GLP-1 Agonists in Cardiovascular Diseases: Mechanisms, Clinical Evidence, and Emerging Therapies
Abstract
1. Introduction
2. Mechanisms of GLP-1 Agonists in Cardiovascular Protection
2.1. Anti-Atherogenic Effects
2.1.1. Lipid Metabolism Modulation
2.1.2. Plaque Stabilization
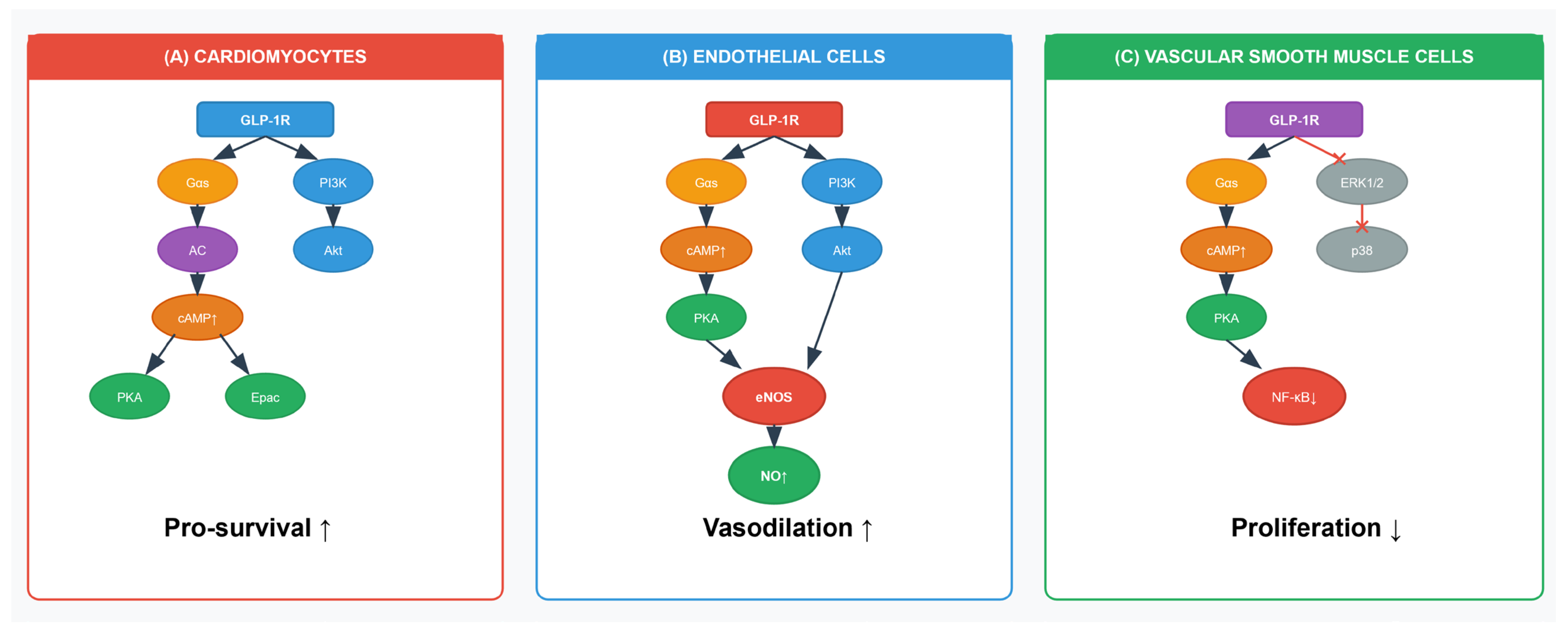
2.1.3. Signaling in VSMC
2.1.4. Signaling in Monocyte Adhesion and Foam Cell Formation
2.2. Endothelial Function and Vascular Homeostasis
2.2.1. Endothelial Nitric Oxide Synthase (eNOS)/Nitric Oxide (NO) Signalling in Endothelial Cells
2.2.2. Blood Pressure Reduction
2.2.3. Anti-Thrombotic Effects
2.2.4. Blood–Brain Barrier (BBB) Protection
2.3. Anti-Inflammatory Properties
2.3.1. Cytokine Modulation
2.3.2. Signaling in Macrophages and Other Immune Cells
2.3.3. Macrophage Polarization
2.3.4. Microglial Activation
2.4. Direct Cardioprotective Effects
2.4.1. Pro-Survival Signalling in Cardiomyocytes: Myocardial Ischemia Protection
2.4.2. Anti-Apoptotic and Anti-Oxidative Effects
2.4.3. Myocardial Metabolism
2.4.4. Anti-Fibrotic Effects
2.5. Weight Loss and Metabolic Benefits
2.6. Neuroprotective Effects in Cerebrovascular Disease
2.6.1. Cerebral Blood Flow (CBF) Improvement
2.6.2. Anti-Oxidative Stress
2.6.3. Synaptic Protection and Neurogenesis
2.7. Interaction and Convergence of Signalling Pathways
3. Clinical Evidence from Cardiovascular Outcome Trials
3.1. Liraglutide
3.2. Semaglutide
3.3. Dulaglutide
3.4. Other GLP-1 Agonists
- Albiglutide: The HARMONY Outcomes trial (2018) reported a 22% MACE reduction (HR 0.78, 95% CI 0.68–0.90) in 9463 T2DM patients, driven by reduced MI [18].
- Efpeglenatide: The AMPLITUDE-O trial (2021) demonstrated a 27% MACE reduction (HR 0.73, 95% CI 0.58–0.92) in 4076 T2DM patients, with significant reductions in stroke and HF events [19].
- Lixisenatide: The ELIXA trial (2015) showed no significant MACE reduction (HR 1.02, 95% CI 0.89–1.17), indicating heterogeneity among GLP-1 agonists [20].
3.5. Heart Failure Outcomes
- STEP-HFpEF and STEP-HFpEF DM: Semaglutide improved KCCQ scores (+16.6 points), reduced HF hospitalizations (HR 0.79), and enhanced physical function in HFpEF patients with or without T2DM [11,46]. Benefits were attributed to weight loss, reduced inflammation, and improved myocardial metabolism [11].
- FLOW Trial: In T2DM patients with chronic kidney disease (CKD), semaglutide reduced the composite of kidney-disease progression or HF events by 27% (HR 0.73, 95% CI 0.54–0.98) alongside renal benefits [58].
