As the RV morphology is complex due to its crescent shape, the definition of RV dysfunction can be somewhat vague [35,36]. Main echocardiographic findings suggestive of RV dysfunction include the following: 1. RV dilatation and systolic dysfunction, often with preserved contractility of the RV apex, a pattern known as McConnell’s sign, which has a specificity of 94% [37]. It should be kept in mind that the crescent shape of the RV may lead to high inter-observer variation in assessment of RV function [38]. 2. Signs suggestive of RV pressure overload such as shift of the interventricular septum to the left, which can be seen as a D-shape in the short-axis view (Figure 1B).
| Echocardiographic Finding | Description | Comments |
|---|---|---|
| McConnell’s sign | Normal excursion of the right ventricular apex with hypokinesis of the mid-free wall segment [37] | There is a poor PPV (45–67%) for acute PE diagnosis [39]. An additional study found the McConnell’s sign to be correlated with a high thrombotic burden in particular central or multi-lobar PE, which may be associated with a higher risk of clinical deterioration. This association is still debatable [40]. |
| RV dysfunction | No standard definition | Shown to be a marker of adverse events [41,42]. There is a high inter-observer variability. |
| RV dilatation Figure 1A | End diastolic diameter > 30 mm, RV/LV end diastolic diameter > 0.9 [43] | 30% of patients with normotensive pulmonary embolism are diagnosed with RV dilation [11]—an independent risk of in-hospital mortality. High inter-observer variability in measurements. |
| Interventricular septum shift Figure 1B | “D”-shaped septum in the short-axis view is a secondary sign for increased RV pressures | Leftward interventricular septal shift may be associated with increased mortality |
| Tricuspid annular pane systolic excursion (TAPSE) | TAPSE < 1.6 cm | Shown to predict both 30-day and all-cause mortality as well as PE-specific mortality [44,45]. Main limitation—low inter-observer variability |
| Systolic pulmonary artery pressure (SPAP) and mean PAP (MPAP) estimation Figure 1D | Measured using the simplified Bernoulli equation: PAP = 4V2 + right atrial pressure (RAP) | Elevated PAPs are associated with a higher risk of both in-hospital and ICU mortality [46]. Easily obtained when there is an adequate tricuspid regurgitation signal. (Figure 1C). Evaluation of RAP via IVC parameters [47] can be problematic and a main cause for over/under estimation of PAPs Elevated PAP is not part of risk stratification among PE patients according ESC guidelines [1] |
| Visible Right Sided thrombi or thrombus in transit Figure 1E | An infrequent finding | A large registry found it to be associated with more complicated presenting symptoms (tachycardia, lower blood pressure) as well as higher short-term mortality among PE patients [33] |
5.2. Additional Echocardiographic Parameters
5.2.1. Tricuspid Annular Plane Systolic Excursion (TAPSE)
5.2.2. Pulmonary Artery Pressure (PAP)
Right Ventricular–Pulmonary Artery Coupling
5.2.3. Echocardiographic Response to Therapy
5.3. Computed Tomography (CT) (Figure 2)
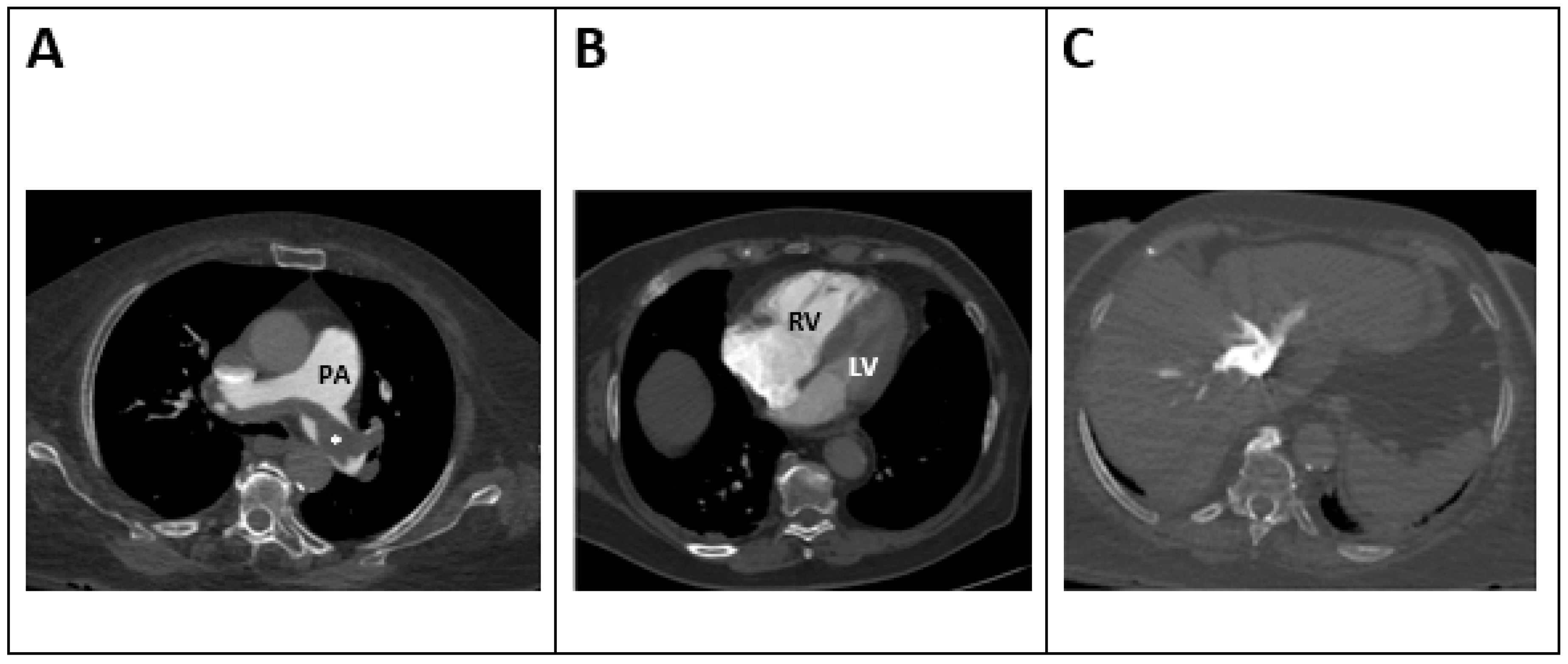
5.3.1. RV/LV Ratio
5.3.2. Evaluation of Thrombotic Clot Burden
5.4. Ventilation-Perfusion (V/Q) Scan
6. Laboratory Findings
7. Treatment of Patients with Intermediate-Risk/Submassive PE
7.1. Medical Therapy
7.2. Anticoagulation Therapy
7.2.1. Parenteral Anticoagulants
7.2.2. Oral Anticoagulants
7.2.3. Thrombolysis
7.2.4. Percutaneous Catheter-Directed Interventions (CDIs)
7.3. Early Reperfusion Treatment in Unique or High-Risk Circumstances
7.4. Inferior Vena Cava (IVC) Filter
8. Patient Outcomes
9. Future Directions
10. Summary
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.-J.; Harjola, V.-P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the Diagnosis and Management of Acute Pulmonary Embolism Developed in Collaboration with the European Respiratory Society (ERS): The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef]
- Becattini, C.; Agnelli, G.; Lankeit, M.; Masotti, L.; Pruszczyk, P.; Casazza, F.; Vanni, S.; Nitti, C.; Kamphuisen, P.; Vedovati, M.C.; et al. Acute Pulmonary Embolism: Mortality Prediction by the 2014 European Society of Cardiology Risk Stratification Model. Eur. Respir. J. 2016, 48, 780–786. [Google Scholar] [CrossRef]
- Nobre, C.; Mesquita, D.; Thomas, B.; Ponte, T.; Santos, L.; Tavares, J. A Clinical Audit of Thrombolytic Therapy in Patients with Normotensive Pulmonary Embolism and Intermediate Risk. Acute Card. Care 2014, 16, 63–66. [Google Scholar] [CrossRef]
- Daley, M.J.; Lat, I. Clinical Controversies in Thrombolytic Therapy for the Management of Acute Pulmonary Embolism. Pharmacotherapy 2012, 32, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Condliffe, R.; Elliot, C.A.; Hughes, R.J.; Hurdman, J.; Maclean, R.M.; Sabroe, I.; van Veen, J.J.; Kiely, D.G. Management Dilemmas in Acute Pulmonary Embolism. Thorax 2014, 69, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Heit, J.A. The Epidemiology of Venous Thromboembolism in the Community. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 370–372. [Google Scholar] [CrossRef] [PubMed]
- CDC Data and Statistics on Venous Thromboembolism. Available online: https://www.cdc.gov/blood-clots/data-research/facts-stats/index.html (accessed on 31 May 2025).
