Promising Preventive Strategies for Intraventricular Hemorrhage in Preterm Neonates: A Critical Review
Abstract
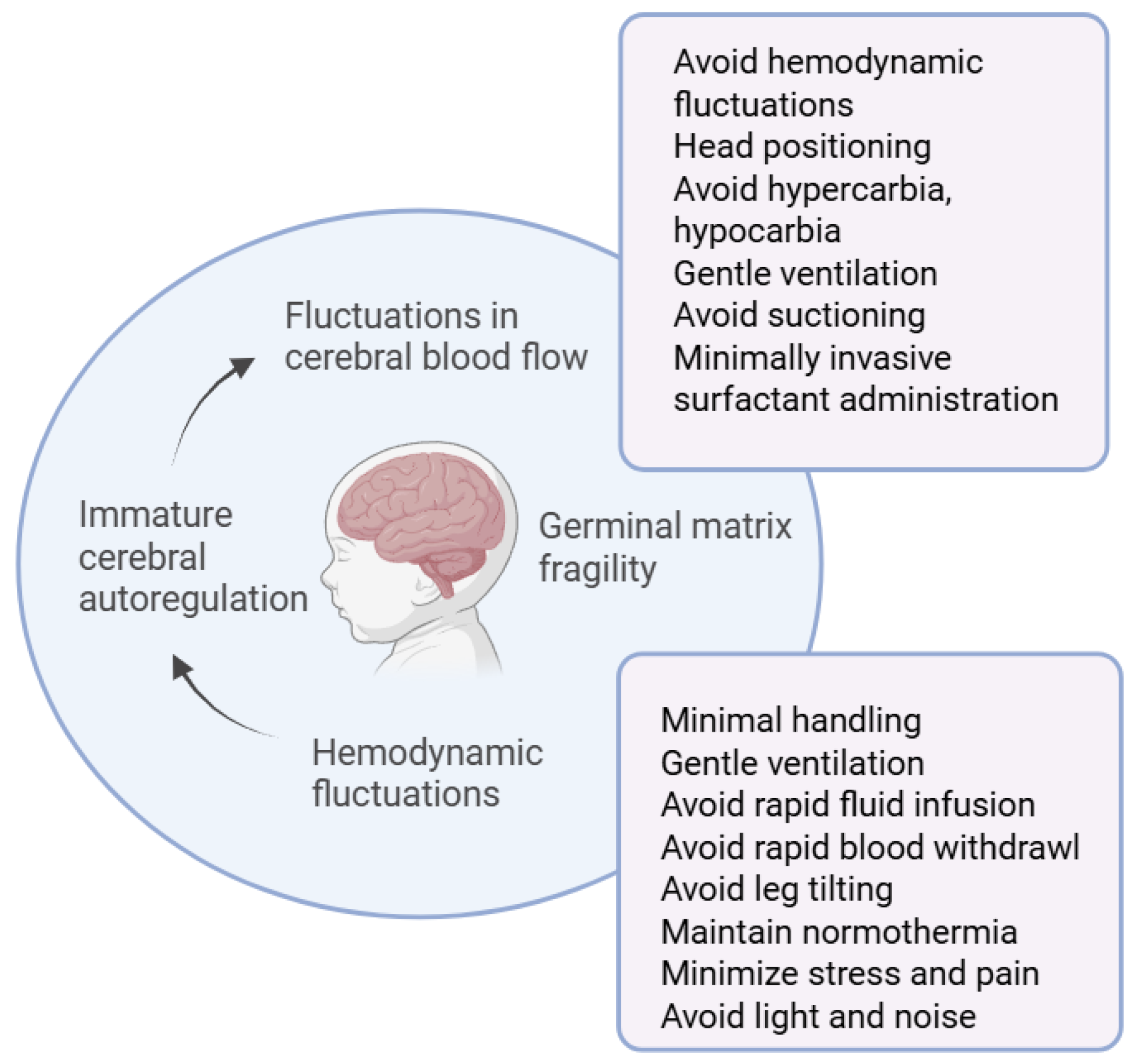
1. Introduction
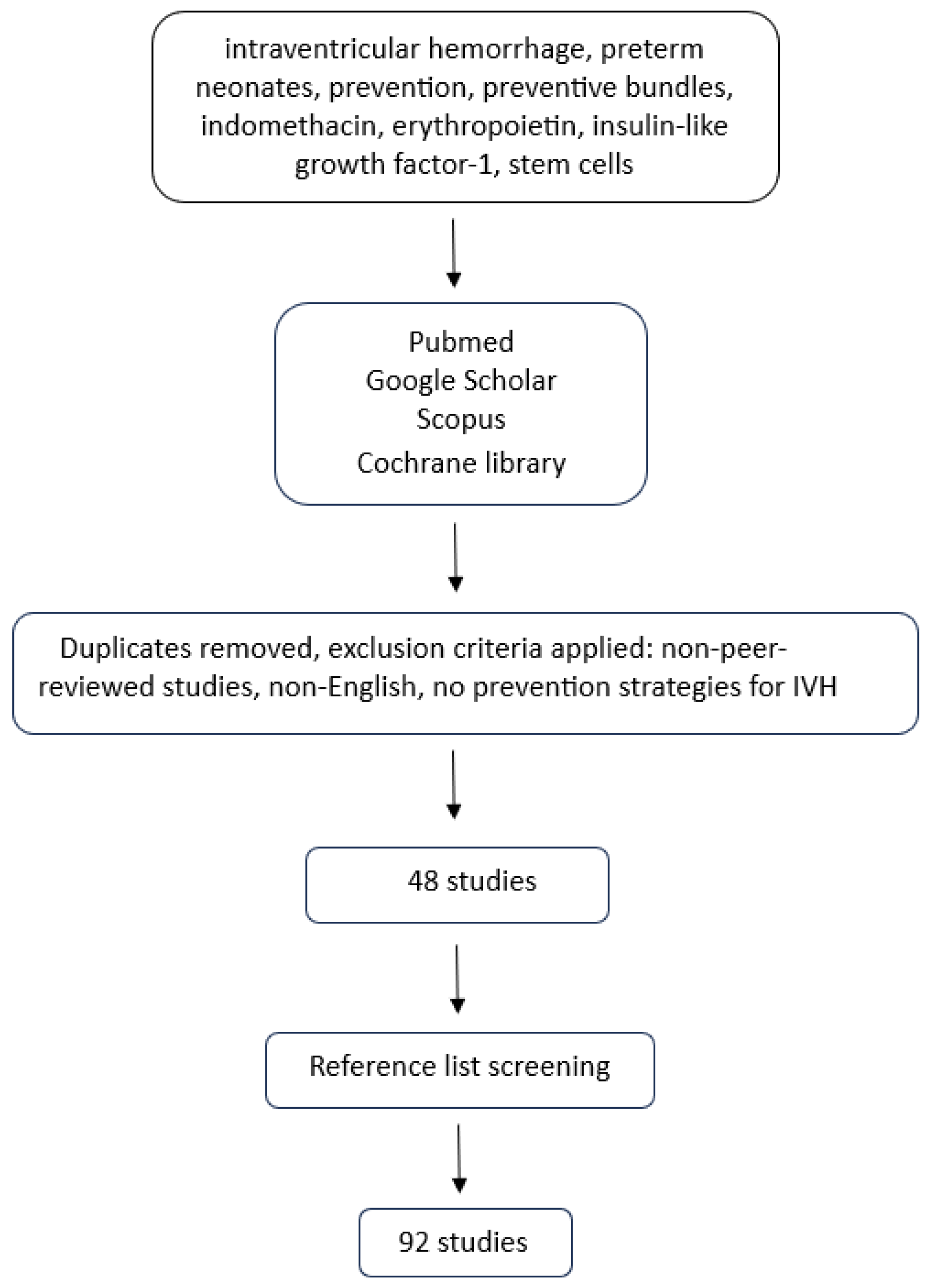
2. Methods
3. Results
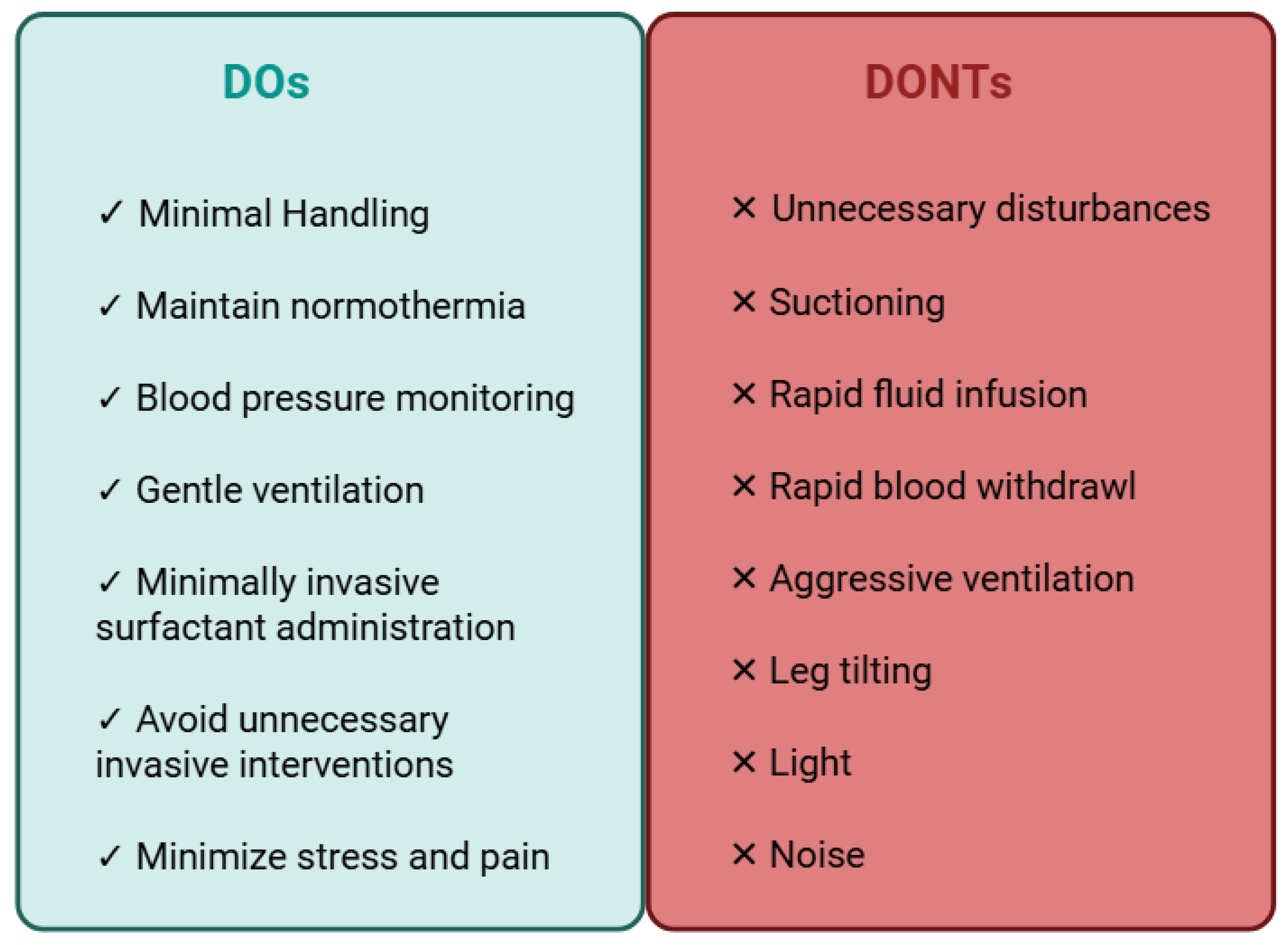
3.1. IVH Prevention Bundles (IVHPB)
| Author | Study Design | Population | Groups | Intervention | Outcome Measures | Main Findings of Interest | Limitations |
|---|---|---|---|---|---|---|---|
| de Bijl-Marcus, 2020 [43] | Multicenter cohort | 561 neonates, GA < 30 weeks | 281 intervention group 280 pre-intervention | Positioning (tilted MHP) Avoidance of rapid fluid infusion or blood withdrawal Avoidance of leg elevation | IVH rate cPVL in-hospital mortality | IVH any grade, cPVL and/or mortality: OR 0.42, 95% CI 0.27–0.65 sIVH, cPVL, and/or mortality: OR 0.54, 95% CI 0.33–0.91 | Not randomized The recruitment period was different in each center. The effectiveness of each intervention could not be assessed. Potential variation in reporting of cUS and determining the grade of the IVH |
| Persad, 2021 [46] | Single-center retrospective cohort | 404 neonates, GA < 30 weeks | 215 intervention group 189 pre-intervention | Optimization of antenatal care Positioning (tilted MHP) Minimal handling | IVH rate | sIVH: 9.8% vs. 6.9%, p = 0.37 | The effectiveness of each intervention could not be assessed. No monitoring of adherence Potential variation in reporting of cUS and determining the grade of the IVH. |
| Gross, 2021 [45] | Single-center retrospective cohort | 229 neonates, GA < 30 weeks or BW< 1250 g | 107 intervention group 122 pre-intervention | Positioning (tilted MHP) Minimal handling | IVH rate | IVH any grade: OR 1.02; 95% CI 0.57–1.84 sIVH: OR 1.0; 95% CI 0.67–1.55 | Single-center, small sample Not randomized CUS on the fourth day |
| Kolnik, 2023 [32] | Single-center quality-improvement | 425 neonates, GA < 30 weeks or BW< 1250 g | 185 intervention group 240 pre-intervention | Improvement of adherence to IVHPB (positioning, minimal handling, thermoregulation) | IVH rate | IVH any grade: OR 0.30; 95% CI 0.10–0.90 | Single-center No monitoring of adherence Severity of illness bias (reduced adherence in critically ill) |
| Tang, 2025 [33] | Single-center quality-improvement | 86 neonates, GA < 26 weeks | 21 intervention group 65 pre-intervention | Improvement of adherence to IVHPB | IVH rate | sIVH: 18.8% vs. 39.2%, p = 0.14 | Single-center, small sample No monitoring of adherence |
| Peltola, 2025 [34] | Single-center quality-improvement | 122 neonates, GA < 30 weeks | 78 intervention group 44 pre-intervention | Positioning (tilted MHP) Minimal handling | IVH rate | IVH any grade: 9.3% vs. 24.4% | Limited intervention period |
3.2. Head Position
3.3. Minimally Invasive Surfactant Administration
3.4. Indomethacin
| Author | Type of Study | Population | Study Groups | Intervention | Objective | Outcome of Interest | NNT | Main Conclusions | Limitations |
|---|---|---|---|---|---|---|---|---|---|
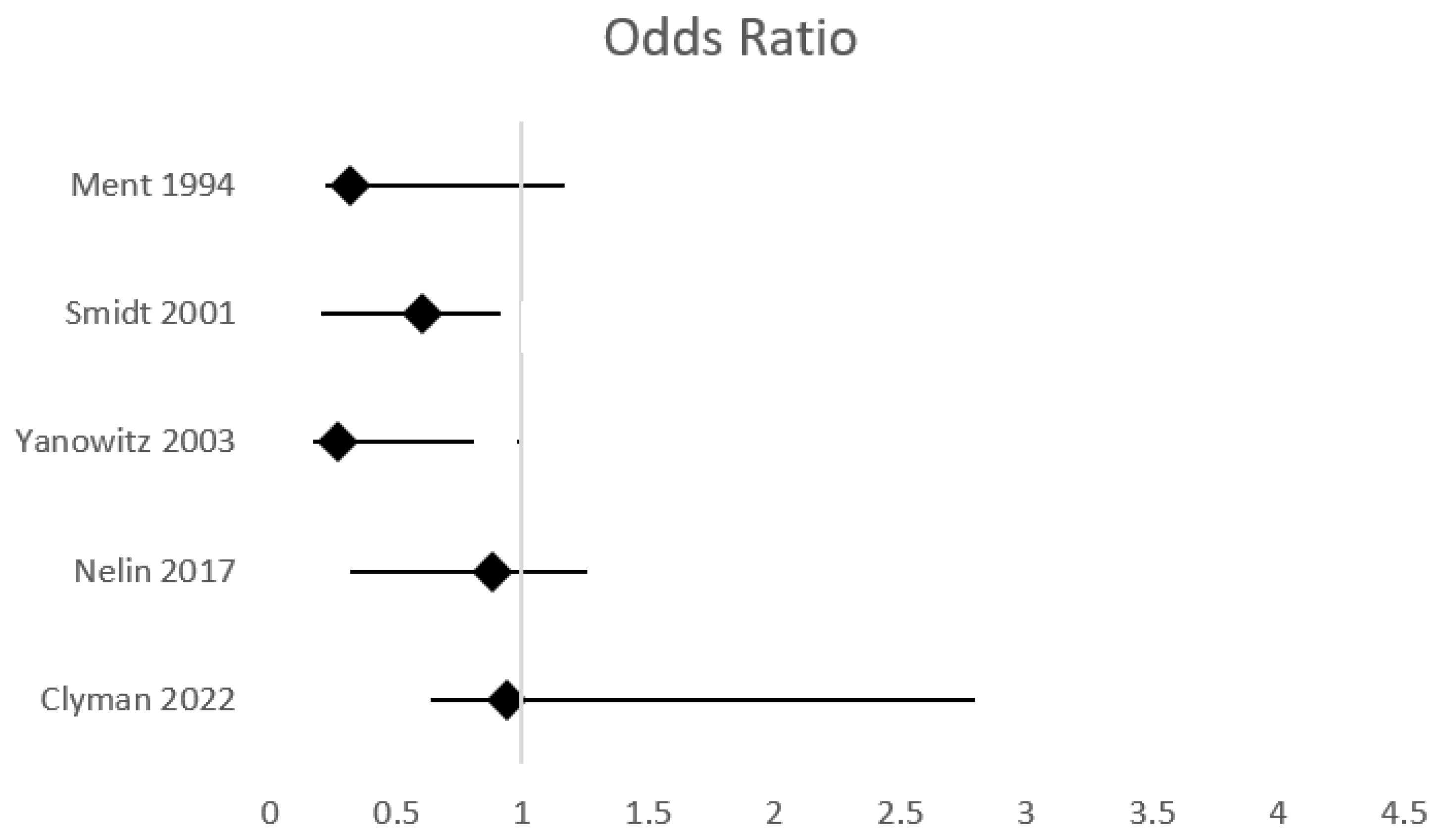
| Ment, 1994 [78] | RCT | 431 neonates, BW 600–1250 g | 209 IP-222 placebo | 0.1 mg/kg at 6–12 h, followed by 0.1 mg/kg/day for 2 days | Incidence of IVH | sIVH: RR 0.32, 95% CI 0.099–1.1 | IVH any grade: 25 sIVH: 25 | IP was associated with reduced rate of IVH and particularly grade IV IVH | Relatively small sample size |
| Smidt, 2001 [77] | RCT | 1202 neonates, BW 500–999 g | 601 IP-601 placebo | 0.1 mg/kg/day for 3 days | Mortality or NDI at CA 18 months Incidence of IVH and other preterm morbidities | IVH any grade: OR 1.0, 95% CI 0.8–1.3 sIVH: OR 0.6, 95% CI 0.4–0.9 | 25 | IP reduced the rate of sIVH and PDA IP did not improve survival without neurosensory impairment at 18 months | Neonates with preexisting IVH were not excluded |
| Yanowitz, 2003 [83] | Retrospective cohort | 260 neonates, GA < 29 weeks, BW < 1350 g | 102 IP-158 evaluated for PDA at 26 h (117 received indomethacin) | 0.1 mg/kg/day at <24 h for 3 days (IP) 0.2 mg/kg at 36 h followed by 2 doses, every 12 h 0.1–0.2 mg/kg (PDA) | Ιncidence of sIVH in patients receiving IP versus indomethacin for confirmed PDA. | sIVH: OR 0.27, 95% CI 0.10–0.77 | 12.5 | Reduced incidence of sIVH with IP compared to early echocardiographic strategy | Retrospective, non-randomized Single-center Lower GA in neonates in the IP group Small sample size |
| Nelin, 2017 [86] | Retrospective cohort | 671 outborn neonates, GA < 28 weeks | 530 IP-141 control | ND | The effect of IP on mortality and preterm morbidities | IVH any grade: 55% vs. 53%, p = 0.63 sIVH: 21% vs. 23%, p = 0.64 | IVH any grade: 50 sIVH: 50 | IP was not associated with lower IVH rates IP was associated with improved survival rates | Retrospective, non-randomized Only neonates transferred to a level IV NICU Prolonged recruitment IP protocols differed in different centers |
| Gillam-Krakauer, 2021 [82] | Retrospective cohort | 384 neonates, GA < 29 weeks | 299 IP-85 control | 0.2 mg/kg at 12 h (single dose) | The effect of IP on IVH, PDA, and motor function | IVH: OR 0.58, 95% CI 0.36–0.94 | IVH any grade: 14.3 sIVH: 50 | Decreased IVH rates with IP, in the gestation-adjusted model IP was associated with decreased mortality No increased risk of acute kidney injury | Retrospective, non-randomized Significantly lower GA in the IP group More neonates in the control group were outborn Adherence to the protocol was not mandatory |
| Clyman, 2022 [87] | Intention-to-treat, cohort-controlled | 106 neonates, GA < 25 weeks | 68 IP-38 controls | 0.2 mg/kg at <24 h, followed by 2–4 doses 0.1 mg/kg | Mortality, incidence of IVH and other preterm morbidities | sIVH: OR 0.94, 95% CI 0.30–2.92 | 12.5 | IP was not associated with a significant reduction in IVH or other prematurity-related morbidities IP was associated with a lower risk of PDA associated morbidities | Single-center, small sample Retrospective, non-randomized Prolonged recruitment |
| Hanke, 2023 [80] | Observational multicenter cohort | 1767 neonates, GA < 26 weeks with AIS | 195 IP-1572 controls | 0.1 mg/kg/day for up to 3 days | Incidence of IVH | IVH any grade: OR 0.47, 95% CI 0.27–0.79 sIVH: OR 0.24, 95% CI: 0.09–0.61 | IVH any grade: 23.8 sIVH: 15.9 | Significantly reduced IVH rates in preterm neonates with amniotic infection syndrome | Non-randomized Clinical diagnosis of AIS Selection bias Potential cofounders |
3.5. Erythropoietin (EPO)
| Author | Type of Study | Population | Study Groups | Intervention | Objectives | Outcome of Interest | Main Conclusions | Limitations |
|---|---|---|---|---|---|---|---|---|
| Ohls, 2014 [122] | RCT | 99 neonates, BW 500–1250 g | 33 rhEPO-33 darbepoetin-33 placebo | 400 U/kg rhEPO sc three times per week until 35 weeks PMA | Preterm morbidities Neurodevelopmental outcome at 18–22 months | sIVH (EPO vs. control): 9.4% vs. 23% | No statistically significant difference in the rate of sIVH and other prematurity complications. Fewer transfusions and exposure to fewer donors. | Small sample size Short follow-up period |
| Fauchere, 2015 [120] | RCT | 443 neonates, GA 26–32 weeks | 229 rhEPO-214 placebo | 3000 U/kg iv rhEPO 3 doses (<3, 12–18, and 36–42 h) | Neonatal morbidities Neurodevelopmental outcome at 24 months | IVH any grade: OR 1.0, 95% CI 0.6–1.6 | No adverse effects. No significant differences in prematurity complications. | Short follow-up period |
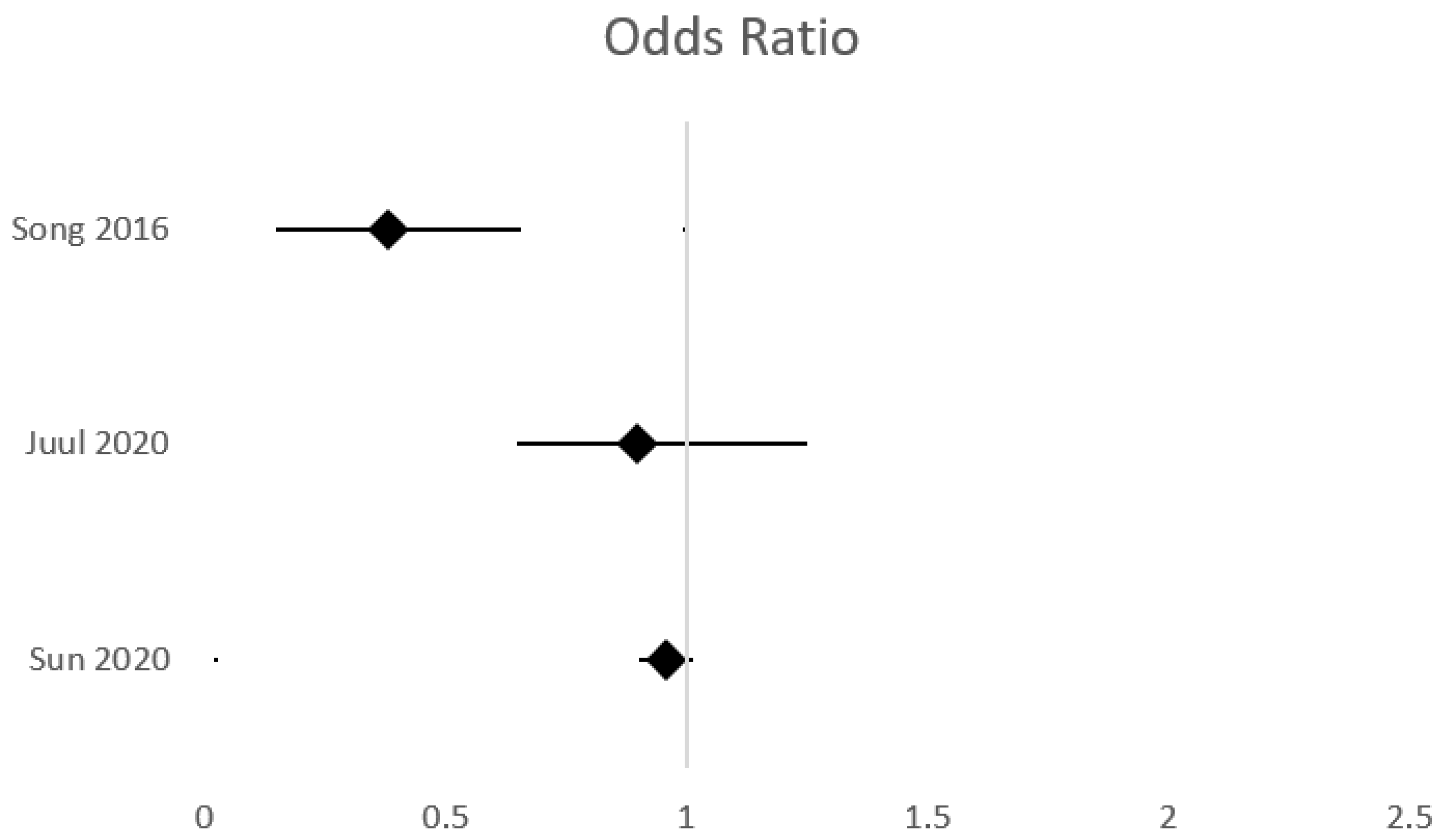
| Song, 2016 [124] | RCT | 743 neonates, GA < 32 weeks | 336 rhEPO-377 placebo | 500 U/kg iv rhEPO, initial dose <72 h, every 48 h for 2 weeks | Mortality/neurodevelopmental outcome at 18 months | sIVH: OR 0.38, 95% CI 0.23–0.62 | Significantly reduced incidence of sIVH. Better neurodevelopmental outcome. | Limited number of neonates with GA < 28 weeks and BW < 1000 g More males Short follow-up period |