3.6. Meta-Analyses and Real-World Evidence
3.7. Critical Appraisal of CVOTs and Evidence Gaps
4. Emerging and Combination Therapies
4.1. Dual GLP-1/GIP Agonists
4.2. CagriSema (Cagrilintide/Semaglutide)
4.3. Retatrutide (GLP-1/GIP/Glucagon Triple Agonist)
5. Applications in Specific Cardiovascular Conditions
5.1. Atherosclerotic Cardiovascular Disease (ASCVD)
5.2. Heart Failure
5.3. Stroke and Cerebrovascular Disease
5.4. Vascular Dementia and Cognitive Impairment
5.4.1. Reducing White Matter Injury
5.4.2. Enhancing Neurogenesis
5.4.3. Anti-Inflammatory Effects
5.5. Non-Diabetic Populations
5.6. Cardio–Renal–Metabolic Axis
5.7. Diabetic Cardiomyopathy
6. Challenges, Limitations, and Future Directions
6.1. Safety, Tolerability, and Adverse Effects
6.2. Implementation Challenges: Cost, Access, and Adherence
6.3. Unanswered Mechanistic and Clinical Questions
6.4. Future Directions and Precision Medicine
7. Conclusions
Funding
Conflicts of Interest
Abbreviations
| CVD | Cardiovascular Diseases |
| T2DM | Type 2 Diabetes Mellitus |
| GLP-1 | Glucagon-Like Peptide-1 |
| GIP | Glucose-Dependent Insulinotropic Polypeptide |
| CVOTs | Cardiovascular Outcome Trials |
| MACE | Major Adverse Cardiovascular Events |
| MI | Myocardial Infarction |
| HFpEF | Heart Failure with Preserved Ejection Fraction |
| ASCVD | Atherosclerotic Cardiovascular Disease |
| VSMCs | Vascular Smooth Muscle Cells |
| LDL-C | Low-Density Lipoprotein Cholesterol |
| HDL-C | High-Density Lipoprotein Cholesterol |
| MMP-9 | Matrix Metalloproteinase-9 |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| LDLR | Low-Density Lipoprotein Receptor |
| cAMP | Cyclic Adenosine Monophosphate |
| NF-κB | Nuclear Factor Kappa-B |
| MAPK | Mitogen-Activated Protein Kinase |
| VCAM-1 | Vascular Cell Adhesion Molecule-1 |
| ICAM-1 | Intercellular Adhesion Molecule-1 |
| ATP | Adenosine Triphosphate |
| ABCA1 | ATP-Binding Cassette Transporter A1 |
| eNOS | endothelial Nitric Oxidase Synthase |
| NO | Nitric Oxide |
| SBP | Systolic Blood Pressure |
| PAI-1 | Plasminogen Activator Inhibitor-1 |
| BBB | Blood-Brain Barrier |
| TNF-α | Tumor Necrosis Factor Alpha |
| IL-6 | Interleukin 6 |
| IL-1β | Interleukin 1 Beta |
| hs-CRP | High-Sensitivity C-Reactive Protein |
| NLRP3 | NOD-, LRR-, and Pyrin Domain-Containing Protein 3 |
| PI3K | Phosphatidylinositol 3-kinase (PI3K) |
| ROS | Reactive Oxygen Species |
| MnSOD | Manganese Superoxide Dismutase |
| GPx-1 | Glutathione Peroxidase-1 |
| Nrf2 | Nuclear Factor Erythroid 2-related Factor 2 |