- Lucena, J.; Rico, A.; Vázquez, R.; Marín, R.; Martínez, C.; Salguero, M.; Miguel, L. Pulmonary Embolism and Sudden–Unexpected Death: Prospective Study on 2477 Forensic Autopsies Performed at the Institute of Legal Medicine in Seville. J. Forensic Leg. Med. 2009, 16, 196–201. [Google Scholar] [CrossRef]
- Goldhaber, S.Z. Pulmonary Embolism. Lancet 2004, 363, 1295–1305. [Google Scholar] [CrossRef]
- Taylor, R.A.; Davis, J.; Liu, R.; Gupta, V.; Dziura, J.; Moore, C.L. Point-of-Care Focused Cardiac Ultrasound for Prediction of Pulmonary Embolism Adverse Outcomes. J. Emerg. Med. 2013, 45, 392–399. [Google Scholar] [CrossRef]
- Pruszczyk, P.; Goliszek, S.; Lichodziejewska, B.; Kostrubiec, M.; Ciurzyński, M.; Kurnicka, K.; Dzikowska-Diduch, O.; Palczewski, P.; Wyzgal, A. Prognostic Value of Echocardiography in Normotensive Patients with Acute Pulmonary Embolism. JACC Cardiovasc. Imaging 2014, 7, 553–560. [Google Scholar] [CrossRef]
- Grifoni, S.; Olivotto, I.; Cecchini, P.; Pieralli, F.; Camaiti, A.; Santoro, G.; Conti, A.; Agnelli, G.; Berni, G. Short-Term Clinical Outcome of Patients with Acute Pulmonary Embolism, Normal Blood Pressure, and Echocardiographic Right Ventricular Dysfunction. Circulation 2000, 101, 2817–2822. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.; Lindmarker, P.; Juhlin-Dannfelt, A.; Johnsson, H.; Jorfeldt, L. Echocardiography Doppler in Pulmonary Embolism: Right Ventricular Dysfunction as a Predictor of Mortality Rate. Am. Heart J. 1997, 134, 479–487. [Google Scholar] [CrossRef]
- Jaff, M.R.; McMurtry, M.S.; Archer, S.L.; Cushman, M.; Goldenberg, N.; Goldhaber, S.Z.; Jenkins, J.S.; Kline, J.A.; Michaels, A.D.; Thistlethwaite, P.; et al. Management of Massive and Submassive Pulmonary Embolism, Iliofemoral Deep Vein Thrombosis, and Chronic Thromboembolic Pulmonary Hypertension: A Scientific Statement from the American Heart Association. Circulation 2011, 123, 1788–1830. [Google Scholar] [CrossRef]
- McIntyre, K.M.; Sasahara, A.A. The Hemodynamic Response to Pulmonary Embolism in Patients without Prior Cardiopulmonary Disease. Am. J. Cardiol. 1971, 28, 288–294. [Google Scholar] [CrossRef]
- Torbicki, A.; Perrier, A.; Konstantinides, S.; Agnelli, G.; Galiè, N.; Pruszczyk, P.; Bengel, F.; Brady, A.J.B.; Ferreira, D.; Janssens, U.; et al. Guidelines on the Diagnosis and Management of Acute Pulmonary Embolism: The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur. Heart J. 2008, 29, 2276–2315. [Google Scholar] [CrossRef] [PubMed]
- Kearon, C.; Akl, E.A.; Ornelas, J.; Blaivas, A.; Jimenez, D.; Bounameaux, H.; Huisman, M.; King, C.S.; Morris, T.A.; Sood, N.; et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. CHEST 2016, 149, 315–352. [Google Scholar] [CrossRef]
- Busse, L.W.; Vourlekis, J.S. Submassive Pulmonary Embolism. Crit. Care Clin. 2014, 30, 447–473. [Google Scholar] [CrossRef]
- Aujesky, D.; Obrosky, D.S.; Stone, R.A.; Auble, T.E.; Perrier, A.; Cornuz, J.; Roy, P.-M.; Fine, M.J. Derivation and Validation of a Prognostic Model for Pulmonary Embolism. Am. J. Respir. Crit. Care Med. 2005, 172, 1041–1046. [Google Scholar] [CrossRef]
- Righini, M.; Roy, P.-M.; Meyer, G.; Verschuren, F.; Aujesky, D.; Le Gal, G. The Simplified Pulmonary Embolism Severity Index (PESI): Validation of a Clinical Prognostic Model for Pulmonary Embolism. J. Thromb. Haemost. JTH 2011, 9, 2115–2117. [Google Scholar] [CrossRef] [PubMed]
- Aujesky, D.; Roy, P.-M.; Le Manach, C.P.; Verschuren, F.; Meyer, G.; Obrosky, D.S.; Stone, R.A.; Cornuz, J.; Fine, M.J. Validation of a Model to Predict Adverse Outcomes in Patients with Pulmonary Embolism. Eur. Heart J. 2006, 27, 476–481. [Google Scholar] [CrossRef]
- Chan, C.M.; Woods, C.; Shorr, A.F. The Validation and Reproducibility of the Pulmonary Embolism Severity Index. J. Thromb. Haemost. JTH 2010, 8, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- Dentali, F.; Riva, N.; Turato, S.; Grazioli, S.; Squizzato, A.; Steidl, L.; Guasti, L.; Grandi, A.M.; Ageno, W. Pulmonary Embolism Severity Index Accurately Predicts Long-Term Mortality Rate in Patients Hospitalized for Acute Pulmonary Embolism. J. Thromb. Haemost. JTH 2013, 11, 2103–2110. [Google Scholar] [CrossRef]
- Donzé, J.; Le Gal, G.; Fine, M.J.; Roy, P.-M.; Sanchez, O.; Verschuren, F.; Cornuz, J.; Meyer, G.; Perrier, A.; Righini, M.; et al. Prospective Validation of the Pulmonary Embolism Severity Index. A Clinical Prognostic Model for Pulmonary Embolism. Thromb. Haemost. 2008, 100, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Aujesky, D.; Roy, P.-M.; Verschuren, F.; Righini, M.; Osterwalder, J.; Egloff, M.; Renaud, B.; Verhamme, P.; Stone, R.A.; Legall, C.; et al. Outpatient versus Inpatient Treatment for Patients with Acute Pulmonary Embolism: An International, Open-Label, Randomised, Non-Inferiority Trial. Lancet 2011, 378, 41–48. [Google Scholar] [CrossRef]
- Wicki, J.; Perrier, A.; Perneger, T.V.; Bounameaux, H.; Junod, A.F. Predicting Adverse Outcome in Patients with Acute Pulmonary Embolism: A Risk Score. Thromb. Haemost. 2000, 84, 548–552. [Google Scholar] [CrossRef]
- Hellenkamp, K.; Kaeberich, A.; Schwung, J.; Konstantinides, S.; Lankeit, M. Risk Stratification of Normotensive Pulmonary Embolism Based on the sPESI—Does It Work for All Patients? Int. J. Cardiol. 2015, 197, 162–163. [Google Scholar] [CrossRef]
- Cordeanu, M.; Gaertner, S.; Faller, A.; Mirea, C.; Le Ray, I.; Stephan, D. Prognostic Value of the Simplified PESI Score in Comparison with the 2014 ESC Risk Model in Pulmonary Embolism. Int. J. Cardiol. 2016, 220, 623–624. [Google Scholar] [CrossRef]
- Ferrer, M.; Morillo, R.; Elías, T.; Jara, L.; García, L.; Nieto, R.; Sandoval, E.; Uresandi, F.; Otero, R.; Jiménez, D. Validation of Two Clinical Prognostic Models in Patients with Acute Symptomatic Pulmonary Embolism. Arch. Bronconeumol. 2013, 49, 427–431. [Google Scholar] [CrossRef]
- Masotti, L.; Panigada, G.; Landini, G.; Pieralli, F.; Corradi, F.; Lenti, S.; Migliacci, R.; TUSCAN-PE Investigators. Predictive Ability of the New 2014 ESC Prognostic Model in Acute Pulmonary Embolism. Int. J. Cardiol. 2016, 202, 801–803. [Google Scholar] [CrossRef]
- Barco, S.; Vicaut, E.; Klok, F.A.; Lankeit, M.; Meyer, G.; Konstantinides, S.V.; PEITHO Investigators. Improved Identification of Thrombolysis Candidates amongst Intermediate-Risk Pulmonary Embolism Patients: Implications for Future Trials. Eur. Respir. J. 2018, 51, 1701775. [Google Scholar] [CrossRef] [PubMed]
- Natanzon, S.S.; Fardman, A.; Chernomordik, F.; Mazin, I.; Herscovici, R.; Goitein, O.; Ben-Zekry, S.; Younis, A.; Grupper, A.; Matetzky, S.; et al. PESI Score for Predicting Clinical Outcomes in PE Patients with Right Ventricular Involvement. Heart Vessel. 2022, 37, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Torbicki, A.; Galié, N.; Covezzoli, A.; Rossi, E.; De Rosa, M.; Goldhaber, S.Z.; ICOPER Study Group. Right Heart Thrombi in Pulmonary Embolism: Results from the International Cooperative Pulmonary Embolism Registry. J. Am. Coll. Cardiol. 2003, 41, 2245–2251. [Google Scholar] [CrossRef] [PubMed]
- Chartier, L.; Béra, J.; Delomez, M.; Asseman, P.; Beregi, J.P.; Bauchart, J.J.; Warembourg, H.; Théry, C. Free-Floating Thrombi in the Right Heart: Diagnosis, Management, and Prognostic Indexes in 38 Consecutive Patients. Circulation 1999, 99, 2779–2783. [Google Scholar] [CrossRef] [PubMed]
- Alsoos, F.; Khaddam, A. Echocardiographic Evaluation Methods for Right Ventricular Function. J. Echocardiogr. 2015, 13, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, O.; Trinquart, L.; Colombet, I.; Durieux, P.; Huisman, M.V.; Chatellier, G.; Meyer, G. Prognostic Value of Right Ventricular Dysfunction in Patients with Haemodynamically Stable Pulmonary Embolism: A Systematic Review. Eur. Heart J. 2008, 29, 1569–1577. [Google Scholar] [CrossRef]
- McConnell, M.V.; Solomon, S.D.; Rayan, M.E.; Come, P.C.; Goldhaber, S.Z.; Lee, R.T. Regional Right Ventricular Dysfunction Detected by Echocardiography in Acute Pulmonary Embolism. Am. J. Cardiol. 1996, 78, 469–473. [Google Scholar] [CrossRef]
- Gripari, P.; Muratori, M.; Fusini, L.; Tamborini, G.; Ali, S.G.; Brusoni, D.; Pepi, M. Right Ventricular Dimensions and Function: Why Do We Need a More Accurate and Quantitative Imaging? J. Cardiovasc. Echogr. 2015, 25, 19–25. [Google Scholar] [CrossRef]
- Vaid, U.; Singer, E.; Marhefka, G.D.; Kraft, W.K.; Baram, M. Poor Positive Predictive Value of McConnell’s Sign on Transthoracic Echocardiography for the Diagnosis of Acute Pulmonary Embolism. Hosp. Pract. 1995 2013, 41, 23–27. [Google Scholar] [CrossRef]
- Tuzovic, M.; Adigopula, S.; Amsallem, M.; Kobayashi, Y.; Kadoch, M.; Boulate, D.; Krishnan, G.; Liang, D.; Schnittger, I.; Fleischmann, D.; et al. Regional Right Ventricular Dysfunction in Acute Pulmonary Embolism: Relationship with Clot Burden and Biomarker Profile. Int. J. Cardiovasc. Imaging 2016, 32, 389–398. [Google Scholar] [CrossRef]
- Keller, K.; Beule, J.; Schulz, A.; Coldewey, M.; Dippold, W.; Balzer, J.O. Right Ventricular Dysfunction in Hemodynamically Stable Patients with Acute Pulmonary Embolism. Thromb. Res. 2014, 133, 555–559. [Google Scholar] [CrossRef]
- Coutance, G.; Cauderlier, E.; Ehtisham, J.; Hamon, M.; Hamon, M. The Prognostic Value of Markers of Right Ventricular Dysfunction in Pulmonary Embolism: A Meta-Analysis. Crit. Care 2011, 15, R103. [Google Scholar] [CrossRef]
- Frémont, B.; Pacouret, G.; Jacobi, D.; Puglisi, R.; Charbonnier, B.; de Labriolle, A. Prognostic Value of Echocardiographic Right/Left Ventricular End-Diastolic Diameter Ratio in Patients with Acute Pulmonary Embolism: Results from a Monocenter Registry of 1,416 Patients. Chest 2008, 133, 358–362. [Google Scholar] [CrossRef]
- Miller, D.; Farah, M.G.; Liner, A.; Fox, K.; Schluchter, M.; Hoit, B.D. The Relation between Quantitative Right Ventricular Ejection Fraction and Indices of Tricuspid Annular Motion and Myocardial Performance. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2004, 17, 443–447. [Google Scholar] [CrossRef]