| Peltoniemi, 2017 [126] | RCT | 39 neonates, BW 700–1500 g, GA < 30 weeks | 21 rhEPO-18 placebo | 250 U/kg/day iv rhEPO for 6 days | Effect of rhEPO administration without iron supplementation in neonatal morbidities and 2 year outcome | IVH any grade: OR 0.83, 95% CI 0.15–4.75 | No benefit on IVH incidence or neurodevelopmental outcome at 2 years. No significant difference in other prematurity complications. | Short follow-up period |
| Juul, 2020 [119] | RCT | 941 neonates, GA 24–28 weeks | 376 rhEPO, 365 placebo | 1000 U/kg iv every 48 h for 6 doses, followed by 400 U/kg sc three times per week until 32 weeks PMA | Neonatal morbidities Neurodevelopmental outcome at 24 months | sIVH: OR 0.90, 95% CI 0.25–1.26 | No benefit on neurodevelopmental outcome at 2 years. No significant difference in the rate of prematurity complications. | Short follow-up period |
| Sun, 2020 [125] | RCTs reanalysis | 1898 neonates, GA 24–32 weeks | 950 rhEPO, 948 placebo | 500 U/kg iv rhEPO, initial dose <72 h, every 48 h for 2 weeks | Effect on ROP and other neonatal morbidities | sIVH: 0.96, 95%CI 0.94–0.98 | Significantly lower rates of IVH, NEC and mortality. No significant impact on the incidence of ROP | Limited number of neonates with GA < 28 weeks and BW < 1000 g More males Participants from studies with different objectives |
| Fernandez, 2025 [115] | Pilot study | 40 neonates, GA < 32 weeks | 33 rhEPO, 7 placebo | 400 U/kg iv three times per week until 32 weeks PMA | Incidence of IVH | IVH any grade: 3 days: 6.5% vs. 71.4%, 10 days: 6% vs. 28.6% | Significantly reduced incidence of IVH. | Small sample study Limited generalized ability |
3.6. Insulin-like Growth Factor-1 (IGF-1)
3.7. Stem Cells
3.8. Hemostatic and Anticoagulant Agents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Care Practices, Morbidity and Mortality of Preterm Neonates in China, 2013–2014: A Retrospective study. Sci. Rep. 2019, 9, 19863. [CrossRef]
- Nagy, Z.; Obeidat, M.; Máté, V.; Nagy, R.; Szántó, E.; Veres, D.S.; Kói, T.; Hegyi, P.; Major, G.S.; Garami, M.; et al. Occurrence and Time of Onset of Intraventricular Hemorrhage in Preterm Neonates: A Systematic Review and Meta-Analysis of Individual Patient Data. JAMA Pediatr. 2025, 179, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Siffel, C.; Kistler, K.D.; Sarda, S.P. Global incidence of intraventricular hemorrhage among extremely preterm infants: A systematic literature review. J. Perinat. Med. 2021, 49, 1017–1026. [Google Scholar] [CrossRef]
- Al-Abdi, S.Y.; Al-Aamri, M.A. A Systematic Review and Meta-analysis of the Timing of Early Intraventricular Hemorrhage in Preterm Neonates: Clinical and Research Implications. J. Clin. Neonatol. 2014, 3, 76–88. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, W.; Tian, X. Analysis of risk factors of early intraventricular hemorrhage in very-low-birth-weight premature infants: A single center retrospective study. BMC Pregnancy Childbirth 2022, 1, 890. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yeo, K.T.; Thomas, R.; Chow, S.S.; Bolisetty, S.; Haslam, R.; Tarnow-Mordi, W.; Lui, K.; Australian and New Zealand Neonatal Network. Improving incidence trends of severe intraventricular haemorrhages in preterm infants <32 weeks gestation: A cohort study. Arch. Dis. Child. Fetal Neonatal Ed. 2020, 105, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Handley, S.C.; Passarella, M.; Lee, H.C.; Lorch, S.A. Incidence Trends and Risk Factor Variation in Severe Intraventricular Hemorrhage across a Population Based Cohort. J. Pediatr. 2018, 200, 24–29.e3. [Google Scholar] [CrossRef]
- Razak, A.; Johnston, E.; Stewart, A.; Clark, M.A.T.; Stevens, P.; Charlton, M.; Wong, F.; McDonald, C.; Hunt, R.W.; Miller, S.; et al. Temporal Trends in Severe Brain Injury and Associated Outcomes in Very Preterm Infants. Neonatology 2024, 121, 440–449. [Google Scholar] [CrossRef]
- Özek, E.; Kersin, S.G. Intraventricular hemorrhage in preterm babies. Turk. Pediatri Ars. 2020, 55, 215–221. [Google Scholar] [CrossRef]
- Egesa, W.I.; Odoch, S.; Odong, R.J.; Nakalema, G.; Asiimwe, D.; Ekuk, E.; Twesigemukama, S.; Turyasiima, M.; Lokengama, R.K.; Waibi, W.M.; et al. Germinal Matrix-Intraventricular Hemorrhage: A Tale of Preterm Infants. Int. J. Pediatr. 2021, 2021, 6622598. [Google Scholar] [CrossRef] [PubMed]
- Tsao, P.C. Pathogenesis and Prevention of Intraventricular Hemorrhage in Preterm Infants. J. Korean Neurosurg. Soc. 2023, 66, 228–238. [Google Scholar] [CrossRef]
- Papile, L.A.; Burstein, J.; Burstein, R.; Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1500 gm. J. Pediatr. 1978, 92, 529–534. [Google Scholar] [CrossRef]
- Kuban, K.; Teele, R.L. Rationale for grading intracranial hemorrhage in premature infants. Pediatrics 1984, 74, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wang, S.; Zhang, T.; Duan, S.; Wang, H. Neurodevelopmental outcomes in preterm or low birth weight infants with germinal matrix-intraventricular hemorrhage: A meta-analysis. Pediatr. Res. 2024, 95, 625–633. [Google Scholar] [CrossRef]
- Wang, Y.; Song, J.; Zhang, X.; Kang, W.; Li, W.; Yue, Y.; Zhang, S.; Xu, F.; Wang, X.; Zhu, C. The Impact of Different Degrees of Intraventricular Hemorrhage on Mortality and Neurological Outcomes in Very Preterm Infants: A Prospective Cohort Study. Front. Neurol. 2022, 13, 853417. [Google Scholar] [CrossRef]
- Rees, P.; Callan, C.; Chadda, K.R.; Vaal, M.; Diviney, J.; Sabti, S.; Harnden, F.; Gardiner, J.; Battersby, C.; Gale, C.; et al. Preterm Brain Injury and Neurodevelopmental Outcomes: A Meta-analysis. Pediatrics 2022, 150, e2022057442. [Google Scholar] [CrossRef]
- Payne, A.H.; Hintz, S.R.; Hibbs, A.M.; Walsh, M.C.; Vohr, B.R.; Bann, C.M.; Wilson-Costello, D.E.; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neurodevelopmental outcomes of extremely low-gestational-age neonates with low-grade periventricular-intraventricular hemorrhage. JAMA Pediatr. 2013, 167, 451–459. [Google Scholar] [CrossRef]
- Ann Wy, P.; Rettiganti, M.; Li, J.; Yap, V.; Barrett, K.; Whiteside-Mansell, L.; Casey, P. Impact of intraventricular hemorrhage on cognitive and behavioral outcomes at 18 years of age in low birth weight preterm infants. J. Perinatol. 2015, 35, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Reubsaet, P.; Brouwer, A.J.; van Haastert, I.C.; Brouwer, M.J.; Koopman, C.; Groenendaal, F.; de Vries, L.S. The Impact of Low-Grade Germinal Matrix-Intraventricular Hemorrhage on Neurodevelopmental Outcome of Very Preterm Infants. Neonatology 2017, 112, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Argyropoulou, M.I.; Xydis, V.G.; Drougia, A.; Giantsouli, A.S.; Giapros, V.; Astrakas, L.G. Structural and functional brain connectivity in moderate-late preterm infants with low-grade intraventricular hemorrhage. Neuroradiology 2022, 64, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Argyropoulou, M.I.; Astrakas, L.G.; Xydis, V.G.; Drougia, A.; Mouka, V.; Goel, I.; Giapros, V.; Andronikou, S. Is Low-Grade Intraventricular Hemorrhage in Very Preterm Infants an Innocent Condition? Structural and Functional Evaluation of the Brain Reveals Regional Neurodevelopmental Abnormalities. AJNR Am. J. Neuroradiol. 2020, 41, 542–547. [Google Scholar] [CrossRef]