| ARE | Enhanced Antioxidant Response |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate |
| NOX | NADPH Oxidase |
| TGF-β | Transforming Growth Factor Beta |
| CBF | Cerebral Blood Flow |
| BDNF | Brain-Derived Neurotrophic Factor |
| KCCQ | Kansas City Cardiomyopathy Questionnaire |
| CKD | Chronic Kidney Disease |
| HFrEF | Heart Failure with Reduced Ejection Fraction |
| LVEF | Left Ventricular Ejection Fraction |
| VEGF | Vascular Endothelial Growth Factor |
| SOD | Superoxide Dismutase |
References
- World Health Organization. Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 1 July 2025).
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Drucker, D.J. The cardiovascular biology of glucagon-like peptide-1. Cell Metab. 2016, 24, 15–30. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 10. Cardiovascular disease and risk management: Standards of care in diabetes—2024. Diabetes Care 2024, 47 (Suppl. 1), S179–S218. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Meier, J.J. Incretin hormones: Their role in health and disease. Diabetes Obes. Metab. 2018, 20, 5–21. [Google Scholar] [CrossRef]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.E.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Riesmeyer, J.S.; Riddle, M.C.; Rydén, L.; et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomised placebo-controlled trial. Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Marx, N.; Husain, M.; Lehrke, M.; Verma, S.; Sattar, N. GLP-1 Receptor Agonists for the Reduction of Atheroscle-rotic Cardiovascular Risk in Patients with Type 2 Diabetes. Circulation 2022, 146, 1882–1894. [Google Scholar] [CrossRef]
- Drucker, D.J. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef] [PubMed]
- Kosiborod, M.N.; Abildstrøm, S.Z.; Borlaug, B.A.; Butler, J.; Rasmussen, S.; Davies, M.; Hovingh, G.K.; Kitzman, D.W.; Lindegaard, M.L.; Møller, D.V.; et al. Semaglutide in Patients with Heart Failure with Preserved Ejection Fraction and Obesity. N. Engl. J. Med. 2023, 389, 1069–1084. [Google Scholar] [CrossRef]
- Ryan, D.H.; Lingvay, I.; Deanfield, J.; Kahn, S.E.; Barros, E.; Burguera, B.; Colhoun, H.M.; Cercato, C.; Dicker, D.; Horn, D.B.; et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N. Engl. J. Med. 2023, 389, 2221–2232. [Google Scholar] [CrossRef]
- Salcedo, I.; Tweedie, D.; Li, Y.; Greig, N.H. Neuroprotective and neurotrophic actions of glucagon-like peptide-1: An emerging opportunity to treat neurodegenerative and cerebrovascular disorders. Br. J. Pharmacol. 2012, 166, 1586–1599. [Google Scholar] [CrossRef] [PubMed]