- Lobo, J.L.; Holley, A.; Tapson, V.; Moores, L.; Oribe, M.; Barrón, M.; Otero, R.; Nauffal, D.; Valle, R.; Monreal, M.; et al. Prognostic Significance of Tricuspid Annular Displacement in Normotensive Patients with Acute Symptomatic Pulmonary Embolism. J. Thromb. Haemost. JTH 2014, 12, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Khemasuwan, D.; Yingchoncharoen, T.; Tunsupon, P.; Kusunose, K.; Moghekar, A.; Klein, A.; Tonelli, A.R. Right Ventricular Echocardiographic Parameters Are Associated with Mortality after Acute Pulmonary Embolism. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2015, 28, 355–362. [Google Scholar] [CrossRef]
- Beigel, R.; Cercek, B.; Luo, H.; Siegel, R.J. Noninvasive Evaluation of Right Atrial Pressure. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2013, 26, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Kopecna, D.; Briongos, S.; Castillo, H.; Moreno, C.; Recio, M.; Navas, P.; Lobo, J.L.; Alonso-Gomez, A.; Obieta-Fresnedo, I.; Fernández-Golfin, C.; et al. Interobserver Reliability of Echocardiography for Prognostication of Normotensive Patients with Pulmonary Embolism. Cardiovasc. Ultrasound 2014, 12, 29. [Google Scholar] [CrossRef]
- Lyhne, M.D.; Bikdeli, B.; Jiménez, D.; Kabrhel, C.; Dudzinski, D.M.; Moisés, J.; Lobo, J.L.; Armestar, F.; Guirado, L.; Ballaz, A.; et al. Right Ventricular-Pulmonary Artery Coupling for Prognostication in Acute Pulmonary Embolism. Eur. Heart J. Acute Cardiovasc. Care 2024, 13, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Goldhaber, S.Z.; Haire, W.D.; Feldstein, M.L.; Miller, M.; Toltzis, R.; Smith, J.L.; Taveira da Silva, A.M.; Come, P.C.; Lee, R.T.; Parker, J.A. Alteplase versus Heparin in Acute Pulmonary Embolism: Randomised Trial Assessing Right-Ventricular Function and Pulmonary Perfusion. Lancet 1993, 341, 507–511. [Google Scholar] [CrossRef]
- Kline, J.A.; Steuerwald, M.T.; Marchick, M.R.; Hernandez-Nino, J.; Rose, G.A. Prospective Evaluation of Right Ventricular Function and Functional Status 6 Months after Acute Submassive Pulmonary Embolism: Frequency of Persistent or Subsequent Elevation in Estimated Pulmonary Artery Pressure. Chest 2009, 136, 1202–1210. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Vicaut, E.; Danays, T.; Becattini, C.; Bertoletti, L.; Beyer-Westendorf, J.; Bouvaist, H.; Couturaud, F.; Dellas, C.; Duerschmied, D.; et al. Impact of Thrombolytic Therapy on the Long-Term Outcome of Intermediate-Risk Pulmonary Embolism. J. Am. Coll. Cardiol. 2017, 69, 1536–1544. [Google Scholar] [CrossRef]
- Araoz, P.A.; Haramati, L.B.; Mayo, J.R.; Barbosa, E.J.M.; Rybicki, F.J.; Colletti, P.M. Panel Discussion: Pulmonary Embolism Imaging and Outcomes. Am. J. Roentgenol. 2012, 198, 1313–1319. [Google Scholar] [CrossRef]
- Hariharan, P.; Dudzinski, D.M.; Rosovsky, R.; Haddad, F.; MacMahon, P.; Parry, B.; Chang, Y.; Kabrhel, C. Relation Among Clot Burden, Right-Sided Heart Strain, and Adverse Events After Acute Pulmonary Embolism. Am. J. Cardiol. 2016, 118, 1568–1573. [Google Scholar] [CrossRef] [PubMed]
- Meinel, F.G.; Nance, J.W.; Schoepf, U.J.; Hoffmann, V.S.; Thierfelder, K.M.; Costello, P.; Goldhaber, S.Z.; Bamberg, F. Predictive Value of Computed Tomography in Acute Pulmonary Embolism: Systematic Review and Meta-Analysis. Am. J. Med. 2015, 128, 747–759.e2. [Google Scholar] [CrossRef]
- Mansencal, N.; Joseph, T.; Vieillard-Baron, A.; Langlois, S.; El Hajjam, M.; Qanadli, S.D.; Lacombe, P.; Jardin, F.; Dubourg, O. Diagnosis of Right Ventricular Dysfunction in Acute Pulmonary Embolism Using Helical Computed Tomography. Am. J. Cardiol. 2005, 95, 1260–1263. [Google Scholar] [CrossRef]
- Mastora, I.; Remy-Jardin, M.; Masson, P.; Galland, E.; Delannoy, V.; Bauchart, J.-J.; Remy, J. Severity of Acute Pulmonary Embolism: Evaluation of a New Spiral CT Angiographic Score in Correlation with Echocardiographic Data. Eur. Radiol. 2003, 13, 29–35. [Google Scholar] [CrossRef]
- Engelke, C.; Rummeny, E.J.; Marten, K. Acute Pulmonary Embolism on MDCT of the Chest: Prediction of Cor Pulmonale and Short-Term Patient Survival from Morphologic Embolus Burden. AJR Am. J. Roentgenol. 2006, 186, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Bach, A.G.; Nansalmaa, B.; Kranz, J.; Taute, B.-M.; Wienke, A.; Schramm, D.; Surov, A. CT Pulmonary Angiography Findings That Predict 30-Day Mortality in Patients with Acute Pulmonary Embolism. Eur. J. Radiol. 2015, 84, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Apfaltrer, P.; Henzler, T.; Meyer, M.; Roeger, S.; Haghi, D.; Gruettner, J.; Süselbeck, T.; Wilson, R.B.; Schoepf, U.J.; Schoenberg, S.O.; et al. Correlation of CT Angiographic Pulmonary Artery Obstruction Scores with Right Ventricular Dysfunction and Clinical Outcome in Patients with Acute Pulmonary Embolism. Eur. J. Radiol. 2012, 81, 2867–2871. [Google Scholar] [CrossRef]
- Qanadli, S.D.; El Hajjam, M.; Vieillard-Baron, A.; Joseph, T.; Mesurolle, B.; Oliva, V.L.; Barré, O.; Bruckert, F.; Dubourg, O.; Lacombe, P. New CT Index to Quantify Arterial Obstruction in Pulmonary Embolism: Comparison with Angiographic Index and Echocardiography. AJR Am. J. Roentgenol. 2001, 176, 1415–1420. [Google Scholar] [CrossRef]
- van der Meer, R.W.; Pattynama, P.M.T.; van Strijen, M.J.L.; van den Berg-Huijsmans, A.A.; Hartmann, I.J.C.; Putter, H.; de Roos, A.; Huisman, M.V. Right Ventricular Dysfunction and Pulmonary Obstruction Index at Helical CT: Prediction of Clinical Outcome during 3-Month Follow-up in Patients with Acute Pulmonary Embolism. Radiology 2005, 235, 798–803. [Google Scholar] [CrossRef]
- Araoz, P.A.; Gotway, M.B.; Trowbridge, R.L.; Bailey, R.A.; Auerbach, A.D.; Reddy, G.P.; Dawn, S.K.; Webb, W.R.; Higgins, C.B. Helical CT Pulmonary Angiography Predictors of In-Hospital Morbidity and Mortality in Patients with Acute Pulmonary Embolism. J. Thorac. Imaging 2003, 18, 207–216. [Google Scholar] [CrossRef]
- Bailis, N.; Lerche, M.; Meyer, H.J.; Wienke, A.; Surov, A. Contrast Reflux into the Inferior Vena Cava on Computer Tomographic Pulmonary Angiography Is a Predictor of 24-Hour and 30-Day Mortality in Patients with Acute Pulmonary Embolism. Acta Radiol. Stockh. Swed. 1987 2021, 62, 34–41. [Google Scholar] [CrossRef]
- Aviram, G.; Soikher, E.; Bendet, A.; Shmueli, H.; Ziv-Baran, T.; Amitai, Y.; Friedensohn, L.; Berliner, S.; Meilik, A.; Topilsky, Y. Prediction of Mortality in Pulmonary Embolism Based on Left Atrial Volume Measured on CT Pulmonary Angiography. Chest 2016, 149, 667–675. [Google Scholar] [CrossRef]
- Oz, I.I.; Altınsoy, B.; Serifoglu, I.; Sayın, R.; Buyukuysal, M.C.; Erboy, F.; Akduman, E.I. Evaluation of Right Atrium-to-Right Ventricle Diameter Ratio on Computed Tomography Pulmonary Angiography: Prediction of Adverse Outcome and 30-Day Mortality. Eur. J. Radiol. 2015, 84, 2526–2532. [Google Scholar] [CrossRef] [PubMed]
- Nural, M.S.; Elmali, M.; Findik, S.; Yapici, O.; Uzun, O.; Sunter, A.T.; Erkan, L. Computed Tomographic Pulmonary Angiography in the Assessment of Severity of Acute Pulmonary Embolism and Right Ventricular Dysfunction. Acta Radiol. Stockh. Swed. 1987 2009, 50, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.M.; Nardelli, P.; Minhas, J.K.; Ash, S.Y.; Estépar, R.S.J.; Antkowiak, M.C.; Badlam, J.B.; Piazza, G.; Estépar, R.S.J.; Washko, G.R.; et al. CT Imaging Determinants of Persistent Hypoxemia in Acute Intermediate-Risk Pulmonary Embolism. J. Thromb. Thrombolysis 2023, 56, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Bajc, M.; Schümichen, C.; Grüning, T.; Lindqvist, A.; Le Roux, P.-Y.; Alatri, A.; Bauer, R.W.; Dilic, M.; Neilly, B.; Verberne, H.J.; et al. EANM Guideline for Ventilation/Perfusion Single-Photon Emission Computed Tomography (SPECT) for Diagnosis of Pulmonary Embolism and Beyond. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2429–2451. [Google Scholar] [CrossRef]
- Hurwitz, L.M.; Reiman, R.E.; Yoshizumi, T.T.; Goodman, P.C.; Toncheva, G.; Nguyen, G.; Lowry, C. Radiation Dose from Contemporary Cardiothoracic Multidetector CT Protocols with an Anthropomorphic Female Phantom: Implications for Cancer Induction. Radiology 2007, 245, 742–750. [Google Scholar] [CrossRef]
- Le Roux, P.-Y.; Robin, P.; Salaun, P.-Y. New Developments and Future Challenges of Nuclear Medicine and Molecular Imaging for Pulmonary Embolism. Thromb. Res. 2018, 163, 236–241. [Google Scholar] [CrossRef]
- Sostman, H.D.; Miniati, M.; Gottschalk, A.; Matta, F.; Stein, P.D.; Pistolesi, M. Sensitivity and Specificity of Perfusion Scintigraphy Combined with Chest Radiography for Acute Pulmonary Embolism in PIOPED II. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2008, 49, 1741–1748. [Google Scholar] [CrossRef]
- Bajaj, A.; Rathor, P.; Sehgal, V.; Kabak, B.; Shetty, A.; Al Masalmeh, O.; Hosur, S. Prognostic Value of Biomarkers in Acute Non-Massive Pulmonary Embolism: A Systematic Review and Meta-Analysis. Lung 2015, 193, 639–651. [Google Scholar] [CrossRef]
- Daquarti, G.; March Vecchio, N.; Mitrione, C.S.; Furmento, J.; Ametrano, M.C.; Dominguez Pace, M.P.; Costabel, J.P. High-Sensitivity Troponin and Right Ventricular Function in Acute Pulmonary Embolism. Am. J. Emerg. Med. 2016, 34, 1579–1582. [Google Scholar] [CrossRef] [PubMed]
- Kaeberich, A.; Seeber, V.; Jiménez, D.; Kostrubiec, M.; Dellas, C.; Hasenfuß, G.; Giannitsis, E.; Pruszczyk, P.; Konstantinides, S.; Lankeit, M. Age-Adjusted High-Sensitivity Troponin T Cut-off Value for Risk Stratification of Pulmonary Embolism. Eur. Respir. J. 2015, 45, 1323–1331. [Google Scholar] [CrossRef]
- Keller, K.; Beule, J.; Schulz, A.; Coldewey, M.; Dippold, W.; Balzer, J.O. Cardiac Troponin I for Predicting Right Ventricular Dysfunction and Intermediate Risk in Patients with Normotensive Pulmonary Embolism. Neth. Heart J. Mon. J. Neth. Soc. Cardiol. Neth. Heart Found. 2015, 23, 55–61. [Google Scholar] [CrossRef]