- Périsset, A.; Natalucci, G.; Adams, M.; Karen, T.; Bassler, D.; Hagmann, C. Impact of low-grade intraventricular hemorrhage on neurodevelopmental outcome in very preterm infants at two years of age. Early Hum. Dev. 2023, 177–178, 105721. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.C.; Catalano, R.; Profit, J.; Gould, J.B.; Lee, H.C. Impact of antenatal steroids on intraventricular hemorrhage in very-low-birth weight infants. J. Perinatol. 2016, 36, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Fortmann, I.; Mertens, L.; Boeckel, H.; Grüttner, B.; Humberg, A.; Astiz, M.; Roll, C.; Rickleffs, I.; Rody, A.; Härtel, C.; et al. A Timely Administration of Antenatal Steroids Is Highly Protective Against Intraventricular Hemorrhage: An Observational Multicenter Cohort Study of Very Low Birth Weight Infants. Front. Pediatr. 2022, 10, 721355. [Google Scholar] [CrossRef] [PubMed]
- Ayed, M.; Ahmed, J.; More, K.; Ayed, A.; Husain, H.; AlQurashi, A.; Alrajaan, N. Antenatal Magnesium Sulfate for Preterm Neuroprotection: A Single-Center Experience from Kuwait Tertiary NICU. Biomed. Hub 2022, 7, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Bansal, V.; Desai, A. Efficacy of Antenatal Magnesium Sulfate for Neuroprotection in Extreme Prematurity: A Comparative Observational Study. J. Obstet. Gynaecol. India 2022, 72 (Suppl. 1), 36–47. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric Care consensus No. 6: Periviable Birth. Obstet. Gynecol. 2017, 130, e187–e199. [Google Scholar] [CrossRef]
- Odd, D.; Reeve, N.F.; Barnett, J.; Cutter, J.; Daniel, R.; Gale, C.; Siasakos, D. PRECIOUS study (PREterm Caesarean/vaginal birth and IVH/OUtcomeS): Does mode of birth reduce the risk of death or brain injury in very preterm babies? A cohort and emulated target trial protocol. BMJ Open 2024, 14, e089722. [Google Scholar] [CrossRef]
- Hemmati, F.; Sharma, D.; Namavar Jahromi, B.; Salarian, L.; Farahbakhsh, N. Delayed cord clamping for prevention of intraventricular hemorrhage in preterm neonates: A randomized control trial. J. Matern. Fetal Neonatal Med. 2022, 35, 3633–3639. [Google Scholar] [CrossRef]
- Ikeda, T.; Ito, Y.; Mikami, R.; Matsuo, K.; Kawamura, N.; Yamoto, A.; Ito, E. Fluctuations in internal cerebral vein and central side veins of preterm infants. Pediatr. Int. 2021, 63, 1319–1326. [Google Scholar] [CrossRef]
- Helwich, E.; Rutkowska, M.; Bokiniec, R.; Gulczyńska, E.; Hożejowski, R. Intraventricular hemorrhage in premature infants with Respiratory Distress Syndrome treated with surfactant: Incidence and risk factors in the prospective cohort study. Dev. Period. Med. 2017, 21, 328–335. [Google Scholar]
- Kolnik, S.E.; Upadhyay, K.; Wood, T.R.; Juul, S.E.; Valentine, G.C. Reducing Severe Intraventricular Hemorrhage in Preterm Infants With Improved Care Bundle Adherence. Pediatrics 2023, 152, e2021056104. [Google Scholar] [CrossRef]
- Tang, I.; Huntingford, S.; Zhou, L.; Fox, C.; Miller, T.; Krishnamurthy, M.B.; Wong, F.Y. Reducing severe intraventricular haemorrhage rates in <26-week preterm infants with bedside assessment and care bundle implementation. Acta Paediatr. 2025, 114, 1179–1188. [Google Scholar] [CrossRef]
- Peltola, S.D.; Akpan, U.S.; Tumin, D.; Huffman, P. Quality improvement initiative to decrease severe intraventricular hemorrhage rates in preterm infants by implementation of a care bundle. J. Perinatol. 2025, 45, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Limperopoulos, C.; Gauvreau, K.K.; O’Leary, H.; Moore, M.; Bassan, H.; Eichenwald, E.C.; Soul, J.S.; Ringer, S.A.; Di Salvo, D.N.; du Plessis, A.J. Cerebral hemodynamic changes during intensive care of preterm infants. Pediatrics 2008, 5, e1006–e1013. [Google Scholar] [CrossRef] [PubMed]
- Cabral, L.A.; Velloso, M. Comparing the effects of minimal handling protocols on the physiological parameters of preterm infants receiving exogenous surfactant therapy. Braz. J. Phys. Ther. 2014, 18, 152–164. [Google Scholar] [CrossRef] [PubMed]
- de Bijl-Marcus, K.A.; Brouwer, A.J.; de Vries, L.S.; van Wezel-Meijler, G. The Effect of Head Positioning and Head Tilting on the Incidence of Intraventricular Hemorrhage in Very Preterm Infants: A Systematic Review. Neonatology 2017, 111, 267–279. [Google Scholar] [CrossRef]
- Chen, Y.T.; Wu, H.P.; Lan, H.Y.; Peng, H.F.; Chen, S.J.; Yin, T.; Liaw, J.J.; Chang, Y.C. Effects of intubation and hypoxemia on intraventricular hemorrhage in preterm infants during the first week: An observational study. Heart Lung 2025, 69, 78–86. [Google Scholar] [CrossRef]
- Elser, H.E.; Holditch-Davis, D.; Levy, J.; Brandon, D.H. The effects of environmental noise and infant position on cerebral oxygenation. Adv. Neonatal Care 2012, 12 (Suppl. 5), S18–S27. [Google Scholar] [CrossRef]
- Karen, T.; Kleiser, S.; Ostojic, D.; Isler, H.; Guglielmini, S.; Bassler, D.; Wolf, M.; Scholkmann, F. Cerebral hemodynamic responses in preterm-born neonates to visual stimulation: Classification according to subgroups and analysis of frontotemporal-occipital functional connectivity. Neurophotonics 2019, 6, 045005. [Google Scholar] [CrossRef]
- Roychoudhury, S.; Yusuf, Κ. Thermoregulation: Advances in Preterm Infants. Neoreviews 2017, 18, e692–e702. [Google Scholar] [CrossRef]
- Brophy, H.; Tan, G.M.; Yoxall, C.W. Very Low Birth Weight Outcomes and Admission Temperature: Does Hyperthermia Matter? Children 2022, 9, 1706. [Google Scholar] [CrossRef]
- de Bijl-Marcus, K.; Brouwer, A.J.; De Vries, L.S.; Groenendaal, F.; Wezel-Meijler, G.V. Neonatal care bundles are associated with a reduction in the incidence of intraventricular haemorrhage in preterm infants: A multicentre cohort study. Arch. Dis. Child. Fetal Neonatal Ed. 2020, 105, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.E.; Sampson, L.; Dunn, M.; Rolnitsky, A.; Ng, E. Sustained Reduction in Severe Intraventricular Hemorrhage in Micropremature Infants: A Quality Improvement Intervention. Children 2025, 12, 264. [Google Scholar] [CrossRef] [PubMed]
- Gross, M.; Engel, C.; Trotter, A. Evaluating the Effect of a Neonatal Care Bundle for the Prevention of Intraventricular Hemorrhage in Preterm Infants. Children 2021, 8, 257. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Persad, N.; Kelly, E.; Amaral, N.; Neish, A.; Cheng, C.; Fan, C.S.; Runeckles, K.; Shah, V. Impact of a “Brain Protection Bundle” in Reducing Severe Intraventricular Hemorrhage in Preterm Infants <30 Weeks GA: A Retrospective Single Centre Study. Children 2021, 8, 983. [Google Scholar] [CrossRef]
- Edwards, E.M.; Ehret, D.E.Y.; Cohen, H.; Zayack, D.; Soll, R.F.; Horbar, J.D. Quality Improvement Interventions to Prevent Intraventricular Hemorrhage: A Systematic Review. Pediatrics 2024, 154, e2023064431. [Google Scholar] [CrossRef] [PubMed]
- Goyen, T.A.; Jani, P.R.; Skelton, H.; Pussell, K.; Manley, B.; Tarnow-Mordi, W.; Positioning the Preterm Infant for Neuroprotection (PIN) Trial Investigator Collaborative Group. Does Midline Head Positioning Decrease Intraventricular Hemorrhage or Is It Futile? Without a Definitive Trial, We Will Never Know. World J. Pediatr. 2025, 21, 533–536. [Google Scholar] [CrossRef]
- Emery, J.R.; Peabody, J.L. Head Position Affects Intracranial Pressure in Newborn Infants. J. Pediatr. 1983, 103, 950–953. [Google Scholar] [CrossRef]
- Cowan, F.; Thoresen, M. Changes in Superior Sagittal Sinus Blood Velocities Due to Postural Alterations and Pressure on the Head of the Newborn Infant. Pediatrics 1985, 75, 1038–1047. [Google Scholar] [CrossRef]
- Goldberg, R.N.; Joshi, A.; Moscoso, P.; Castillo, T. The Effect of Head Position on Intracranial Pressure in the Neonate. Crit. Care Med. 1983, 11, 428–430. [Google Scholar] [CrossRef]