- Frías, J.P.; Davies, M.J.; Rosenstock, J.; Pérez Manghi, F.C.; Fernández Landó, L.; Bergman, B.K.; Liu, B.; Cui, X.; Brown, K. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 503–515. [Google Scholar] [CrossRef]
- Young, K.G.; McInnes, E.H.; Massey, R.J.; Kahkoska, A.R.; Pilla, S.J.; Raghavan, S.; Stanislawski, M.A.; Tobias, D.K.; McGovern, A.P.; Dawed, A.Y.; et al. Treatment effect heterogeneity following type 2 diabetes treatment with GLP1-receptor agonists and SGLT2-inhibitors: A systematic review. Commun. Med. 2023, 3, 131. [Google Scholar] [CrossRef] [PubMed]
- Sposito, A.C.; Berwanger, O.; de Carvalho, L.S.F.; Saraiva, J.F.K. GLP-1RAs in type 2 diabetes: Mechanisms that underlie cardiovascular effects and overview of cardiovascular outcome data. Cardiovasc. Diabetol. 2018, 17, 157. [Google Scholar] [CrossRef] [PubMed]
- Holman, R.R.; Bethel, M.A.; Mentz, R.J.; Thompson, V.P.; Lokhnygina, Y.; Buse, J.B.; Chan, J.C.; Choi, J.; Gustavson, S.M.; Iqbal, N.; et al. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 1228–1239. [Google Scholar] [CrossRef]
- Hernandez, A.F.; Green, J.B.; Janmohamed, S.; D’Agostino, R.B.; Granger, C.B.; Jones, N.P.; Leiter, L.A.; Rosenberg, A.E.; Sigmon, K.N.; Somerville, M.C.; et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): A double-blind, randomised placebo-controlled trial. Lancet 2018, 392, 1519–1529. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Sattar, N.; Rosenstock, J.; Ramasundarahettige, C.; Pratley, R.; Lopes, R.D.; Lam, C.S.; Khurmi, N.S.; Heenan, L.; Del Prato, S.; et al. Cardiovascular and Renal Outcomes with Efpeglenatide in Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 896–907. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Claggett, B.; Diaz, R.; Dickstein, K.; Gerstein, H.C.; Køber, L.V.; Lawson, F.C.; Ping, L.; Wei, X.; Lewis, E.F.; et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N. Engl. J. Med. 2015, 373, 2247–2257. [Google Scholar] [CrossRef] [PubMed]
- Eli Lilly and Company. Lilly’s Mounjaro® (Tirzepatide), a GIP/GLP-1 Dual Agonist, Demonstrated Cardiovascular Protection in Landmark Head-to-Head Trial, Reinforcing Its Benefit in Patients with Type 2 Diabetes and Heart Disease. Lilly News Release. 31 July 2025. Available online: https://investor.lilly.com/news-releases/news-release-details/lillys-mounjaro-tirzepatide-gipglp-1-dual-agonist-demonstrated (accessed on 21 July 2025).