- Hakemi, E.U.; Alyousef, T.; Dang, G.; Hakmei, J.; Doukky, R. The Prognostic Value of Undetectable Highly Sensitive Cardiac Troponin I in Patients with Acute Pulmonary Embolism. Chest 2015, 147, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Becattini, C.; Vedovati, M.C.; Agnelli, G. Prognostic Value of Troponins in Acute Pulmonary Embolism: A Meta-Analysis. Circulation 2007, 116, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Stein, P.D.; Matta, F.; Janjua, M.; Yaekoub, A.Y.; Jaweesh, F.; Alrifai, A. Outcome in Stable Patients with Acute Pulmonary Embolism Who Had Right Ventricular Enlargement and/or Elevated Levels of Troponin I. Am. J. Cardiol. 2010, 106, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.; Binder, L.; Hruska, N.; Luthe, H.; Buchwald, A.B. Cardiac Troponin I Elevation in Acute Pulmonary Embolism Is Associated with Right Ventricular Dysfunction. J. Am. Coll. Cardiol. 2000, 36, 1632–1636. [Google Scholar] [CrossRef]
- Pruszczyk, P.; Bochowicz, A.; Torbicki, A.; Szulc, M.; Kurzyna, M.; Fijałkowska, A.; Kuch-Wocial, A. Cardiac Troponin T Monitoring Identifies High-Risk Group of Normotensive Patients with Acute Pulmonary Embolism. Chest 2003, 123, 1947–1952. [Google Scholar] [CrossRef]
- Lega, J.-C.; Lacasse, Y.; Lakhal, L.; Provencher, S. Natriuretic Peptides and Troponins in Pulmonary Embolism: A Meta-Analysis. Thorax 2009, 64, 869–875. [Google Scholar] [CrossRef] [PubMed]
- ten Wolde, M.; Tulevski, I.I.; Mulder, J.W.M.; Söhne, M.; Boomsma, F.; Mulder, B.J.M.; Büller, H.R. Brain Natriuretic Peptide as a Predictor of Adverse Outcome in Patients with Pulmonary Embolism. Circulation 2003, 107, 2082–2084. [Google Scholar] [CrossRef]
- Pieralli, F.; Olivotto, I.; Vanni, S.; Conti, A.; Camaiti, A.; Targioni, G.; Grifoni, S.; Berni, G. Usefulness of Bedside Testing for Brain Natriuretic Peptide to Identify Right Ventricular Dysfunction and Outcome in Normotensive Patients with Acute Pulmonary Embolism. Am. J. Cardiol. 2006, 97, 1386–1390. [Google Scholar] [CrossRef]
- Kucher, N.; Printzen, G.; Goldhaber, S.Z. Prognostic Role of Brain Natriuretic Peptide in Acute Pulmonary Embolism. Circulation 2003, 107, 2545–2547. [Google Scholar] [CrossRef]
- Lankeit, M.; Jiménez, D.; Kostrubiec, M.; Dellas, C.; Kuhnert, K.; Hasenfuß, G.; Pruszczyk, P.; Konstantinides, S. Validation of N-Terminal pro-Brain Natriuretic Peptide Cut-off Values for Risk Stratification of Pulmonary Embolism. Eur. Respir. J. 2014, 43, 1669–1677. [Google Scholar] [CrossRef]
- Ruan, L.B.; He, L.; Zhao, S.; Zhu, P.; Li, W.Y. Prognostic Value of Plasma Heart-Type Fatty Acid-Binding Protein in Patients with Acute Pulmonary Embolism: A Meta-Analysis. Chest 2014, 146, 1462–1467. [Google Scholar] [CrossRef]
- Liu, M.; Yuan, X.; Qiu, X.; Shan, X.; Lin, D.; Zhu, L. Prognostic Role of Heart-Type Fatty Acid Binding Protein in Pulmonary Embolism: A Meta-Analysis. Thromb. Res. 2015, 135, 20–25. [Google Scholar] [CrossRef]
- Bajaj, A.; Rathor, P.; Sehgal, V.; Shetty, A.; Kabak, B.; Hosur, S. Risk Stratification in Acute Pulmonary Embolism with Heart-Type Fatty Acid-Binding Protein: A Meta-Analysis. J. Crit. Care 2015, 30, 1151.e1–1151.e7. [Google Scholar] [CrossRef]
- Dellas, C.; Puls, M.; Lankeit, M.; Schäfer, K.; Cuny, M.; Berner, M.; Hasenfuss, G.; Konstantinides, S. Elevated Heart-Type Fatty Acid-Binding Protein Levels on Admission Predict an Adverse Outcome in Normotensive Patients with Acute Pulmonary Embolism. J. Am. Coll. Cardiol. 2010, 55, 2150–2157. [Google Scholar] [CrossRef] [PubMed]
- Boscheri, A.; Wunderlich, C.; Langer, M.; Schoen, S.; Wiedemann, B.; Stolte, D.; Elmer, G.; Barthel, P.; Strasser, R.H. Correlation of Heart-Type Fatty Acid-Binding Protein with Mortality and Echocardiographic Data in Patients with Pulmonary Embolism at Intermediate Risk. Am. Heart J. 2010, 160, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Vanni, S.; Viviani, G.; Baioni, M.; Pepe, G.; Nazerian, P.; Socci, F.; Bartolucci, M.; Bartolini, M.; Grifoni, S. Prognostic Value of Plasma Lactate Levels among Patients with Acute Pulmonary Embolism: The Thrombo-Embolism Lactate Outcome Study. Ann. Emerg. Med. 2013, 61, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Vanni, S.; Nazerian, P.; Bova, C.; Bondi, E.; Morello, F.; Pepe, G.; Paladini, B.; Liedl, G.; Cangioli, E.; Grifoni, S.; et al. Comparison of Clinical Scores for Identification of Patients with Pulmonary Embolism at Intermediate-High Risk of Adverse Clinical Outcome: The Prognostic Role of Plasma Lactate. Intern. Emerg. Med. 2017, 12, 657–665. [Google Scholar] [CrossRef]
- Becattini, C.; Lignani, A.; Masotti, L.; Forte, M.B.; Agnelli, G. D-Dimer for Risk Stratification in Patients with Acute Pulmonary Embolism. J. Thromb. Thrombolysis 2012, 33, 48–57. [Google Scholar] [CrossRef]
- Hellenkamp, K.; Schwung, J.; Rossmann, H.; Kaeberich, A.; Wachter, R.; Hasenfuß, G.; Konstantinides, S.; Lankeit, M. Risk Stratification of Normotensive Pulmonary Embolism: Prognostic Impact of Copeptin. Eur. Respir. J. 2015, 46, 1701–1710. [Google Scholar] [CrossRef] [PubMed]
- Hellenkamp, K.; Pruszczyk, P.; Jiménez, D.; Wyzgał, A.; Barrios, D.; Ciurzyński, M.; Morillo, R.; Hobohm, L.; Keller, K.; Kurnicka, K.; et al. Prognostic Impact of Copeptin in Pulmonary Embolism: A Multicentre Validation Study. Eur. Respir. J. 2018, 51, 1702037. [Google Scholar] [CrossRef] [PubMed]
- Müller-Bardorff, M.; Weidtmann, B.; Giannitsis, E.; Kurowski, V.; Katus, H.A. Release Kinetics of Cardiac Troponin T in Survivors of Confirmed Severe Pulmonary Embolism. Clin. Chem. 2002, 48, 673–675. [Google Scholar] [CrossRef]
- Jiménez, D.; Díaz, G.; Molina, J.; Martí, D.; Del Rey, J.; García-Rull, S.; Escobar, C.; Vidal, R.; Sueiro, A.; Yusen, R.D. Troponin I and Risk Stratification of Patients with Acute Nonmassive Pulmonary Embolism. Eur. Respir. J. 2008, 31, 847–853. [Google Scholar] [CrossRef]
- Konstantinides, S.; Geibel, A.; Olschewski, M.; Kasper, W.; Hruska, N.; Jäckle, S.; Binder, L. Importance of Cardiac Troponins I and T in Risk Stratification of Patients with Acute Pulmonary Embolism. Circulation 2002, 106, 1263–1268. [Google Scholar] [CrossRef]
- Kucher, N.; Goldhaber, S.Z. Cardiac Biomarkers for Risk Stratification of Patients with Acute Pulmonary Embolism. Circulation 2003, 108, 2191–2194. [Google Scholar] [CrossRef]
- Viswanathan, K.; Kilcullen, N.; Morrell, C.; Thistlethwaite, S.J.; Sivananthan, M.U.; Hassan, T.B.; Barth, J.H.; Hall, A.S. Heart-Type Fatty Acid-Binding Protein Predicts Long-Term Mortality and Re-Infarction in Consecutive Patients with Suspected Acute Coronary Syndrome Who Are Troponin-Negative. J. Am. Coll. Cardiol. 2010, 55, 2590–2598. [Google Scholar] [CrossRef]
- van Belle, A.; Büller, H.R.; Huisman, M.V.; Huisman, P.M.; Kaasjager, K.; Kamphuisen, P.W.; Kramer, M.H.H.; Kruip, M.J.H.A.; Kwakkel-van Erp, J.M.; Leebeek, F.W.G.; et al. Effectiveness of Managing Suspected Pulmonary Embolism Using an Algorithm Combining Clinical Probability, D-Dimer Testing, and Computed Tomography. JAMA 2006, 295, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Ghanima, W.; Abdelnoor, M.; Holmen, L.O.; Nielssen, B.E.; Ross, S.; Sandset, P.M. D-Dimer Level Is Associated with the Extent of Pulmonary Embolism. Thromb. Res. 2007, 120, 281–288. [Google Scholar] [CrossRef]
- Gutte, H.; Mortensen, J.; Jensen, C.V.; von der Recke, P.; Petersen, C.L.; Kristoffersen, U.S.; Kjaer, A. ANP, BNP and D-Dimer Predict Right Ventricular Dysfunction in Patients with Acute Pulmonary Embolism. Clin. Physiol. Funct. Imaging 2010, 30, 466–472. [Google Scholar] [CrossRef]
- Jeebun, V.; Doe, S.J.; Singh, L.; Worthy, S.A.; Forrest, I.A. Are Clinical Parameters and Biomarkers Predictive of Severity of Acute Pulmonary Emboli on CTPA? QJM Mon. J. Assoc. Physicians 2010, 103, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Türedi, S.; Karahan, S.C.; Menteşe, A.; Gündüz, A.; Topbaş, M.; Koşucu, P.; Oztuna, F.; Tatli, O. Investigation of Relationship between the D-Dimer and Ischemia-Modified Albumin Levels with the Radiological Imaging-Based Pulmonary Embolism Severity Score in Acute Pulmonary Embolism. Anadolu Kardiyol. Derg. AKD Anatol. J. Cardiol. 2010, 10, 346–352. [Google Scholar] [CrossRef]
- Lankeit, M.; Jiménez, D.; Kostrubiec, M.; Dellas, C.; Hasenfuss, G.; Pruszczyk, P.; Konstantinides, S. Predictive Value of the High-Sensitivity Troponin T Assay and the Simplified Pulmonary Embolism Severity Index in Hemodynamically Stable Patients with Acute Pulmonary Embolism: A Prospective Validation Study. Circulation 2011, 124, 2716–2724. [Google Scholar] [CrossRef] [PubMed]
- Bolignano, D.; Cabassi, A.; Fiaccadori, E.; Ghigo, E.; Pasquali, R.; Peracino, A.; Peri, A.; Plebani, M.; Santoro, A.; Settanni, F.; et al. Copeptin (CTproAVP), a New Tool for Understanding the Role of Vasopressin in Pathophysiology. Clin. Chem. Lab. Med. 2014, 52, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Finocchiaro, S.; Mauro, M.S.; Rochira, C.; Spagnolo, M.; Laudani, C.; Landolina, D.; Mazzone, P.M.; Agnello, F.; Ammirabile, N.; Faro, D.C.; et al. Percutaneous Interventions for Pulmonary Embolism. EuroIntervention J. Eur. Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2024, 20, e408–e424. [Google Scholar] [CrossRef]
- Schulman, S.; Kearon, C.; Kakkar, A.K.; Mismetti, P.; Schellong, S.; Eriksson, H.; Baanstra, D.; Schnee, J.; Goldhaber, S.Z.; RE-COVER Study Group. Dabigatran versus Warfarin in the Treatment of Acute Venous Thromboembolism. N. Engl. J. Med. 2009, 361, 2342–2352. [Google Scholar] [CrossRef]
- Schulman, S.; Kakkar, A.K.; Goldhaber, S.Z.; Schellong, S.; Eriksson, H.; Mismetti, P.; Christiansen, A.V.; Friedman, J.; Le Maulf, F.; Peter, N.; et al. Treatment of Acute Venous Thromboembolism with Dabigatran or Warfarin and Pooled Analysis. Circulation 2014, 129, 764–772. [Google Scholar] [CrossRef]
- EINSTEIN–PE Investigators; Büller, H.R.; Prins, M.H.; Lensin, A.W.A.; Decousus, H.; Jacobson, B.F.; Minar, E.; Chlumsky, J.; Verhamme, P.; Wells, P.; et al. Oral Rivaroxaban for the Treatment of Symptomatic Pulmonary Embolism. N. Engl. J. Med. 2012, 366, 1287–1297. [Google Scholar] [CrossRef]
- Agnelli, G.; Buller, H.R.; Cohen, A.; Curto, M.; Gallus, A.S.; Johnson, M.; Masiukiewicz, U.; Pak, R.; Thompson, J.; Raskob, G.E.; et al. Oral Apixaban for the Treatment of Acute Venous Thromboembolism. N. Engl. J. Med. 2013, 369, 799–808. [Google Scholar] [CrossRef]
- Hokusai-VTE Investigators; Büller, H.R.; Décousus, H.; Grosso, M.A.; Mercuri, M.; Middeldorp, S.; Prins, M.H.; Raskob, G.E.; Schellong, S.M.; Schwocho, L.; et al. Edoxaban versus Warfarin for the Treatment of Symptomatic Venous Thromboembolism. N. Engl. J. Med. 2013, 369, 1406–1415. [Google Scholar] [CrossRef] [PubMed]