- Pichler, G.; van Boetzelar, M.C.; Müller, W.; Urlesberger, B. Effect of Tilting on Cerebral Hemodynamics in Preterm and Term Infants. Biol. Neonate. 2001, 80, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.M.; Rao, R.; Mathur, A.M. Head Position Change Is Not Associated with Acute Changes in Bilateral Cerebral Oxygenation in Stable Preterm Infants during the First 3 Days of Life. Am. J. Perinatol. 2015, 32, 645–652. [Google Scholar] [CrossRef]
- Ancora, G.; Maranella, E.; Aceti, A.; Pierantoni, L.; Grandi, S.; Corvaglia, L.; Faldella, G. Effect of Posture on Brain Hemodynamics in Preterm Newborns Not Mechanically Ventilated. Neonatology 2010, 97, 212–217. [Google Scholar] [CrossRef]
- Pellicer, A.; Gayá, F.; Madero, R.; Quero, J.; Cabañas, F. Noninvasive Continuous Monitoring of the Effects of Head Position on Brain Hemodynamics in Ventilated Infants. Pediatrics 2002, 109, 434–440. [Google Scholar] [CrossRef]
- Spengler, D.; Loewe, E.; Krause, M.F. Supine vs. Prone Position with Turn of the Head Does Not Affect Cerebral Perfusion and Oxygenation in Stable Preterm Infants ≤32 Weeks Gestational Age. Front. Physiol. 2018, 9, 1664. [Google Scholar] [CrossRef]
- Al-Abdi, S.Y.; Nojoom, M.S.; Alshaalan, H.M.; Al-Aamri, M.A. Pilot-Randomized Study on Intraventricular Hemorrhage with Midline versus Lateral Head Positions. Saudi Med. J. 2011, 32, 420–421. [Google Scholar]
- Al-Abdi, S.; Alallah, J.; Al Omran, A.; Al Alwan, Q.; Al Hashimi, H.; Haidar, S. The Risk of Intraventricular Hemorrhage with Flat Midline versus Flat Right Lateral Head Positions: A Prematurely Terminated Multicenter Randomized Clinical Trial. In The Pediatric Academic Societies (PAS); Pediatric Research: San Diego, CA, USA, 2015. [Google Scholar]
- Kochan, M.; Leonardi, B.; Firestine, A.; McPadden, J.; Cobb, D.; Shah, T.A.; Vazifedan, T.; Bass, W.T. Elevated Midline Head Positioning of Extremely Low Birth Weight Infants: Effects on Cardiopulmonary Function and the Incidence of Periventricular-Intraventricular Hemorrhage. J. Perinatol. 2019, 39, 54–62. [Google Scholar] [CrossRef]
- Kumar, P.; Carroll, K.F.; Prazad, P.; Raghavan, A.; Waruingi, W.; Wang, H. Elevated Supine Midline Head Position for Prevention of Intraventricular Hemorrhage in VLBW and ELBW Infants: A Retrospective Multicenter Study. J. Perinatol. 2021, 41, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Romantsik, O.; Calevo, M.G.; Bruschettini, M. Head Midline Position for Preventing the Occurrence or Extension of Germinal Matrix-Intraventricular Haemorrhage in Preterm Infants. Cochrane Database Syst. Rev. 2020, 7, CD012362. [Google Scholar] [CrossRef] [PubMed]
- Tana, M.; Tirone, C.; Aurilia, C.; Lio, A.; Paladini, A.; Fattore, S.; Esposito, A.; De Tomaso, D.; Vento, G. Respiratory Management of the Preterm Infant: Supporting Evidence-Based Practice at the Bedside. Children 2023, 10, 535. [Google Scholar] [CrossRef]
- Sweet, D.G.; Carnielli, V.P.; Greisen, G.; Hallman, M.; Klebermass-Schrehof, K.; Ozek, E.; Te Pas, A.; Plavka, R.; Roehr, C.C.; Saugstad, O.D.; et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome: 2022 Update. Neonatology 2023, 120, 3–23. [Google Scholar] [CrossRef]
- Kurepa, D.; Perveen, S.; Lipener, Y.; Kakkilaya, V. The use of less invasive surfactant administration (LISA) in the United States with review of the literature. J. Perinatol. 2019, 39, 426–432. [Google Scholar] [CrossRef]
- Sayed, M.K.M.; Hassanien, F.E.; Khalaf, M.S.; Ali Badawy, A. MIST or INSURE in Preterm Infants with Respiratory Distress Syndrome. J. Child Sci. 2024, 14, e66–e74. [Google Scholar] [CrossRef]
- Langhammer, K.; Roth, B.; Kribs, A.; Göpel, W.; Kuntz, L.; Miedaner, F. Treatment and outcome data of very low birth weight infants treated with less invasive surfactant administration in comparison to intubation and mechanical ventilation in the clinical setting of a cross-sectional observational multicenter study. Eur. J. Pediatr. 2018, 177, 1207–1217. [Google Scholar] [CrossRef]
- Pérez-Iranzo, A.; Jarque, A.; Toledo, J.D.; Tosca, R. Less invasive surfactant administration reduces incidence of severe intraventricular haemorrage in preterms with respiratory distress syndrome: A cohort study. J. Perinatol. 2020, 8, 1185–1192. [Google Scholar] [CrossRef]
- Kribs, A.; Roll, C.; Göpel, W.; Wieg, C.; Groneck, P.; Laux, R.; Teig, N.; Hoehn, T.; Böhm, W.; Welzing, L.; et al. Nonintubated Surfactant Application vs Conventional Therapy in Extremely Preterm Infants: A Randomized Clinical Trial. JAMA Pediatr. 2015, 169, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Latif, M.E.; Davis, P.G.; Wheeler, K.I.; De Paoli, A.G.; Dargaville, P.A. Surfactant therapy via thin catheter in preterm infants with or at risk of respiratory distress syndrome. Cochrane Database Syst. Rev. 2021, 5, CD011672. [Google Scholar] [CrossRef] [PubMed]
- Aldana-Aguirre, J.C.; Pinto, M.; Featherstone, R.M.; Kumar, M. Less invasive surfactant administration versus intubation for surfactant delivery in preterm infants with respiratory distress syndrome: A systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2017, 102, F17–F23. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.H.T.; Sekulich, D.C.; Scott, A.; Nolte, W.M.; Gibson, K.; Su, R.; Alrifai, M.W.; Lopata, S.M.; Lewis, T.; Reese, J.; et al. The Relationship of Indomethacin Exposure with Efficacy and Renal Toxicity Outcomes for Preterm Infants in the Neonatal Intensive Care Unit. Clin. Transl. Sci. 2025, 18, e70251. [Google Scholar] [CrossRef]
- Stark, A.; Smith, P.B.; Hornik, C.P.; Zimmerman, K.O.; Hornik, C.D.; Pradeep, S.; Clark, R.H.; Benjamin, D.K., Jr.; Laughon, M.; Greenberg, R.G. Medication Use in the Neonatal Intensive Care Unit and Changes from 2010 to 2018. J. Pediatr. 2022, 240, 66–71.e4. [Google Scholar] [CrossRef]
- Leffler, C.W.; Mirro, R.; Shibata, M.; Parfenova, H.; Armstead, W.M.; Zuckerman, S. Effects of Indomethacin on Cerebral Vasodilator Responses to Arachidonic Acid and Hypercapnia in Newborn Pigs. Pediatr. Res. 1993, 33, 609–614. [Google Scholar] [CrossRef][Green Version]
- Coyle, M.G.; Oh, W.; Petersson, K.H.; Stonestreet, B.S. Effects of Indomethacin on Brain Blood Flow, Cerebral Metabolism, and Sagittal Sinus Prostanoids after Hypoxia. Am. J. Physiol. 1995, 269, H1450–H1459. [Google Scholar] [CrossRef] [PubMed]
- McCalden, T.A.; Nath, R.G.; Thiele, K. The role of prostacyclin in the hypercapnic and hypoxic cerebrovascular dilations. Life Sci. 1984, 34, 1801–1807. [Google Scholar] [CrossRef]
- Ment, L.R.; Stewart, W.B.; Ardito, T.A.; Huang, E.; Madri, J.A. Indomethacin promotes germinal matrix microvessel maturation in the newborn beagle pup. Stroke 1992, 23, 1132–1137. [Google Scholar] [CrossRef]
- Smidt, B.; Davis, P.; Moddemann, D.; Ohlsson, A.; Roberts, R.S.; Saigal, S.; Solimano, A.; Vincer, M.; Wright, L.L.; Trial of Indomethacin Prophylaxis in Preterms Investigators. Long-term effects of indomethacin prophylaxis in extremely-low-birth-weight infants. N. Engl. J. Med. 2001, 344, 1966–1972. [Google Scholar] [CrossRef]
- Ment, L.R.; Oh, W.; Ehrenkranz, R.A.; Philip, A.G.; Vohr, B.; Allan, W.; Duncan, C.C.; Scott, D.T.; Taylor, K.J.; Katz, K.H.; et al. Low-dose indomethacin and prevention of intraventricular hemorrhage: A multicenter randomized trial. Pediatrics 1994, 93, 543–550. [Google Scholar] [CrossRef]
- Bandstra, E.S.; Montalvo, B.M.; Goldberg, R.N.; Pacheco, I.; Ferrer, P.L.; Flynn, J.; Gregorios, J.B.; Bancalari, E. Prophylactic indomethacin for prevention of intraventricular hemorrhage in premature infants. Pediatrics 1988, 82, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Hanke, K.; Fortmann, I.; Humberg, A.; Faust, K.; Kribs, A.; Prager, S.; Felderhoff-Müser, U.; Krüger, M.; Heckmann, M.; Jäger, A.; et al. Indomethacin Prophylaxis Is Associated with Reduced Risk of Intraventricular Hemorrhage in Extremely Preterm Infants Born in the Context of Amniotic Infection Syndrome. Neonatology 2023, 120, 334–343. [Google Scholar] [CrossRef]