- McGuire, D.K.; Marx, N.; Mulvagh, S.L.; Deanfield, J.E.; Inzucchi, S.E.; Pop-Busui, R.; Mann, J.F.; Emerson, S.S.; Poulter, N.R.; Engelmann, M.D.; et al. Oral Semaglutide and Cardiovascular Outcomes in High-Risk Type 2 Diabetes. N. Engl. J. Med. 2025, 392, 2001–2012. [Google Scholar] [CrossRef]
- Heuvelman, V.D.; Van Raalte, D.H.; Smits, M.M. Cardiovascular effects of glucagon-like peptide 1 receptor agonists: From mechanistic studies in humans to clinical outcomes. Cardiovasc. Res. 2019, 116, 916–930. [Google Scholar] [CrossRef]
- Gaspari, T.; Welungoda, I.; Widdop, R.E.; Simpson, R.W.; Dear, A.E. The GLP-1 receptor agonist liraglutide inhibits progression of vascular disease via effects on atherogenesis, plaque stability and endothelial function in an ApoE−/− mouse model. Diab. Vasc. Dis. Res. 2013, 10, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Rakipovski, G.; Rolin, B.; Nøhr, J.; Klewe, I.; Frederiksen, K.S.; Augustin, R.; Hecksher-Sørensen, J.; Ingvorsen, C.; Polex-Wolf, J.; Knudsen, L.B. The GLP-1 analogs liraglutide and semaglutide reduce atherosclerosis in ApoE−/− and LDLr−/− mice by a mechanism that includes inflammatory pathways. JACC Basic Transl. Sci. 2018, 3, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Ussher, J.R.; Drucker, D.J. Cardiovascular Actions of Incretin-Based Therapies. Circ. Res. 2014, 114, 1788–1803. [Google Scholar] [CrossRef]
- Irace, C.; De Luca, S.; Shehaj, E.; Carallo, C.; Loprete, A.; Scavelli, F.; Gnasso, A. Exenatide improves endothelial function assessed by flow mediated dilation technique in subjects with type 2 diabetes: Results from an observational research. Diab. Vasc. Dis. Res. 2013, 10, 72–77. [Google Scholar] [CrossRef]
- Husain, M.; Birkenfeld, A.L.; Donsmark, M.; Dungan, K.; Eliaschewitz, F.G.; Franco, D.R.; Jeppesen, O.K.; Lingvay, I.; Mosenzon, O.; Pedersen, S.D.; et al. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ding, J.; Cheng, X.; Yang, Q.; Hu, P. Glucagon-like peptide-1 receptor agonists: New strategies and therapeutic targets to treat atherosclerotic cardiovascular disease. Front. Pharmacol. 2024, 15, 1396656. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Luo, Y.; Wen, Y.; Wang, D.; Li, J.; Fan, Z. Semaglutide inhibits ischemia/reperfusion-induced cardiomyocyte apoptosis through activating PKG/PKCε/ERK1/2 pathway. Biochem. Biophys. Res. Commun. 2023, 647, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Timmers, L.; Henriques, J.P.; de Kleijn, D.P.; DeVries, J.H.; Kemperman, H.; Steendijk, P.; Verlaan, C.W.; Kerver, M.; Piek, J.J.; Doevendans, P.A.; et al. Exenatide Reduces Infarct Size and Improves Cardiac Function in a Porcine Model of Ischemia and Reperfusion Injury. J. Am. Coll. Cardiol. 2009, 53, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Ceriello, A.; Esposito, K.; Testa, R.; Bonfigli, A.R.; Marra, M.; Giugliano, D. The possible protective role of glucagon-like peptide 1 on endothelium during the meal and evidence for an “endothelial resistance” to glucagon-like peptide 1 in diabetes. Diabetes Care 2011, 34, 697–702. [Google Scholar] [CrossRef]