- Agnelli, G.; Prandoni, P.; Becattini, C.; Silingardi, M.; Taliani, M.R.; Miccio, M.; Imberti, D.; Poggio, R.; Ageno, W.; Pogliani, E.; et al. Extended Oral Anticoagulant Therapy after a First Episode of Pulmonary Embolism. Ann. Intern. Med. 2003, 139, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Tromeur, C.; Sanchez, O.; Presles, E.; Pernod, G.; Bertoletti, L.; Jego, P.; Duhamel, E.; Provost, K.; Parent, F.; Robin, P.; et al. Risk Factors for Recurrent Venous Thromboembolism after Unprovoked Pulmonary Embolism: The PADIS-PE Randomised Trial. Eur. Respir. J. 2018, 51, 1701202. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Quinlan, D.J.; Agnelli, G.; Eikelboom, J.W. Thrombolysis Compared with Heparin for the Initial Treatment of Pulmonary Embolism: A Meta-Analysis of the Randomized Controlled Trials. Circulation 2004, 110, 744–749. [Google Scholar] [CrossRef]
- Stein, P.D.; Matta, F. Thrombolytic Therapy in Unstable Patients with Acute Pulmonary Embolism: Saves Lives but Underused. Am. J. Med. 2012, 125, 465–470. [Google Scholar] [CrossRef]
- Marti, C.; John, G.; Konstantinides, S.; Combescure, C.; Sanchez, O.; Lankeit, M.; Meyer, G.; Perrier, A. Systemic Thrombolytic Therapy for Acute Pulmonary Embolism: A Systematic Review and Meta-Analysis. Eur. Heart J. 2015, 36, 605–614. [Google Scholar] [CrossRef]
- Kline, J.A.; Nordenholz, K.E.; Courtney, D.M.; Kabrhel, C.; Jones, A.E.; Rondina, M.T.; Diercks, D.B.; Klinger, J.R.; Hernandez, J. Treatment of Submassive Pulmonary Embolism with Tenecteplase or Placebo: Cardiopulmonary Outcomes at 3 Months: Multicenter Double-Blind, Placebo-Controlled Randomized Trial. J. Thromb. Haemost. JTH 2014, 12, 459–468. [Google Scholar] [CrossRef]
- Gao, G.; Yang, P.; Liu, M.; Ding, M.; Liu, G.; Tong, Y.; Yang, C.; Meng, F. Thrombolysis for Acute Intermediate-Risk Pulmonary Embolism: A Meta-Analysis. Thromb. Res. 2015, 136, 932–937. [Google Scholar] [CrossRef]
- Meyer, G.; Vicaut, E.; Danays, T.; Agnelli, G.; Becattini, C.; Beyer-Westendorf, J.; Bluhmki, E.; Bouvaist, H.; Brenner, B.; Couturaud, F.; et al. Fibrinolysis for Patients with Intermediate-Risk Pulmonary Embolism. N. Engl. J. Med. 2014, 370, 1402–1411. [Google Scholar] [CrossRef]
- Mikkola, K.M.; Patel, S.R.; Parker, J.A.; Grodstein, F.; Goldhaber, S.Z. Increasing Age Is a Major Risk Factor for Hemorrhagic Complications after Pulmonary Embolism Thrombolysis. Am. Heart J. 1997, 134, 69–72. [Google Scholar] [CrossRef]
- Konstantinides, S.; Geibel, A.; Heusel, G.; Heinrich, F.; Kasper, W.; Management Strategies and Prognosis of Pulmonary Embolism-3 Trial Investigators. Heparin plus Alteplase Compared with Heparin Alone in Patients with Submassive Pulmonary Embolism. N. Engl. J. Med. 2002, 347, 1143–1150. [Google Scholar] [CrossRef]
- Fasullo, S.; Scalzo, S.; Maringhini, G.; Ganci, F.; Cannizzaro, S.; Basile, I.; Cangemi, D.; Terrazzino, G.; Parrinello, G.; Sarullo, F.M.; et al. Six-Month Echocardiographic Study in Patients with Submassive Pulmonary Embolism and Right Ventricle Dysfunction: Comparison of Thrombolysis with Heparin. Am. J. Med. Sci. 2011, 341, 33–39. [Google Scholar] [CrossRef]
- Xu, Q.; Huang, K.; Zhai, Z.; Yang, Y.; Wang, J.; Wang, C. Initial Thrombolysis Treatment Compared with Anticoagulation for Acute Intermediate-Risk Pulmonary Embolism: A Meta-Analysis. J. Thorac. Dis. 2015, 7, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Stevens, S.M.; Woller, S.C.; Kreuziger, L.B.; Bounameaux, H.; Doerschug, K.; Geersing, G.-J.; Huisman, M.V.; Kearon, C.; King, C.S.; Knighton, A.J.; et al. Antithrombotic Therapy for VTE Disease: Second Update of the CHEST Guideline and Expert Panel Report. CHEST 2021, 160, e545–e608. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, M.; Bay, C.; Skrocki, L.; Rahimi, F.; Mehdipour, M.; “MOPETT” Investigators. Moderate Pulmonary Embolism Treated with Thrombolysis (from the “MOPETT” Trial). Am. J. Cardiol. 2013, 111, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Takano, H.; Kubota, Y.; Asai, K.; Shimizu, W. Impact of the Efficacy of Thrombolytic Therapy on the Mortality of Patients with Acute Submassive Pulmonary Embolism: A Meta-Analysis. J. Thromb. Haemost. JTH 2014, 12, 1086–1095. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chakraborty, A.; Weinberg, I.; Kadakia, M.; Wilensky, R.L.; Sardar, P.; Kumbhani, D.J.; Mukherjee, D.; Jaff, M.R.; Giri, J. Thrombolysis for Pulmonary Embolism and Risk of All-Cause Mortality, Major Bleeding, and Intracranial Hemorrhage: A Meta-Analysis. JAMA 2014, 311, 2414–2421. [Google Scholar] [CrossRef]
- Kucher, N.; Boekstegers, P.; Müller, O.J.; Kupatt, C.; Beyer-Westendorf, J.; Heitzer, T.; Tebbe, U.; Horstkotte, J.; Müller, R.; Blessing, E.; et al. Randomized, Controlled Trial of Ultrasound-Assisted Catheter-Directed Thrombolysis for Acute Intermediate-Risk Pulmonary Embolism. Circulation 2014, 129, 479–486. [Google Scholar] [CrossRef]
- Sadeghipour, P.; Jenab, Y.; Moosavi, J.; Hosseini, K.; Mohebbi, B.; Hosseinsabet, A.; Chatterjee, S.; Pouraliakbar, H.; Shirani, S.; Shishehbor, M.H.; et al. Catheter-Directed Thrombolysis vs Anticoagulation in Patients with Acute Intermediate-High-Risk Pulmonary Embolism: The CANARY Randomized Clinical Trial. JAMA Cardiol. 2022, 7, 1189–1197. [Google Scholar] [CrossRef]
- Avgerinos, E.D.; Jaber, W.; Lacomis, J.; Markel, K.; McDaniel, M.; Rivera-Lebron, B.N.; Ross, C.B.; Sechrist, J.; Toma, C.; Chaer, R.; et al. Randomized Trial Comparing Standard Versus Ultrasound-Assisted Thrombolysis for Submassive Pulmonary Embolism: The SUNSET sPE Trial. JACC Cardiovasc. Interv. 2021, 14, 1364–1373. [Google Scholar] [CrossRef]
- Piazza, G.; Hohlfelder, B.; Jaff, M.R.; Ouriel, K.; Engelhardt, T.C.; Sterling, K.M.; Jones, N.J.; Gurley, J.C.; Bhatheja, R.; Kennedy, R.J.; et al. A Prospective, Single-Arm, Multicenter Trial of Ultrasound-Facilitated, Catheter-Directed, Low-Dose Fibrinolysis for Acute Massive and Submassive Pulmonary Embolism: The SEATTLE II Study. JACC Cardiovasc. Interv. 2015, 8, 1382–1392. [Google Scholar] [CrossRef]
- Tapson, V.F.; Sterling, K.; Jones, N.; Elder, M.; Tripathy, U.; Brower, J.; Maholic, R.L.; Ross, C.B.; Natarajan, K.; Fong, P.; et al. A Randomized Trial of the Optimum Duration of Acoustic Pulse Thrombolysis Procedure in Acute Intermediate-Risk Pulmonary Embolism: The OPTALYSE PE Trial. JACC Cardiovasc. Interv. 2018, 11, 1401–1410. [Google Scholar] [CrossRef]
- Sterling, K.M.; Goldhaber, S.Z.; Sharp, A.S.P.; Kucher, N.; Jones, N.; Maholic, R.; Meneveau, N.; Zlotnick, D.; Sayfo, S.; Konstantinides, S.V.; et al. Prospective Multicenter International Registry of Ultrasound-Facilitated Catheter-Directed Thrombolysis in Intermediate-High and High-Risk Pulmonary Embolism (KNOCOUT PE). Circ. Cardiovasc. Interv. 2024, 17, e013448. [Google Scholar] [CrossRef]
- Pasha, A.K.; Siddiqui, M.U.; Siddiqui, M.D.; Ahmed, A.; Abdullah, A.; Riaz, I.; Murad, M.H.; Bjarnason, H.; Wysokinski, W.E.; McBane, R.D. Catheter Directed Compared to Systemically Delivered Thrombolysis for Pulmonary Embolism: A Systematic Review and Meta-Analysis. J. Thromb. Thrombolysis 2022, 53, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Tu, T.; Toma, C.; Tapson, V.F.; Adams, C.; Jaber, W.A.; Silver, M.; Khandhar, S.; Amin, R.; Weinberg, M.; Engelhardt, T.; et al. A Prospective, Single-Arm, Multicenter Trial of Catheter-Directed Mechanical Thrombectomy for Intermediate-Risk Acute Pulmonary Embolism: The FLARE Study. JACC Cardiovasc. Interv. 2019, 12, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Toma, C.; Jaber, W.A.; Weinberg, M.D.; Bunte, M.C.; Khandhar, S.; Stegman, B.; Gondi, S.; Chambers, J.; Amin, R.; Leung, D.A.; et al. Acute Outcomes for the Full US Cohort of the FLASH Mechanical Thrombectomy Registry in Pulmonary Embolism. EuroIntervention J. Eur. Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2023, 18, 1201–1212. [Google Scholar] [CrossRef]
- Jaber, W.A.; Gonsalves, C.F.; Stortecky, S.; Horr, S.; Pappas, O.; Gandhi, R.T.; Pereira, K.; Giri, J.; Khandhar, S.J.; Ammar, K.A.; et al. Large-Bore Mechanical Thrombectomy Versus Catheter-Directed Thrombolysis in the Management of Intermediate-Risk Pulmonary Embolism: Primary Results of the PEERLESS Randomized Controlled Trial. Circulation 2025, 151, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Sista, A.K.; Horowitz, J.M.; Tapson, V.F.; Rosenberg, M.; Elder, M.D.; Schiro, B.J.; Dohad, S.; Amoroso, N.E.; Dexter, D.J.; Loh, C.T.; et al. Indigo Aspiration System for Treatment of Pulmonary Embolism: Results of the EXTRACT-PE Trial. JACC Cardiovasc. Interv. 2021, 14, 319–329. [Google Scholar] [CrossRef]
- Klok, F.A.; Piazza, G.; Sharp, A.S.P.; Ní Ainle, F.; Jaff, M.R.; Chauhan, N.; Patel, B.; Barco, S.; Goldhaber, S.Z.; Kucher, N.; et al. Ultrasound-Facilitated, Catheter-Directed Thrombolysis vs Anticoagulation Alone for Acute Intermediate-High-Risk Pulmonary Embolism: Rationale and Design of the HI-PEITHO Study. Am. Heart J. 2022, 251, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Kjærgaard, J.; Carlsen, J.; Sonne-Holm, E.; Wiberg, S.; Holmvang, L.; Lassen, J.F.; Sørensen, R.; Høfsten, D.; Ulriksen, P.S.; Jawad, S.; et al. A Randomized Trial of Low-Dose Thrombolysis, Ultrasound-Assisted Thrombolysis, or Heparin for Intermediate-High Risk Pulmonary Embolism-the STRATIFY Trial: Design and Statistical Analysis Plan. Trials 2024, 25, 853. [Google Scholar] [CrossRef]
- Sista, A.K.; Troxel, A.B.; Tarpey, T.; Parpia, S.; Goldhaber, S.Z.; Stringer, W.W.; Magnuson, E.A.; Cohen, D.J.; Kahn, S.R.; Rao, S.V.; et al. Rationale and Design of the PE-TRACT Trial: A Multicenter Randomized Trial to Evaluate Catheter-Directed Therapy for the Treatment of Intermediate-Risk Pulmonary Embolism. Am. Heart J. 2025, 281, 112–122. [Google Scholar] [CrossRef]
- Penumbra Inc. STRIKE-PE: A Prospective, Multicenter Study of the IndigoTM Aspiration System Seeking to Evaluate the Long-Term Safety and Outcomes of Treating Pulmonary Embolism. 2025. Available online: https://clinicaltrials.gov/ (accessed on 20 June 2025).