- Luque, M.J.; Tapia, J.L.; Villarroel, L.; Marshall, G.; Musante, G.; Carlo, W.; Kattan, J.; Neocosur Neonatal Network. A risk prediction model for severe intraventricular hemorrhage in very low birth weight infants and the effect of prophylactic indomethacin. J. Perinatol. 2014, 34, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Gillam-Krakauer, M.; Slaughter, J.C.; Cotton, R.B.; Robinson, B.E.; Reese, J.; Maitre, N.L. Outcomes in infants <29 weeks of gestation following single-dose prophylactic indomethacin. J. Perinatol. 2021, 41, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Yanowitz, T.D.; Baker, R.W.; Sobchak Brozanski, B. Prophylactic indomethacin reduces grades III and IV intraventricular hemorrhages when compared to early indomethacin treatment of a patent ductus arteriosus. J. Perinatol. 2003, 23, 317–322. [Google Scholar] [CrossRef]
- Razak, A.; Patel, W.; Durrani, N.U.R.; Pullattayil, A.K. Interventions to Reduce Severe Brain Injury Risk in Preterm Neonates: A Systematic Review and Meta-analysis. JAMA Netw. Open 2023, 6, e237473. [Google Scholar] [CrossRef]
- Mitra, S.; Gardner, C.E.; MacLellan, A.; Disher, T.; Styranko, D.M.; Campbell-Yeo, M.; Kuhle, S.; Johnston, B.C.; Dorling, J. Prophylactic cyclo-oxygenase inhibitor drugs for the prevention of morbidity and mortality in preterm infants: A network meta-analysis. Cochrane Database Syst. Rev. 2022, 4, CD013846. [Google Scholar] [CrossRef]
- Nelin, T.D.; Pena, E.; Giacomazzi, T.; Lee, S.; Logan, J.W.; Moallem, M.; Bapat, R.; Shepherd, E.G.; Nelin, L.D. Outcomes following indomethacin prophylaxis in extremely preterm infants in an all-referral NICU. J. Perinatol. 2017, 37, 932–937. [Google Scholar] [CrossRef]
- Clyman, R.I.; Hills, N.K. Effects of prophylactic indomethacin on morbidity and mortality in infants <25 weeks’ gestation: A protocol driven intention to treat analysis. J. Perinatol. 2022, 42, 1662–1668. [Google Scholar] [CrossRef]
- Szakmar, E.; Harrison, S.; Elshibiny, H.; Munster, C.; El-Dib, M. Effect of implementing a clinical practice guideline for prophylactic indomethacin on reduction of severe IVH in extremely preterm infants. J. Neonatal-Perinat. Med. 2025, 18, 449–455. [Google Scholar] [CrossRef]
- Al-Matary, A.; Abu Shaheen, A.; Abozaid, S. Use of Prophylactic Indomethacin in Preterm Infants: A Systematic Review and Meta-Analysis. Front. Pediatr. 2022, 10, 760029. [Google Scholar] [CrossRef]
- Singh, R.; Gorstein, S.V.; Bednarek, F.; Chou, J.H.; McGowan, E.C.; Visintainer, P.F. A predictive model for SIVH risk in preterm infants and targeted indomethacin therapy for prevention. Sci. Rep. 2013, 3, 2539. [Google Scholar] [CrossRef] [PubMed]
- Lea, C.L.; Smith-Collins, A.; Luyt, K. Protecting the premature brain: Current evidence-based strategies for minimising perinatal brain injury in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2017, 102, F176–F182. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.; Natarajan, G.; Laptook, A.R.; Chowdhury, D.; Bell, E.F.; Ambalavanan, N.; Carlo, W.A.; Gantz, M.; Das, A.; Tapia, J.L.; et al. Model for severe intracranial hemorrhage and role of early indomethacin in extreme preterm infants. Pediatr. Res. 2022, 92, 1648–1656. [Google Scholar] [CrossRef]
- Foglia, E.E.; Roberts, R.S.; Stoller, J.Z.; Davis, P.G.; Haslam, R.; Schmidt, B.; Trial of Indomethacin Prophylaxis in Preterms Investigators. Effect of Prophylactic Indomethacin in Extremely Low Birth Weight Infants Based on the Predicted Risk of Severe Intraventricular Hemorrhage. Neonatology 2018, 113, 183–186. [Google Scholar] [CrossRef]
- Bhat, R.; Zayek, M.; Maertens, P.; Eyal, F. A single-dose indomethacin prophylaxis for reducing perinatal brain injury in extremely low birth weight infants: A non-inferiority analysis. J. Perinatol. 2019, 39, 1462–1471. [Google Scholar] [CrossRef]
- Mirza, H.; Oh, W.; Laptook, A.; Vohr, B.; Tucker, R.; Stonestreet, B.S. Indomethacin prophylaxis to prevent intraventricular hemorrhage: Association between incidence and timing of drug administration. J. Pediatr. 2013, 163, 706–710.e1. [Google Scholar] [CrossRef]
- Mirza, H.; Laptook, A.R.; Oh, W.; Vohr, B.R.; Stoll, B.J.; Kandefer, S.; Stonestreet, B.S.; Generic Database Subcommittee of the NICHD Neonatal Research Network. Effects of indomethacin prophylaxis timing on intraventricular haemorrhage and patent ductus arteriosus in extremely low birth weight infants. Arch. Dis. Child. Fetal Neonatal Ed. 2016, 101, F418–F422. [Google Scholar] [CrossRef]
- Ment, L.R.; Vohr, B.; Allan, W.; Westerveld, M.; Sparrow, S.S.; Schneider, K.C.; Katz, K.H.; Duncan, C.C.; Makuch, R.W. Outcome of children in the indomethacin intraventricular hemorrhage prevention trial. Pediatrics 2000, 105, 485–491. [Google Scholar] [CrossRef]
- Ment, L.R.; Vohr, B.; Oh, W.; Scott, D.T.; Allan, W.C.; Westerveld, M.; Duncan, C.C.; Ehrenkranz, R.A.; Katz, K.H.; Schneider, K.C.; et al. Neurodevelopmental outcome at 36 months’ corrected age of preterm infants in the Multicenter Indomethacin Intraventricular Hemorrhage Prevention Trial. Pediatrics 1996, 98, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Fowlie, P.W.; Davis, P.G.; McGuire, W. Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants. Cochrane Database Syst. Rev. 2010, 7, CD000174. [Google Scholar] [CrossRef]
- Sangem, M.; Asthana, S.; Amin, S. Multiple courses of indomethacin and neonatal outcomes in premature infants. Pediatr. Cardiol. 2008, 29, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Couser, R.J.; Hoekstra, R.E.; Ferrara, T.B.; Wright, G.B.; Cabalka, A.K.; Connett, J.E. Neurodevelopmental follow-up at 36 months’ corrected age of preterm infants treated with prophylactic indomethacin. Arch. Pediatr. Adolesc. Med. 2000, 154, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Stavel, M.; Wong, J.; Cieslak, Z.; Sherlock, R.; Claveau, M.; Shah, P.S. Effect of prophylactic indomethacin administration and early feeding on spontaneous intestinal perforation in extremely low-birth-weight infants. J. Perinatol. 2017, 37, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Attridge, J.T.; Clark, R.; Walker, M.W.; Gordon, P.V. New insights into spontaneous intestinal perforation using a national data set: (2) two populations of patients with perforations. J. Perinatol. 2006, 26, 185–188. [Google Scholar] [CrossRef]
- Sharma, R.; Hudak, M.L.; Tepas, J.J., 3rd; Wludyka, P.S.; Teng, R.J.; Hastings, L.K.; Renfro, W.H.; Marvin, W.J., Jr. Prenatal or postnatal indomethacin exposure and neonatal gut injury associated with isolated intestinal perforation and necrotizing enterocolitis. J. Perinatol. 2010, 30, 786–793. [Google Scholar] [CrossRef]
- Kelleher, J.; Salas, A.A.; Bhat, R.; Ambalavanan, N.; Saha, S.; Stoll, B.J.; Bell, E.F.; Walsh, M.C.; Laptook, A.R.; Sánchez, P.J.; et al. Prophylactic indomethacin and intestinal perforation in extremely low birth weight infants. Pediatrics 2014, 134, e1369–e1377. [Google Scholar] [CrossRef]
- Maruyama, K.; Fujiu, T. Effects of prophylactic indomethacin on renal and intestinal blood flows in premature infants. Pediatr. Int. 2012, 54, 480–485. [Google Scholar] [CrossRef]
- Akima, S.; Kent, A.; Reynolds, G.J.; Gallagher, M.; Falk, M.C. Indomethacin and renal impairment in neonates. Pediatr. Nephrol. 2004, 19, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Abdi, H.H.; Backes, C.H.; Ball, M.K.; Talavera-Barber, M.M.; Klebanoff, M.A.; Jadcherla, S.R.; Mohamed, T.H.; Slaughter, J.L. Prophylactic Indomethacin in extremely preterm infants: Association with death or BPD and observed early serum creatinine levels. J. Perinatol. 2021, 4, 749–755. [Google Scholar] [CrossRef]