- Li, H.; Yao, W.; Liu, Z.; Xu, A.; Huang, Y.; Ma, X.L.; Irwin, M.G.; Xia, Z. Hyperglycemia Abrogates Ischemic Postconditioning Cardioprotection by Impairing AdipoR1/Caveolin-3/STAT3 Signaling in Diabetic Rats. Diabetes 2016, 65, 942–955. [Google Scholar] [CrossRef]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Sun, F.; Wu, S.; Wang, J.; Guo, S.; Chai, S.; Yang, Z.; Li, L.; Zhang, Y.; Ji, L.; Zhan, S. Effect of Glucagon-like Peptide-1 Receptor Agonists on Lipid Profiles Among Type 2 Diabetes: A Systematic Review and Network Meta-analysis. Clin. Ther. 2015, 37, 225–241.e8. [Google Scholar] [CrossRef]
- Rizzo, M.; Chandalia, M.; Patti, A.M.; Di Bartolo, V.; Rizvi, A.A.; Montalto, G.; Abate, N. Liraglutide decreases carotid intima-media thickness in patients with type 2 diabetes: 8-month prospective pilot study. Cardiovasc. Diabetol. 2014, 13, 49. [Google Scholar] [CrossRef]
- Hirata, Y.; Kurobe, H.; Nishio, C.; Tanaka, K.; Fukuda, D.; Uematsu, E.; Nishimoto, S.; Soeki, T.; Harada, N.; Sakaue, H.; et al. Exendin-4, a glucagon-like peptide-1 receptor agonist, attenuates neointimal hyperplasia after vascular injury. Eur. J. Pharmacol. 2013, 699, 106–111. [Google Scholar] [CrossRef]
- Vanhoutte, P.M.; Shimokawa, H.; Feletou, M.; Tang, E.H.C. Endothelial dysfunction and vascular disease - a 30th anniversary update. Acta Physiol. 2017, 219, 22–96. [Google Scholar] [CrossRef] [PubMed]
- Cameron-Vendrig, A.; Reheman, A.; Siraj, M.A.; Xu, X.R.; Wang, Y.; Lei, X.; Afroze, T.; Shikatani, E.; El-Mounayri, O.; Noyan, H.; et al. Glucagon-Like Peptide 1 Receptor Activation Attenuates Platelet Aggregation and Thrombosis. Diabetes 2016, 65, 1714–1723. [Google Scholar] [CrossRef]
- Loganathan, J.; Cohen, A.C.; Kaloupis, G.M.; Harris, C.; Chronopoulos, A.; James, V.; Hamilton, J.; Green, S.; Wallis, A.; Morgan, S.; et al. A pilot clinical study to Evaluate Liraglutide-mediated Anti-platelet activity in patients with type-2 Diabetes (ELAID study). J. Diabetes Its Complicat. 2022, 36, 108188. [Google Scholar] [CrossRef]
- Ridker, P.M. From C-reactive protein to interleukin-6 to interleukin-1: Moving upstream to identify novel targets for atheroprotection. Circ. Res. 2016, 118, 145–156. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Jun, H.-S. Anti-Inflammatory Effects of GLP-1-Based Therapies beyond Glucose Control. Mediat. Inflamm. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Marcos, P.J.; Nobrega-Pereira, S. NADPH: New oxygen for the ROS theory of aging. Oncotarget 2016, 7, 50814–50815. [Google Scholar] [CrossRef]
- Hendarto, H.; Inoguchi, T.; Maeda, Y.; Ikeda, N.; Zheng, J.; Takei, R.; Yokomizo, H.; Hirata, E.; Sonoda, N.; Takayanagi, R. GLP-1 analog liraglutide protects against oxidative stress and albuminuria in streptozotocin-induced diabetic rats via protein kinase A-mediated inhibition of renal NAD(P)H oxidases. Metabolism 2012, 61, 1422–1434. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, Y.; Matsui, T.; Takeuchi, M.; Yamagishi, S.-I. Glucagon-like peptide-1 (GLP-1) inhibits advanced glycation end product (AGE)-induced up-regulation of VCAM-1 mRNA levels in endothelial cells by suppressing AGE receptor (RAGE) expression. Biochem. Biophys. Res. Commun. 2010, 391, 1405–1408. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef]
- Davies, M.; Færch, L.; Jeppesen, O.K.; Pakseresht, A.; Pedersen, S.D.; Perreault, L.; Rosenstock, J.; Shimomura, I.; Viljoen, A.; Wadden, T.A.; et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): A randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet 2021, 397, 971–984. [Google Scholar] [CrossRef]