- Moriarty, J.M.; Dohad, S.Y.; Schiro, B.J.; Tamaddon, H.; Heithaus, R.E.; Iliadis, E.A.; Dexter, D.J.; Shavelle, D.M.; Leal, S.R.N.; Attallah, A.S.; et al. Clinical, Functional, and Quality-of-Life Outcomes after Computer Assisted Vacuum Thrombectomy for Pulmonary Embolism: Interim Analysis of the STRIKE-PE Study. J. Vasc. Interv. Radiol. 2024, 35, 1154–1165.e6. [Google Scholar] [CrossRef]
- Sanchez, O.; Charles-Nelson, A.; Ageno, W.; Barco, S.; Binder, H.; Chatellier, G.; Duerschmied, D.; Empen, K.; Ferreira, M.; Girard, P.; et al. Reduced-Dose Intravenous Thrombolysis for Acute Intermediate-High-Risk Pulmonary Embolism: Rationale and Design of the Pulmonary Embolism International THrOmbolysis (PEITHO)-3 Trial. Thromb. Haemost. 2022, 122, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhai, Z.; Liang, L.; Liu, F.; Yang, Y.; Wang, C. Lower Dosage of Recombinant Tissue-Type Plasminogen Activator (Rt-PA) in the Treatment of Acute Pulmonary Embolism: A Systematic Review and Meta-Analysis. Thromb. Res. 2014, 133, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Götzinger, F.; Lauder, L.; Sharp, A.S.P.; Lang, I.M.; Rosenkranz, S.; Konstantinides, S.; Edelman, E.R.; Böhm, M.; Jaber, W.; Mahfoud, F. Interventional Therapies for Pulmonary Embolism. Nat. Rev. Cardiol. 2023, 20, 670–684. [Google Scholar] [CrossRef]
- Pruszczyk, P.; Klok, F.; Kucher, N.; Roik, M.; Meneveau, N.; Sharp, A.S.P.; Nielsen-Kudsk, J.; Obradović, S.; Barco, S.; Giannini, F.; et al. Percutaneous Treatment Options for Acute Pulmonary Embolism: A Clinical Consensus Statement by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function and the European Association of Percutaneous Cardiovascular Interventions. EuroIntervention 2022, 18, e623–e638. [Google Scholar] [CrossRef]
- Kuo, W.T.; Banerjee, A.; Kim, P.S.; DeMarco, F.J.; Levy, J.R.; Facchini, F.R.; Unver, K.; Bertini, M.J.; Sista, A.K.; Hall, M.J.; et al. Pulmonary Embolism Response to Fragmentation, Embolectomy, and Catheter Thrombolysis (PERFECT): Initial Results from a Prospective Multicenter Registry. Chest 2015, 148, 667–673. [Google Scholar] [CrossRef]
- Rivera-Lebron, B.; McDaniel, M.; Ahrar, K.; Alrifai, A.; Dudzinski, D.M.; Fanola, C.; Blais, D.; Janicke, D.; Melamed, R.; Mohrien, K.; et al. Diagnosis, Treatment and Follow Up of Acute Pulmonary Embolism: Consensus Practice from the PERT Consortium. Clin. Appl. Thromb. Off. J. Int. Acad. Clin. Appl. Thromb. 2019, 25, 1076029619853037. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, R.; Marino, S.; Tangianu, F.; Imberti, D. Inferior Vena Cava Filters: A Clinical Review and Future Perspectives. J. Clin. Med. 2024, 13, 1761. [Google Scholar] [CrossRef] [PubMed]
- DeYoung, E.; Minocha, J. Inferior Vena Cava Filters: Guidelines, Best Practice, and Expanding Indications. Semin. Interv. Radiol. 2016, 33, 65–70. [Google Scholar] [CrossRef]
- Asmar, S.; Michael, G.; Gallo, V.; Weinberg, M.D. The Role of IVC Filters in the Management of Acute Pulmonary Embolism. J. Clin. Med. 2024, 13, 1494. [Google Scholar] [CrossRef] [PubMed]
- Ghanima, W.; Nielssen, B.E.; Holmen, L.O.; Witwit, A.; Al-Ashtari, A.; Sandset, P.M. Multidetector Computed Tomography (MDCT) in the Diagnosis of Pulmonary Embolism: Interobserver Agreement among Radiologists with Varied Levels of Experience. Acta Radiol. 2007, 48, 165–170. [Google Scholar] [CrossRef]
- Topff, L.; Ranschaert, E.R.; Bartels-Rutten, A.; Negoita, A.; Menezes, R.; Beets-Tan, R.G.H.; Visser, J.J. Artificial Intelligence Tool for Detection and Worklist Prioritization Reduces Time to Diagnosis of Incidental Pulmonary Embolism at CT. Radiol. Cardiothorac. Imaging 2023, 5, e220163. [Google Scholar] [CrossRef] [PubMed]
- Wildman-Tobriner, B.; Ngo, L.; Mammarappallil, J.G.; Konkel, B.; Johnson, J.M.; Bashir, M.R. Missed Incidental Pulmonary Embolism: Harnessing Artificial Intelligence to Assess Prevalence and Improve Quality Improvement Opportunities. J. Am. Coll. Radiol. JACR 2021, 18, 992–999. [Google Scholar] [CrossRef]
- Batra, K.; Xi, Y.; Al-Hreish, K.M.; Kay, F.U.; Browning, T.; Baker, C.; Peshock, R.M. Detection of Incidental Pulmonary Embolism on Conventional Contrast-Enhanced Chest CT: Comparison of an Artificial Intelligence Algorithm and Clinical Reports. AJR Am. J. Roentgenol. 2022, 219, 895–902. [Google Scholar] [CrossRef]
- Cheikh, A.B.; Gorincour, G.; Nivet, H.; May, J.; Seux, M.; Calame, P.; Thomson, V.; Delabrousse, E.; Crombé, A. How Artificial Intelligence Improves Radiological Interpretation in Suspected Pulmonary Embolism. Eur. Radiol. 2022, 32, 5831–5842. [Google Scholar] [CrossRef]
- Zhou, M.; Hu, Y.; Long, X.; Liu, D.; Liu, L.; Dong, C.; Wang, J.; Kong, X. Diagnostic Performance of Magnetic Resonance Imaging for Acute Pulmonary Embolism: A Systematic Review and Meta-Analysis. J. Thromb. Haemost. JTH 2015, 13, 1623–1634. [Google Scholar] [CrossRef]
- Vitarelli, A.; Barillà, F.; Capotosto, L.; D’Angeli, I.; Truscelli, G.; De Maio, M.; Ashurov, R. Right Ventricular Function in Acute Pulmonary Embolism: A Combined Assessment by Three-Dimensional and Speckle-Tracking Echocardiography. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2014, 27, 329–338. [Google Scholar] [CrossRef]
- Bova, C.; Sanchez, O.; Prandoni, P.; Lankeit, M.; Konstantinides, S.; Vanni, S.; Jiménez, D. Identification of Intermediate-Risk Patients with Acute Symptomatic Pulmonary Embolism. Eur. Respir. J. 2014, 44, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Kabrhel, C.; Rosovsky, R.; Channick, R.; Jaff, M.R.; Weinberg, I.; Sundt, T.; Dudzinski, D.M.; Rodriguez-Lopez, J.; Parry, B.A.; Harshbarger, S.; et al. A Multidisciplinary Pulmonary Embolism Response Team: Initial 30-Month Experience with a Novel Approach to Delivery of Care to Patients with Submassive and Massive Pulmonary Embolism. Chest 2016, 150, 384–393. [Google Scholar] [CrossRef]
- Wright, C.; Goldenberg, I.; Schleede, S.; McNitt, S.; Gosev, I.; Elbadawi, A.; Pietropaoli, A.; Barrus, B.; Chen, Y.L.; Mazzillo, J.; et al. Effect of a Multidisciplinary Pulmonary Embolism Response Team on Patient Mortality. Am. J. Cardiol. 2021, 161, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Gardner, T.A.; Fuher, A.; Longino, A.; Sink, E.M.; Jurica, J.; Park, B.; Lindquist, J.; Bull, T.M.; Hountras, P. Reduced Mortality Associated with Pulmonary Embolism Response Team Consultation for Intermediate and High-Risk Pulmonary Embolism: A Retrospective Cohort Study. Thromb. J. 2024, 22, 38. [Google Scholar] [CrossRef] [PubMed]

| Study | Population | Outcome |
|---|---|---|
| Aujesky et al. (2005) [19] | 367 PE patients divided into 5 risk classes according to PESI score | Low-risk patients (Class I/II) had ≤1% 30 days mortality risk while class V had 24% mortality risk |
| Chan et al. (2010) [22] | 302 PE patients assessed for 30- and 90-day mortality according to the PESI score | No mortality was observed among class I-III risk groups. 30-day and 90-day mortality among the class IV group was 9.2% and 10.5%, respectively. |
| Dentali et al. (2013) [23] | 538 PE patients assessed for long-term mortality according to the PESI score | 12-month mortality risk was <5% among class I patients and 72% among class V patients. The PESI score can be a useful tool for assessing long-term mortality among PE patients |
| Study | CT Finding | Main Findings | Comments |
|---|---|---|---|
| RV/LV RATIO > 0.9 [55] N = 13,162 Figure 2A | RV/LV ratio measured in transverse and 4-chamber view | Association with a 2.5-fold risk of all-cause mortality and a 5-fold risk of PE-related mortality | Validated in assessing RV dysfunction and mortality [56,62], |
| IVC Contrast Reflux [59] N = 365 Figure 2B | Reflux of contrast to IVC and hepatic veins (different degrees of reflux depending on vein involvement) | IVC reflux predicts 30-day mortality [59] | Most trials support its prognostic role in acute PE [59,67]. |
| Decreased left atrial size [65] N = 756 Figure 2C | Left atrium is measured in CT of the pulmonary arteries | Decreased left atrial size (<62 mL) is associated with higher clot load in pulmonary arteries and higher mortality rates [65]. | New technology is emerging evaluating left atrial dimensions and correlation with patient outcome |
| Mastora et al. (2003) [57] N = 36 | Percentage of obstructed central and peripheral pulmonary vessel. | A higher Mastora score was associated with RV dysfunction and higher pulmonary artery pressure and enables quantitative assessment of acute PE based on CT findings. | Mastora score correlation with mortality or short-term prognosis is debatable [59,60] |
| Qanadli et al. (2001) [61] N = 54 | Quantification and degree of obstructed vessel based on anatomic location | High correlation between Qanadli score and pulmonary angiography findings. Higher scores were correlated with RV dilation | Conflicting results regarding prognostic significance of the Qanadli score [60,62,63] |
| Right atrial (RA)/Right ventricle (RV) ratio (2015) [66] N = 79 | Measured in the 4-chamber view. Pathological cut-off was-1.01 | RA/RV diameter ratio was found to correlate with 30-day mortality among normotensive PE patients | More research is needed to validate this parameter. |
| Hassan et al. (2023) [68] N = 703 | Association between CT findings and persistent hypoxemia in intermediate-risk patients | Small arterial vessel fraction and PA/Aorta diameter ratio were associated with higher risk of persistent hypoxemia at discharge in intermediate-risk PE patients | Retrospective single-center study |
| (A) | ||||
| Study | Participants | Outcomes | Comments | |