- Shaffer, C.L.; Gal, P.; Ransom, J.L.; Carlos, R.Q.; Smith, M.S.; Davey, A.M.; Dimaguila, M.A.; Brown, Y.L.; Schall, S.A. Effect of age and birth weight on indomethacin pharmacodynamics in neonates treated for patent ductus arteriosus. Crit. Care Med. 2002, 30, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Kramer, K.P.; Minot, K.; Butler, C.; Haynes, K.; Mason, A.; Nguyen, L.; Wynn, S.; Liebowitz, M.; Rogers, E.E. Reduction of Severe Intraventricular Hemorrhage in Preterm Infants: A Quality Improvement Project. Pediatrics 2022, 149, e2021050652. [Google Scholar] [CrossRef]
- Wood, T.R.; Juul, S.E. Taking Stock After Another Negative Erythropoietin Neuroprotection Trial. JAMA Netw. Open 2022, 5, e2247054. [Google Scholar] [CrossRef] [PubMed]
- Rangarajan, V.; Juul, S.E. Erythropoietin: Emerging Role of Erythropoietin in Neonatal Neuroprotection. Pediatr. Neurol. 2014, 51, 481–488. [Google Scholar] [CrossRef]
- Kimáková, P.; Solár, P.; Solárová, Z.; Komel, R.; Debeljak, N. Erythropoietin and Its Angiogenic Activity. Int. J. Mol. Sci. 2017, 18, 1519. [Google Scholar] [CrossRef] [PubMed]
- Juul, S.E.; Pet, G.C. Erythropoietin and Neonatal Neuroprotection. Clin. Perinatol. 2015, 42, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, D.A.A.; Diaz, H.A.R.; Garnica, A.D.F.; Iturri-Soliz, P.; Arias-Reyes, C.; Schneider Gasser, E.M.; Soliz, J. Low and Sustained Doses of Erythropoietin Prevent Preterm Infants from Intraventricular Hemorrhage. Respir. Physiol. Neurobiol. 2025, 331, 104363. [Google Scholar] [CrossRef]
- Juul, S.E.; McPherson, R.J.; Farrell, F.X.; Jolliffe, L.; Ness, D.J.; Gleason, C.A. Erytropoietin Concentrations in Cerebrospinal Fluid of Nonhuman Primates and Fetal Sheep Following High-Dose Recombinant Erythropoietin. Biol. Neonate 2004, 85, 138–144. [Google Scholar] [CrossRef]
- Demers, E.J.; McPherson, R.J.; Juul, S.E. Erythropoietin Protects Dopaminergic Neurons and Improves Neurobehavioral Outcomes in Juvenile Rats after Neonatal Hypoxia-Ischemia. Pediatr. Res. 2005, 58, 297–301. [Google Scholar] [CrossRef]
- Kellert, B.A.; McPherson, R.J.; Juul, S.E. A Comparison of High-Dose Recombinant Erythropoietin Treatment Regimens in Brain-Injured Neonatal Rats. Pediatr. Res. 2007, 61, 451–455. [Google Scholar] [CrossRef]
- Juul, S.E.; Comstock, B.A.; Wadhawan, R.; Mayock, D.E.; Courtney, S.E.; Robinson, T.; Ahmad, K.A.; Bendel-Stenzel, E.; Baserga, M.; LaGamma, E.F.; et al. A Randomized Trial of Erythropoietin for Neuroprotection in Preterm Infants. N. Engl. J. Med. 2020, 382, 233–243. [Google Scholar] [CrossRef]
- Fauchere, J.C.; Koller, B.M.; Tschopp, A.; Dame, C.; Ruegger, C.; Bucher, H.U.; Swiss Erythropoietin Neuroprotection Trial Group. Safety of Early High-Dose Recombinant Erythropoietin for Neuroprotection in Very Preterm Infants. J. Pediatr. 2015, 167, e1–e3. [Google Scholar] [CrossRef]
- Wellmann, S.; Hagmann, C.F.; von Felten, S.; Held, L.; Klebermass-Schrehof, K.; Truttmann, A.C.; Knöpfli, C.; Fauchère, J.C.; Bührer, C.; Bucher, H.U.; et al. Safety and Short-term Outcomes of High-Dose Erythropoietin in Preterm Infants With Intraventricular Hemorrhage: The EpoRepair Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2244744. [Google Scholar] [CrossRef]
- Ohls, R.K.; Kamath-Rayne, B.D.; Christensen, R.D.; Wiedmeier, S.E.; Rosenberg, A.; Fuller, J.; Lacy, C.B.; Roohi, M.; Lambert, D.K.; Burnett, J.J.; et al. Cognitive outcomes of preterm infants randomized to darbepoetin, erythropoietin, or placebo. Pediatrics 2014, 133, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Eichorst, D.; Lala-Black, B.; Gonzalez, R. Higher cumulative doses of erythropoietin and developmental outcomes in preterm infants. Pediatrics 2009, 124, e681–e687. [Google Scholar] [CrossRef]
- Song, J.; Sun, H.; Xu, F.; Kang, W.; Gao, L.; Guo, J.; Zhang, Y.; Xia, L.; Wang, X.; Zhu, C. Recombinant human erythropoietin improves neurological outcomes in very preterm infants. Ann. Neurol. 2016, 80, 24–34. [Google Scholar] [CrossRef]
- Sun, H.; Song, J.; Kang, W.; Wang, Y.; Sun, X.; Zhou, C.; Xiong, H.; Xu, F.; Li, M.; Zhang, X.; et al. Effect of early prophylactic low-dose recombinant human erythropoietin on retinopathy of prematurity in very preterm infants. J. Transl. Med. 2020, 18, 397. [Google Scholar] [CrossRef]
- Peltoniemi, O.M.; Anttila, E.; Kaukola, T.; Buonocore, G.; Hallman, M. Randomized trial of early erythropoietin supplementation after preterm birth: Iron metabolism and outcome. Early Hum. Dev. 2017, 109, 44–49. [Google Scholar] [CrossRef]
- Ohlsson, A.; Aher, S.M. Early erythropoiesis-stimulating agents in preterm or low birth weight infants. Cochrane Database Syst. Rev. 2020, 2, CD004863. [Google Scholar] [CrossRef]
- Hellstrom, A.; Ley, D.; Hallberg, B.; Lofqvist, C.; Hansen-Pupp, I.; Ramenghi, L.A.; Borg, J.; Smith, L.E.H.; Hard, A.L. IGF-1 as a Drug for Preterm Infants: A Step-Wise Clinical Development. Curr. Pharm. Des. 2017, 23, 5964–5970. [Google Scholar] [CrossRef] [PubMed]
- Hellström, A.; Ley, D.; Hansen-Pupp, I.; Hallberg, B.; Löfqvist, C.; van Marter, L.; van Weissenbruch, M.; Ramenghi, L.A.; Beardsall, K.; Dunger, D.; et al. Insulin-like growth factor 1 has multisystem effects on foetal and preterm infant development. Acta Paediatr. 2016, 105, 576–586. [Google Scholar] [CrossRef]
- Ye, P.; D’Ercole, A.J. Insulin-like growth factor actions during development of neural stem cells and progenitors in the central nervous system. J. Neurosci. Res. 2006, 83, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Jacobo, S.M.; Kazlauskas, A. Insulin-like growth factor 1 (IGF-1) stabilizes nascent blood vessels. J. Biol. Chem. 2015, 290, 6349–6360. [Google Scholar] [CrossRef]
- Gram, M.; Ekström, C.; Holmqvist, B.; Carey, G.; Wang, X.; Vallius, S.; Hellström, W.; Ortenlöf, N.; Agyemang, A.A.; Smith, L.E.H.; et al. Insulin-Like Growth Factor 1 in the Preterm Rabbit Pup: Characterization of Cerebrovascular Maturation following Administration of Recombinant Human Insulin-Like Growth Factor 1/Insulin-Like Growth Factor 1-Binding Protein 3. Dev. Neurosci. 2021, 43, 281–295. [Google Scholar] [CrossRef]
- Löfqvist, C.; Niklasson, A.; Engström, E.; Friberg, L.E.; Camacho-Hübner, C.; Ley, D.; Borg, J.; Smith, L.E.; Hellström, A. A pharmacokinetic and dosing study of intravenous insulin-like growth factor-I and IGF-binding protein-3 complex to preterm infants. Pediatr. Res. 2009, 65, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D. Clinical review 68: The endocrine regulation of fetal growth in late gestation: The role of insulin-like growth factors. J. Clin. Endocrinol. Metab. 1995, 80, 1047–1050. [Google Scholar] [CrossRef] [PubMed]
- Hansen-Pupp, I.; Hellström-Westas, L.; Cilio, C.M.; Andersson, S.; Fellman, V.; Ley, D. Inflammation at birth and the insulin-like growth factor system in very preterm infants. Acta Paediatr. 2007, 96, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Lineham, J.D.; Smith, R.M.; Dahlenburg, G.W.; King, R.A.; Haslam, R.R.; Stuart, M.C.; Faull, L. Circulating insulin-like growth factor I levels in newborn premature and full-term infants followed longitudinally. Early Hum. Dev. 1986, 13, 37–46. [Google Scholar] [CrossRef]
- Christiansen, L.I.; Ventura, G.C.; Holmqvist, B.; Aasmul-Olsen, K.; Lindholm, S.E.H.; Lycas, M.D.; Mori, Y.; Secher, J.B.; Burrin, D.G.; Thymann, T.; et al. Insulin-like growth factor 1 supplementation supports motor coordination and affects myelination in preterm pigs. Front. Neurosci. 2023, 17, 1205819. [Google Scholar] [CrossRef]
- Ley, D.; Hallberg, B.; Hansen-Pupp, I.; Dani, C.; Ramenghi, L.A.; Marlow, N.; Beardsall, K.; Bhatti, F.; Dunger, D.; Higginson, J.D.; et al. rhIGF-1/rhIGFBP-3 in Preterm Infants: A Phase 2 Randomized Controlled Trial. J. Pediatr. 2019, 206, 56–65.e8. [Google Scholar] [CrossRef]
- Ley, D.; Hansen-Pupp, I.; Niklasson, A.; Domellöf, M.; Friberg, L.E.; Borg, J.; Löfqvist, C.; Hellgren, G.; Smith, L.E.; Hård, A.L.; et al. Longitudinal infusion of a complex of insulin-like growth factor-I and IGF-binding protein-3 in five preterm infants: Pharmacokinetics and short-term safety. Pediatr. Res. 2013, 73, 68–74. [Google Scholar] [CrossRef]
- Hansen-Pupp, I.; Hellström, A.; Hamdani, M.; Tocoian, A.; Kreher, N.C.; Ley, D.; Hallberg, B. Continuous longitudinal infusion of rhIGF-1/rhIGFBP-3 in extremely preterm infants: Evaluation of feasibility in a phase II study. Growth Horm. IGF Res. 2017, 36, 44–51. [Google Scholar] [CrossRef]
- Hansen-Pupp, I.; Engström, E.; Niklasson, A.; Berg, A.C.; Fellman, V.; Löfqvist, C.; Hellström, A.; Ley, D. Fresh-frozen plasma as a source of exogenous insulin-like growth factor-I in the extremely preterm infant. J. Clin. Endocrinol. Metab. 2009, 94, 477–482. [Google Scholar] [CrossRef]
- Chung, J.K.; Hallberg, B.; Hansen-Pupp, I.; Graham, M.A.; Fetterly, G.; Sharma, J.; Tocoian, A.; Kreher, N.C.; Barton, N.; Hellström, A.; et al. Development and verification of a pharmacokinetic model to optimize physiologic replacement of rhIGF-1/rhIGFBP-3 in preterm infants. Pediatr. Res. 2017, 81, 504–510. [Google Scholar] [CrossRef]
- Piecewicz, S.M.; Pandey, A.; Roy, B.; Xiang, S.H.; Zetter, B.R.; Sengupta, S. Insulin-like growth factors promote vasculogenesis in embryonic stem cells. PLoS ONE 2012, 7, e32191. [Google Scholar] [CrossRef]
- Horsch, S.; Parodi, A.; Hallberg, B.; Malova, M.; Björkman-Burtscher, I.M.; Hansen-Pupp, I.; Marlow, N.; Beardsall, K.; Dunger, D.; van Weissenbruch, M.; et al. Randomized Control Trial of Postnatal rhIGF-1/rhIGFBP-3 Replacement in Preterm Infants: Post-hoc Analysis of Its Effect on Brain Injury. Front. Pediatr. 2020, 8, 517207. [Google Scholar] [CrossRef]
- Yang, S.E.; Ha, C.W.; Jung, M.; Jin, H.J.; Lee, M.; Song, H.; Choi, S.; Oh, W.; Yang, Y.S. Mesenchymal stem/progenitor cells developed in cultures from UC blood. Cytotherapy 2004, 6, 476–486. [Google Scholar] [CrossRef]
- Kern, S.; Eichler, H.; Stoeve, J.; Klüter, H.; Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Batsali, A.K.; Kastrinaki, M.C.; Papadaki, H.A.; Pontikoglou, C. Mesenchymal stem cells derived from Wharton’s Jelly of the umbilical cord: Biological properties and emerging clinical applications. Curr. Stem Cell Res. Ther. 2013, 8, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; McDonald, C.; Yawno, T.; Jenkin, G.; Miller, S.; Malhotra, A. Umbilical Cord Blood and Cord Tissue-Derived Cell Therapies for Neonatal Morbidities: Current Status and Future Challenges. Stem Cells Transl. Med. 2022, 11, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.S.; Ahn, S.Y.; Yoo, H.S.; Sung, S.I.; Choi, S.J.; Oh, W.I.; Park, W.S. Mesenchymal stem cells for bronchopulmonary dysplasia: Phase 1 dose-escalation clinical trial. J. Pediatr. 2014, 164, 966–972.e6. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Chang, Y.S.; Lee, M.H.; Sung, S.I.; Lee, B.S.; Kim, K.S.; Kim, A.R.; Park, W.S. Stem cells for bronchopulmonary dysplasia in preterm infants: A randomized controlled phase II trial. Stem Cells Transl. Med. 2021, 10, 1129–1137. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Chang, Y.S.; Sung, S.I.; Park, W.S. Mesenchymal Stem Cells for Severe Intraventricular Hemorrhage in Preterm Infants: Phase I Dose-Escalation Clinical Trial. Stem Cells Transl. Med. 2018, 7, 847–856. [Google Scholar] [CrossRef]
- Burkhart, H.M.; Qureshi, M.Y.; Rossano, J.W.; Cantero Peral, S.; O’Leary, P.W.; Hathcock, M.; Kremers, W.; Nelson, T.J.; Breuer, A.; Cavanaugh, K.; et al. Autologous stem cell therapy for hypoplastic left heart syndrome: Safety and feasibility of intraoperative intramyocardial injections. J. Thorac. Cardiovasc. Surg. 2019, 6, 1614–1623. [Google Scholar] [CrossRef]
- Cotten, C.M.; Murtha, A.P.; Goldberg, R.N.; Grotegut, C.A.; Smith, P.B.; Goldstein, R.F.; Fisher, K.A.; Gustafson, K.E.; Waters-Pick, B.; Swamy, G.K.; et al. Feasibility of autologous cord blood cells for infants with hypoxic-ischemic encephalopathy. J. Pediatr. 2014, 164, 973–979.e1. [Google Scholar] [CrossRef] [PubMed]
- Cotten, C.M.; Fisher, K.; Malcolm, W.; Gustafson, K.E.; Cheatham, L.; Marion, A.; Greenberg, R.; Kurtzberg, J. A Pilot Phase I Trial of Allogeneic Umbilical Cord Tissue-Derived Mesenchymal Stromal Cells in Neonates with Hypoxic-Ischemic Encephalopathy. Stem Cells Transl. Med. 2023, 12, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, M.; Sawada, M.; Watabe, S.; Sano, H.; Kanai, M.; Tanaka, E.; Ohnishi, S.; Sato, Y.; Sobajima, H.; Hamazaki, T.; et al. Autologous Cord Blood Cell Therapy for Neonatal Hypoxic-Ischaemic Encephalopathy: A Pilot Study for Feasibility and Safety. Sci. Rep. 2020, 10, 4603. [Google Scholar] [CrossRef]
- Yang, J.; Ren, Z.; Zhang, C.; Rao, Y.; Zhong, J.; Wang, Z.; Liu, Z.; Wei, W.; Lu, L.; Wen, J.; et al. Safety of Autologous Cord Blood Cells for Preterms: A Descriptive Study. Stem Cells Int. 2018, 2018, 5268057. [Google Scholar] [CrossRef]
- Zhou, L.; McDonald, C.A.; Yawno, T.; Razak, A.; Connelly, K.; Novak, I.; Miller, S.L.; Jenkin, G.; Malhotra, A. Feasibility and safety of autologous cord blood derived cell administration in extremely preterm infants: A single-centre, open-label, single-arm, phase I trial (CORD-SaFe study). EBioMedicine 2025, 111, 105492. [Google Scholar] [CrossRef]
- Segler, A.; Braun, T.; Fischer, H.S.; Dukatz, R.; Weiss, C.R.; Schwickert, A.; Jäger, C.; Bührer, C.; Henrich, W. Feasibility of Umbilical Cord Blood Collection in Neonates at Risk of Brain Damage—A Step toward Autologous Cell Therapy for a High-Risk Population. Cell Transplant. 2021, 30, 963689721992065. [Google Scholar] [CrossRef]
- Nguyen, T.; Purcell, E.; Smith, M.J.; Penny, T.R.; Paton, M.C.B.; Zhou, L.; Jenkin, G.; Miller, S.L.; McDonald, C.A.; Malhotra, A. Umbilical Cord Blood-Derived Cell Therapy for Perinatal Brain Injury: A Systematic Review & Meta-Analysis of Preclinical Studies. Int. J. Mol. Sci. 2023, 24, 4351. [Google Scholar] [CrossRef]
- Vaes, J.E.G.; Kosmeijer, C.M.; Kaal, M.; van Vliet, R.; Brandt, M.J.V.; Benders, M.J.N.L.; Nijboer, C.H. Regenerative Therapies to Restore Interneuron Disturbances in Experimental Models of Encephalopathy of Prematurity. Int. J. Mol. Sci. 2020, 22, 211. [Google Scholar] [CrossRef]
- Vaes, J.E.G.; van Kammen, C.M.; Trayford, C.; van der Toorn, A.; Ruhwedel, T.; Benders, M.J.N.L.; Dijkhuizen, R.M.; Möbius, W.; van Rijt, S.H.; Nijboer, C.H. Intranasal mesenchymal stem cell therapy to boost myelination after encephalopathy of prematurity. Glia 2021, 69, 655–680. [Google Scholar] [CrossRef]
- Romantsik, O.; Moreira, A.; Thébaud, B.; Ådén, U.; Ley, D.; Bruschettini, M. Stem cell-based interventions for the prevention and treatment of intraventricular haemorrhage and encephalopathy of prematurity in preterm infants. Cochrane Database Syst. Rev. 2023, 2, CD013201. [Google Scholar] [CrossRef]
- Kotowski, M.; Litwinska, Z.; Klos, P.; Pius-Sadowska, E.; Zagrodnik-Ulan, E.; Ustianowski, P.; Rudnicki, J.; Machalinski, B. Autologous cord blood transfusion in preterm infants—Could its humoral effect be the key to control prematurity-related complications? A preliminary study. J. Physiol. Pharmacol. 2017, 68, 921–927. [Google Scholar] [PubMed]