| Bajaj et al. (2015) [73] | Large meta-analysis of 26 trials. | All-cause mortality was higher in the positive troponin group (10.5% vs. 3.1%). PE-related mortality was higher in the troponin-positive group (OR-3.8, CI 2.74–5.27) Serious adverse events (composite of death, need for thrombolytics, endotracheal intubation, catecholamine infusion for sustained hypotension, cardiopulmonary resuscitation, or recurrent PE) | Elevated mortality in the positive troponin group regardless of type (T/I) | |
| Daquarti et al. (2016) [74] | 40 Patients with PE Median PESI-81 | 30% of patients had RV dysfunction which was associated with higher troponin levels (33.5 ng/L vs. 16 ng/L, p = 0.03) | Troponin levels may be associated with RV dysfunction | |
| Kaeberich et al. (2015) [75] | 682 normotensive PE patients | Objective was adjusting troponin levels to age. Outcome: 30-day adverse events Age < 75—troponin cut off-14 pg/mL Age > 75—troponin cut off-45 pg/ml | Troponin is a useful biomarker predicting adverse events and may be adjusted to age. | |
| Keller et al. (2015) [76] | 129 normotensive PE patients | Troponin was associated with RV dysfunction (OR 3.95, CI 1.95–8.02, p = 0.00014) | Troponin is correlated with submassive PE. NPV-73% | |
| Hakemi et al. (2015) [77] | 298 patients with PE | Patients with a negative high sensitive troponin had better survival rates irrespective of clinical risk | Negative HS-troponin may serve as a tool for identifying low-risk patients | |
| Becattini et al. (2007) [78] | Meta-analysis of 20 trials | Elevated troponin was associated with both worse short-term mortality and adverse outcomes | Elevated troponin is associated with high mortality among hemodynamically stable patients | |
| Stein et al. (2010) [79] | 1273 hemodynamically stable PE patients | Increased troponin+ RV enlargement had higher mortality rate compared to negative troponin and normal RV (10.2% vs. 1.9%) | Positive troponin + RV enlargement are strong predictors of adverse outcome among normotensive PE patients | |
| Meyer et al. (2000) [80] | 36 PE patients | 62% patients with RV dilation had positive troponin. | Positive troponin also correlated with more segmental defects in V/Q scans | |
| Pruczczyk et al. (2003) [81] | 64 normotensive PE patients | Repetitive measurements of cardiac troponin At a 6 h interval. The positive troponin group was at high risk for a complicated course (PE- related death) | Repetitive measurements of elevated cardiac troponin are important for risk stratification in normotensive PE patients | |
| (B) | ||||
| Biomarker | Study | Participants | Outcome | Comments |
| Brain natriuretic peptide (BNP) | Lega et al. (2009) [82] | 23 studies—1127 patients | Elevated natriuretic peptide is associated with all-cause mortality (OR-6.2) and PE-related mortality (OR-5.0) | Natriuretic peptides can be used as risk stratification among PE patients |
| Wolde et al. (2003) [83] | 110 PE patients | BNP cut-off of 21.7 pmol/L predicts an NPV of 99% for an uneventful outcome | ||
| Pieralli et al. (2006) [84] | 61 normotensive PE patients | 57% had evidence of RV dysfunction. A BNP level of <85 pg/mL excluded RV dysfunction. Higher levels were associated with RV dysfunction | BNP was a powerful predictor of adverse outcomes with increased levels associated with RV dysfunction | |
| Kucher et al. (2003) [85] | 73 patients with acute PE | BNP levels of <50 pg/mL predicted a benign clinical course | Low levels of BNP may be considered to identify low-risk patients | |
| Lankeit et al. (2014) [86] | 688 normotensive PE patients | NT pro BNP cut-off levels of 600 pg/mL were associated with adverse outcomes. | Elevated levels of NT pro BNP are associated with mortality or clinical deterioration. Low levels (<600 pg/mL) are not sufficient to define low risk | |
| Heart-type fatty acid-binding protein (H-FABP) | Ruan et al. (2014) [87] | Meta-analysis of 6 trials—618 patients | Elevated H-FABP was associated with 30-day mortality (OR-40). Sensitivity and specificity for death and SAE—98% and 86%, respectively | H-FABP is a useful prognostic factor among PE patients |
| Liu et al. (2015) [88] | Meta-analysis of 6 studies, 594 patients | Elevated H-FABP was associated with elevated risk of death, cardiopulmonary resuscitation, endotracheal intubation, use of vasopressors, thrombolysis, surgical embolectomy, admission to the intensive care unit, or mortality in patients with acute PE. | H-FABP is a predictor of adverse events among PE patients | |
| Bajaj et al. (2015) [89] | Meta-analysis of 11 studies—1680 patients (9 studies with hemodynamically stable patients) | H-FABP was associated with complicated course-death, need for thrombolytics, endotracheal intubation, catecholamine infusion for sustained hypotension, CPR, or recurrent PE, as well as 30-day PE-related mortality and RV dysfunction | Prognostic sensitivity and specificity of H-FABP were 90% and 70%, respectively. In predicting 30-day mortality | |
| Dellas et al. (2010) [90] | 126 normotensive PE patients | Higher H-FABP among patients with short-term complications: 30-day mortality, need for catecholamine use and intubation, as well as long-term mortality | H-FABP can predict both short- and long-term mortality among normotensive PE patients | |
| Boscheri et al. (2010) [91] | 101 patients with intermediate-risk PE (RV dysfunction without signs of shock) | H-FABP was a predictor of mortality | Useful and novel biomarker among intermediate-risk PE | |
| Lactate | Vanni et al. (2013) [92] | 270 PE patients | Higher plasma lactate was associated with higher mortality as well as a composite of mortality, progression to shock, mechanical ventilation, and CPR. | Lactate elevation is associated with tissue hypoperfusion and can be seen prior to clinical hemodynamic instability. |
| Vanni et al. (2017) [93] | 994 normotensive PE patients | Adding plasma lactate to the clinical BOVA score identified more patients who are prone to deterioration (hemodynamic collapse and death within 7 days of diagnosis) | Plasma lactate may be used for risk stratification among normotensive PE patients | |
| D-Dimer | Becattini et al. (2012) [94] | Meta-analysis of 22 studies including both stable and unstable PE patients | Higher D-dimer levels associated with both short- (30-day) and long-term (3-month) mortality. Conflicting results regarding correlation with RV dysfunction or thrombotic burden | Prognostic value of D-dimer could not be obtained. Because of low specificity, D dimer alone cannot be used for risk stratification and decision-making algorithm among intermediate-risk PE patients |
| Copeptin | Hellenkamp et al. (2015) [95] | Prospective single-center study including 268 normotensive PE patients | Elevated copeptin levels were associated with a 5.4-fold increased risk of adverse 30-day outcome | Copeptin might be helpful for risk stratification of normotensive patients with PE, especially if integrated into a biomarker-based algorithm. |
| Hellenkamp et al. (2018) [96] | European multicenter study that validated the prognostic impact of copeptin in 843 normotensive patients with acute PE | Patients with copeptin ≥24 pmol·L had a 6.3-fold increased risk for an adverse outcome and a 7.6-fold increased risk for PE-related death | Supporting the concept that copeptin provides information on the hemodynamic impairment due to acute RV failure | |
| Thrombolytic Therapy | ||||
| Study | Participants | Objective | Outcome | Conclusion |
| Gao et al. (2015) [121] Meta-analysis of RCT | Total of 8 studies, 1755 patients Meta-analysis, RCT | Thrombolytic therapy vs. anticoagulation in intermediate-risk PE | Lower mortality in thrombolytic group (RR 0.52, 95% CI (0.28–0.97) Higher major bleeding in thrombolytic patients (RR 3.35, 95% CI, 2.03–5.54) | Intermediate-risk patient may derive benefit from thrombolytic treatment. Bleeding risk must be taken into consideration. |
| Konstantinides et al. (2002) [124] Prospective RCT | 118-heparin + alteplase. 138-heparin + placebo Prospective, RCT | Thrombolytic therapy vs. anticoagulation in submassive PE | No mortality benefit (3.4% thrombolytic vs. 2.2% heparin, p = 0.71). Less clinical deterioration in thrombolytic group (24.6% vs. 19.2%, p = 0.004) | Thrombolytic therapy can prevent clinical deterioration |
| Fasullo et al. (2011) [125] Prospective RCT | 37-thrombolysis 35-heparin Prospective, RCT | Thrombolytic effect vs. heparin on clinical and echocardiographic parameters within 180 days in submassive PE | Thrombolytic group had significant early improvement in RV function which was sustained after 180 days | Thrombolytic therapy in submassive PE improve RV function both in the short and long term |
| Sharifi et al-MOPETT trial (2013) [128] Prospective RCT | 121 PE patients with “moderate PE” involvement of 2 lobar or right/left main pulmonary arteries | Test “safe dose” thrombolytic therapy—≤50% TPA regular dose. Primary outcomes of pulmonary hypertension and composite of pulmonary hypertension and recurrent PE | Pulmonary hypertension and combined outcomes developed in 57% of control group and 16% of “safe dose” group (p < 0.001) No significant difference in mortality, recurrent PE, or bleeding | “Safe dose” thrombolysis can be considered as safe and effective at reducing pulmonary hypertension among stable PE patients |
| PEITHO (2014) [122] Prospective RCT | 506-tenecteplase 499-placebo Prospective, RCT | Thrombolytic therapy vs. heparin in intermediate-risk PE | Death or hemodynamic decompensation was lower in thrombolytic therapy compared to placebo (2.6% vs. 5.6%, OR 0.44, 95% CI, 0.23–0.87, p = 0.02). | Thrombolytic therapy prevented hemodynamic decompensation but increased the risk of major hemorrhage and stroke |
| Nakamura et al. (2014) [129] Meta-analysis of RCT | Total of 6 studies, 1510 patients. Meta-analysis. Only RCTs were included | Thrombolytic therapy vs. Heparin in submassive PE | No difference in combined outcome of mortality and recurrent PE (3.1% vs. 5.4%, RR 0.64, p = 0.2). Significant reduction in combined all-cause death and clinical deterioration (3.9% vs. 9.4%, RR 0.44, p < 0.001). No significant major bleeding in thrombolytic group. | adjuvant thrombolytic therapy prevents clinical deterioration |
| Kline et al. (2014) [120] Prospective RCT | 40-Tenecteplase 43-Placebo Prospective, RCT | Thrombolysis vs. heparin in submassive PE | Adverse outcome (death, circulatory shock, major bleeding, recurrent PE, poor functional capacity) was higher in the placebo group at 90 days (37% vs. 15%, p = 0.017) | Better outcome in thrombolytic group |
| Xu et al. (2015) [126] Meta-analysis of RCT | Total of 7 trials, 1631 patients Meta-analysis Only RCTs were included | Efficacy and safety of thrombolysis in intermediate-risk PE | Trend to reduction in all-cause mortality (OR 0.6, CI 0.34–1.06, p = 0.08), recurrent PE (OR 0.34, CI 0.15–0.77, p = −0.01), and clinical deterioration (OR 0.27, CI 0.18–0.41, p < 0.01) with higher minor bleeding in thrombolytic group. No difference in major bleeding | Thrombolysis is a viable option in intermediate-risk PE, associated with reduction in PE recurrence. Clinical deterioration without higher major bleeding |
| Chatterjee et al. (2014) [130] Meta-analysis of RCT | Total of 8 trials, 1775 patient. Meta-analysis. Only RCTs were included | Thrombolytic therapy vs. anticoagulation in intermediate-risk PE | Thrombolysis was associated with lower all-cause mortality (OR 0.53, CI 0.32–0.88. NNT = 59. Thrombolysis was associated with higher risk of major bleeding (OR 2.73, CI 1.91–3.91). NNH = 18 | Thrombolysis was associated with lower mortality and increased risk of major bleeding and intracranial hemorrhage. |
| Catheter-Directed Thrombolysis (CDT) | ||||
| TRIAL | Participants | Objective | Outcome | Conclusion |
| ULTIMA (2014) [131] Prospective RCT | 59 patients (30 treated with CDT, 29 with AC) | Ultrasound-assisted catheter-directed thrombolysis vs. anticoagulation | RV/LV ratio was significantly reduced in USA group vs. anti coagulation (0.3 ± 0.2 vs. 0.03 ± 0.16, p < 0.001) | USAT was superior to anti coagulation in reversing RV dilation at 24 h |
| CANARY (2022) [132] Prospective RCT | 94 patients Open-label, RCT Stopped prematurely d/t COVID-19 | CDT vs. anti coagulation | 3-month RV/LV ratio was significantly lower with the CDT (0.7 (0.6–0.7) vs. 0.8 (0.7–0.9), p = 0.01) CDT patients experienced lower rate if composite of death or RV/LV > 0.9 | Hypothesis generating for improvement in efficacy outcome with CDT. Trial was terminated prematurely. |
| SUNSET sPE (2021) [133] Prospective RCT | 81 patients with submassive PE. 1:1 randomization to ultrasound-assisted thrombolysis vs. standard catheter-directed thrombolysis | 1:1 randomization to ultrasound-assisted thrombolysis vs. standard catheter-directed thrombolysis | 48 hours’ thrombus burden was reduced in both groups. No significant difference was seen between the groups. | No significant difference in thrombus clearance between ultrasound-assisted and standard catheter-directed thrombolysis |
| SEATTLE II (2015) [134] Prospective single-arm | 119 patients Single-arm, multicenter | Efficacy and safety of USAT using EKOS system | Mean RV/LV diameter ratio decreased from baseline to 48 h (1.55 vs. 1.13, p < 0.001). Mean PA pressure decreased from 51.4 to 36.9, (p < 0.001) | USAT decreases RV dilation and reduces pulmonary hypertension as well as anatomic thrombus burden |
| OPTALYSE PE (2018) [135] Prospective, parallel group | 101 patients Prospective, multicenter, parallel-group | USAT vs. 1 of 4 USAT thrombolytic regimens | Improvement in RV/LV diameter ratio was seen in all subgroups | USAT with low-dose thrombolysis was associated with improved RV function and reduced clot burden |
| KNOCOUT PE (2024) [136] Prospective, single-arm | 489 patients Prospective Multicenter Single-arm | Safety and efficacy of ultrasound-facilitated catheter-directed thrombolysis | Major bleeding within 72 h in 1.6% of patients. All-cause mortality at 30 days—1.0%. QoL improvement in 41.1% | Low mortality and bleeding using catheter-directed thrombolysis |
| Pasha et al. (2022) [137] Meta-analysis | 11,932 patients 8 observational studies | Safety and efficacy of systemic and catheter-directed thrombolysis | CDT was associated with lower in-hospital mortality (RR 0.52, 95%CI (0.4–0.68)). ICH was lower in CDT group (RR 0.66, 95%CI (0.47–0.94)) | Non randomized trial suggests better efficacy and safety of CDT compared to systemic thrombolysis |
| Mechanical Thrombectomy | ||||
| TRIAL | Participants (Intermediate-Risk PE) | Objective | Outcome | Conclusion |
| FLARE (2019) [138] Prospective, single-arm | 104 patients Single-arm study | Safety and effectiveness of FlowTriever System (Inari) in intermediate–high-risk PE patients | RV/LV ratio reduction was 0.38 (25.1%, p < 0.0001). Major bleeding—1% | Mechanical thrombectomy with FlowTriever was safe and effective in intermediate–high-risk patients |
| FLASH (2023) [139] Prospective, single-arm | 799 patients in US cohort. Single-arm study. 76% of patients had intermediate–high-risk PE | Safety and effectiveness of mechanical thrombectomy using FlowTriever | 23% reduction in mean pulmonary artery pressure (p < 0.001). 63% of patients had no overnight intensive care unit stay. RV/LV ratio decreased from 1.23 ± 0.36 to 0.98 ± 0.31 (p < 0.001) | Mechanical thrombectomy with the FlowTriever system had a favorable safety profile and improvement in hemodynamic and functional outcomes |
| PEERLESS (2025) [140] Prospective, RCT | 550 patients Prospective multicenter RCT | Large bore mechanical thrombectomy (LBMT) vs. catheter-directed thrombolysis | Composite of all-cause mortality, intracranial hemorrhage, major bleeding, clinical deterioration and/or escalation to bailout and postprocedural ICY admission. Primary outcome was lower in LBMT compared with CDT (win ratio 5.91 (95%CI 3.68 = 6.97) | LBMT had lower rates of clinical deterioration and/or bailout and postprocedural ICU use compared with CDT |
| EXTRACT PE (2021) [141] Prospective, single-arm | 119 patients. Intermediate–high-risk PE | Efficacy and safety of thrombus aspiration with the Indigo aspiration system | RV-to-LV ratio decreased on average by 0.43 ± 0.26 (95% CI 0.38–0.47, p < 0.0001), PAP pressure was significantly reduced. 2 patients had major bleeding. Overall, 1.7% major adverse event rate | |
| Ongoing Trials | ||||
| Trial | Participants | Objective | Outcome | |
| HI-PEITHO (2022) [142] RCT | Approximately 406 Intermediate–high-risk PE patients | USCDT vs. anticoagulation | Primary: PE-related mortality, PE recurrence, and hemodynamic decompensation. Secondary: changes in LV/RV ratio, cardiorespiratory support, GUSTO bleeding | |
| STRATIFY (2024) [143] RCT | 210 intermediate–high-risk PE | (1) unfractionated heparin (UFH)/low-molecular-weight heparin (LMWH), (2) UFH/LMWH + 20 mg rtPA/6 h intravenously (IV), or (3) UFH + 20 mg rtPA/6 h via USAT. | Primary: Reduction in clot burden. Secondary: bleeding complications, duration of index admission, FIO2, blood pressure, respiratory and heart rate at follow-up CT, mortality, incidence of tricuspid regurgitation, mortality, QoL and 6 min walk test at 3 months | |
| PE-TRACT (2025) [144] RCT | 500 intermediate-risk PE | CDT + anticoagulation vs. anticoagulation | Primary: Peak Vo2 and NYHA class at 12 months | |
| STRIKE-PE (2025) [145] Prospective Observational | 600 Intermediate- and high-risk PE | Indigo aspiration system | Change in RV/LV ratio, device-related death, major bleeding, device-related clinical deterioration, device-related pulmonary vascular injury, and device-related cardiac injury—at 48 h after procedure. Interim analysis (150 patients) shows 25.7% reduction in RV/LV ratio, rate of combined adverse effect—2.7%, serious adverse effects—1.3%, 30-days all-cause mortality—2.0% [146] | |
| PEITHO 3 (2022) [147] | Planned 650 patients with Intermediate–High-risk PE | Reduced-dose Alteplase vs. placebo with heparin | Composite of all-cause death, hemodynamic decompensation, or objectively confirmed recurrent PE within 30 days of randomization | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Natanzon, S.S.; Mansour, M.; Fardman, A.; Chernomordik, F.; Herscovici, R.; Matetzky, S.; Beigel, R. Intermediate-Risk Pulmonary Embolism: Patients’ Stratification, Prognosis, and Therapeutic Options—Time to Pay Attention to the Middle Child. J. Clin. Med. 2025, 14, 6215. https://doi.org/10.3390/jcm14176215
Natanzon SS, Mansour M, Fardman A, Chernomordik F, Herscovici R, Matetzky S, Beigel R. Intermediate-Risk Pulmonary Embolism: Patients’ Stratification, Prognosis, and Therapeutic Options—Time to Pay Attention to the Middle Child. Journal of Clinical Medicine. 2025; 14(17):6215. https://doi.org/10.3390/jcm14176215
Chicago/Turabian StyleNatanzon, Sharon Shalom, Mahmoud Mansour, Alexander Fardman, Fernando Chernomordik, Romana Herscovici, Shlomi Matetzky, and Roy Beigel. 2025. "Intermediate-Risk Pulmonary Embolism: Patients’ Stratification, Prognosis, and Therapeutic Options—Time to Pay Attention to the Middle Child" Journal of Clinical Medicine 14, no. 17: 6215. https://doi.org/10.3390/jcm14176215
APA StyleNatanzon, S. S., Mansour, M., Fardman, A., Chernomordik, F., Herscovici, R., Matetzky, S., & Beigel, R. (2025). Intermediate-Risk Pulmonary Embolism: Patients’ Stratification, Prognosis, and Therapeutic Options—Time to Pay Attention to the Middle Child. Journal of Clinical Medicine, 14(17), 6215. https://doi.org/10.3390/jcm14176215