- Ren, Z.; Xu, F.; Zhang, X.; Zhang, C.; Miao, J.; Xia, X.; Kang, M.; Wei, W.; Ma, T.; Zhang, Q.; et al. Autologous cord blood cell infusion in preterm neonates safely reduces respiratory support duration and potentially preterm complications. Stem Cells Transl. Med. 2020, 9, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Hoban, R.; Perez, K.M.; Hendrixson, D.T.; Valentine, G.C.; Strobel, K.M. Non-Nutritional Use of Human Milk as a Therapeutic Agent in Neonates: Brain, Gut, and Immunologic Targets. Early Hum. Dev. 2024, 198, 106126. [Google Scholar] [CrossRef]
- Hoban, R.; Gallipoli, A.; Signorile, M.; Mander, P.; Gauthier-Fisher, A.; Librach, C.; Wilson, D.; Unger, S. Feasibility of Intranasal Human Milk as Stem Cell Therapy in Preterm Infants with Intraventricular Hemorrhage. J. Perinatol. 2024, 44, 1652–1657. [Google Scholar] [CrossRef]
- Park, W.S.; Ahn, S.Y.; Sung, S.I.; Ahn, J.Y.; Chang, Y.S. Mesenchymal Stem Cells: The Magic Cure for Intraventricular Hemorrhage? Cell Transplant. 2017, 26, 439–448. [Google Scholar] [CrossRef]
- Malhotra, A. Neurotherapeutic Potential of Intranasal Administration of Human Breast Milk. Pediatr. Res. 2023, 94, 1872–1873. [Google Scholar] [CrossRef]
- Keller, T.; Körber, F.; Oberthuer, A.; Schafmeyer, L.; Mehler, K.; Kuhr, K.; Kribs, A. Intranasal Breast Milk for Premature Infants with Severe Intraventricular Hemorrhage—An Observation. Eur. J. Pediatr. 2019, 178, 199–206. [Google Scholar] [CrossRef]
- Gallipoli, A.; Unger, S.; El Shahed, A.; Fan, C.S.; Signorile, M.; Wilson, D.; Hoban, R. Outcomes after Intranasal Human Milk Therapy in Preterm Infants with Intraventricular Hemorrhage. J. Perinatol. 2025, 45, 202–207. [Google Scholar] [CrossRef]
- Ghazi-Birry, H.S.; Brown, W.R.; Moody, D.M.; Challa, V.R.; Block, S.M.; Reboussin, D.M. Human Germinal Matrix: Venous Origin of Hemorrhage and Vascular Characteristics. AJNR Am. J. Neuroradiol. 1997, 18, 219–229. [Google Scholar] [PubMed] [PubMed Central]
- Petäjä, J.; Hiltunen, L.; Fellman, V. Increased Risk of Intraventricular Hemorrhage in Preterm Infants with Thrombophilia. Pediatr. Res. 2001, 49, 643–646. [Google Scholar] [CrossRef]
- Ramenghi, L.A.; Fumagalli, M.; Groppo, M.; Consonni, D.; Gatti, L.; Bertazzi, P.A.; Mannucci, P.M.; Mosca, F. Germinal Matrix Hemorrhage: Intraventricular Hemorrhage in Very-Low-Birth-Weight Infants: The Independent Role of Inherited Thrombophilia. Stroke 2011, 42, 1889–1893. [Google Scholar] [CrossRef]
- Birch, P.; Ogden, S.; Hewson, M. A Randomised, Controlled Trial of Heparin in Total Parenteral Nutrition to Prevent Sepsis Associated with Neonatal Long Lines: The Heparin in Long Line Total Parenteral Nutrition (HILLTOP) Trial. Arch. Dis. Child. Fetal Neonatal Ed. 2010, 95, F252–F257. [Google Scholar] [CrossRef]
- Lesko, S.M.; Mitchell, A.A.; Epstein, M.F.; Louik, C.; Giacoia, G.P.; Shapiro, S. Heparin Use as a Risk Factor for Intraventricular Hemorrhage in Low-Birth-Weight Infants. N. Engl. J. Med. 1986, 314, 1156–1160. [Google Scholar] [CrossRef]
- Bruschettini, M.; Romantsik, O.; Zappettini, S.; Banzi, R.; Ramenghi, L.A.; Calevo, M.G. Heparin for the Prevention of Intraventricular Haemorrhage in Preterm Infants. Cochrane Database Syst. Rev. 2016, 2016, CD011718. [Google Scholar] [CrossRef] [PubMed]
- Brangenberg, R.; Bodensohn, M.; Bürger, U. Antithrombin-III Substitution in Preterm Infants—Effect on Intracranial Hemorrhage and Coagulation Parameters. Biol. Neonate 1997, 72, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Bruschettini, M.; Romantsik, O.; Zappettini, S.; Banzi, R.; Ramenghi, L.A.; Calevo, M.G. Antithrombin for the Prevention of Intraventricular Hemorrhage in Very Preterm Infants. Cochrane Database Syst. Rev. 2016, 2016, CD011636. [Google Scholar] [CrossRef] [PubMed]
- Fulia, F.; Cordaro, S.; Meo, P.; Gitto, P.; Gitto, E.; Trimarchi, G.; Adelardi, S.; Barberi, I. Can the Administration of Antithrombin III Decrease the Risk of Cerebral Hemorrhage in Premature Infants? Biol. Neonate 2003, 83, 1–5. [Google Scholar] [CrossRef]
- Hunt, R.; Hey, E. Ethamsylate for the Prevention of Morbidity and Mortality in Preterm or Very Low Birth Weight Infants. Cochrane Database Syst. Rev. 2010, 36, CD004343. [Google Scholar] [CrossRef] [PubMed]
- Sanghvi, K.P.; Merchant, R.H.; Karnik, A.; Kulkarni, A. Role of Ethamsylate in Preventing Periventricular-Intraventricular Hemorrhage in Premature Infants below 34 Weeks of Gestation. Indian. Pediatr. 1999, 36, 653–658. [Google Scholar] [PubMed]
- Chen, J.Y. Ethamsylate in the Prevention of Periventricular-Intraventricular Hemorrhage in Premature Infants. J. Formos. Med. Assoc. 1993, 92, 889–893. [Google Scholar] [PubMed]
- Neary, E.; Ni Ainle, F.; El-Khuffash, A.; Cotter, M.; Kirkham, C.; McCallion, N. Plasma Transfusion to Prevent Intraventricular Haemorrhage in Very Preterm Infants. Cochrane Database Syst. Rev. 2016, 9, CD012341. [Google Scholar] [CrossRef]
- Dani, C.; Poggi, C.; Ceciarini, F.; Bertini, G.; Pratesi, S.; Rubaltelli, F.F. Coagulopathy Screening and Early Plasma Treatment for the Prevention of Intraventricular Hemorrhage in Preterm Infants. Transfusion 2009, 12, 2637–2644. [Google Scholar] [CrossRef] [PubMed]
- Beverley, D.W.; Pitts-Tucker, T.J.; Congdon, P.J.; Arthur, R.J.; Tate, G. Prevention of Intraventricular Haemorrhage by Fresh Frozen Plasma. Arch. Dis. Child. 1985, 8, 710–713. [Google Scholar] [CrossRef]
| Author | Type of study | Population | Study Groups | Intervention | Objectives | Outcome of interest | Main conclusions | Limitations |
|---|---|---|---|---|---|---|---|---|
| Ley, 2019 [138] | Multicenter RCT | 121 neonates, GA 23–27+6 | 61 intervention, 60 standard care | 250 μg/kg/day continuous iv until CA 29+6 | Incidence of prematurity complications | sIVH: 13.1% vs. 23.3%, p > 0.05 | Non-significant decrease in sIVH rates Reduction in sBPD | Small proportion of neonates had >70% of serum IGF-1 in the target range Central randomization by GA Inconsistency in adverse events report |
| Horsch, 2020 [144] | RCT reanalysis | 104 neonates, GA 23–27+6, no preexisting IVH | 52 intervention, 52 standard care | 250 μg/kg/day continuous iv until CA 29+6 | Effects on brain injury | IVH any grade: 25% vs. 40.5%, p > 0.05 | Although not significant, the beneficial effect on IVH rates was more pronounced in neonates without pre-existing IVH. | Small sample The primary endpoint of the initial study was the incidence of ROP, it was not powered for IVH reduction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dermitzaki, N.; Baltogianni, M.; Tsiogka, C.M.; Nikolaou, A.; Balomenou, F.; Giapros, V. Promising Preventive Strategies for Intraventricular Hemorrhage in Preterm Neonates: A Critical Review. J. Clin. Med. 2025, 14, 6763. https://doi.org/10.3390/jcm14196763
Dermitzaki N, Baltogianni M, Tsiogka CM, Nikolaou A, Balomenou F, Giapros V. Promising Preventive Strategies for Intraventricular Hemorrhage in Preterm Neonates: A Critical Review. Journal of Clinical Medicine. 2025; 14(19):6763. https://doi.org/10.3390/jcm14196763
Chicago/Turabian StyleDermitzaki, Niki, Maria Baltogianni, Chrysanthi Maria Tsiogka, Aikaterini Nikolaou, Foteini Balomenou, and Vasileios Giapros. 2025. "Promising Preventive Strategies for Intraventricular Hemorrhage in Preterm Neonates: A Critical Review" Journal of Clinical Medicine 14, no. 19: 6763. https://doi.org/10.3390/jcm14196763
APA StyleDermitzaki, N., Baltogianni, M., Tsiogka, C. M., Nikolaou, A., Balomenou, F., & Giapros, V. (2025). Promising Preventive Strategies for Intraventricular Hemorrhage in Preterm Neonates: A Critical Review. Journal of Clinical Medicine, 14(19), 6763. https://doi.org/10.3390/jcm14196763