- Neeland, I.J.; Ross, R.; Després, J.-P.; Matsuzawa, Y.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diabetes Endocrinol. 2019, 7, 715–725. [Google Scholar] [CrossRef]
- An, J.-R.; Liu, J.-T.; Gao, X.-M.; Wang, Q.-F.; Sun, G.-Y.; Su, J.-N.; Zhang, C.; Yu, J.-X.; Yang, Y.-F.; Shi, Y. Effects of liraglutide on astrocyte polarization and neuroinflammation in db/db mice: Focus on iron overload and oxidative stress. Front. Cell. Neurosci. 2023, 17, 1136070. [Google Scholar] [CrossRef]
- Sattar, N.; Lee, M.M.Y.; Kristensen, S.L.; Branch, K.R.H.; Del Prato, S.; Khurmi, N.S.; Lam, C.S.P.; Lopes, R.D.; McMurray, J.J.V.; Pratley, R.E.; et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of randomised controlled trials. Lancet 2021, 398, 2015–2026. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; D‘aLessio, D.A. Tirzepatide, a dual GIP/GLP-1 receptor co-agonist for the treatment of type 2 diabetes with unmatched effectiveness regrading glycaemic control and body weight reduction. Cardiovasc. Diabetol. 2022, 21, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Garvey, W.T.; Blüher, M.; Contreras, C.K.O.; Davies, M.J.; Lehmann, E.W.; Pietiläinen, K.H.; Rubino, D.; Sbraccia, P.; Wadden, T.; Zeuthen, N.; et al. Coadministered Cagrilintide and Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2025. [Google Scholar] [CrossRef]
- Frias, J.P.; Deenadayalan, S.; Erichsen, L.; Knop, F.K.; Lingvay, I.; Macura, S.; Mathieu, C.; Pedersen, S.D.; Davies, M. Efficacy and safety of co-administered once-weekly cagrilintide 2·4 mg with once-weekly semaglutide 2·4 mg in type 2 diabetes: A multicentre, randomised, double-blind, active-controlled, phase 2 trial. Lancet 2023, 402, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Jastreboff, A.M.; Kaplan, L.M.; Frías, J.P.; Wu, Q.; Du, Y.; Gurbuz, S.; Coskun, T.; Haupt, A.; Milicevic, Z.; Milicevic, Z.; et al. Triple–hormone-receptor agonist retatrutide for obesity—A phase 2 trial. N. Engl. J. Med. 2023, 389, 514–526. [Google Scholar] [CrossRef]
- Kosiborod, M.N.; Petrie, M.C.; Borlaug, B.A.; Butler, J.; Davies, M.J.; Hovingh, G.K.; Kitzman, D.W.; Møller, D.V.; Treppendahl, M.B.; Verma, S.; et al. Semaglutide in Patients with Obesity-Related Heart Failure and Type 2 Diabetes. N. Engl. J. Med. 2024, 390, 1394–1407. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Botros, F.T.; Riddle, M.C.; Rydén, L.; et al. Dulaglutide and renal outcomes in type 2 diabetes: An exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet 2019, 394, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Pratley, R.E.; Tuttle, K.R.; Rossing, P.; Rasmussen, S.; Perkovic, V.; Nielsen, O.W.; Mann, J.F.; MacIsaac, R.J.; Kosiborod, M.N.; Kamenov, Z.; et al. Effects of Semaglutide on Heart Failure Outcomes in Diabetes and Chronic Kidney Disease in the FLOW Trial. JACC 2024, 84, 1615–1628. [Google Scholar] [CrossRef] [PubMed]
- Margulies, K.B.; Hernandez, A.F.; Redfield, M.M.; Givertz, M.M.; Oliveira, G.H.; Cole, R.; Mann, D.L.; Whellan, D.J.; Kiernan, M.S.; Felker, G.M.; et al. Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction: A randomized clinical trial (FIGHT). JAMA 2016, 316, 500–508. [Google Scholar] [CrossRef]
- Jorsal, A.; Kistorp, C.; Holmager, P.; Tougaard, R.S.; Nielsen, R.; Hänselmann, A.; Nilsson, B.; Møller, J.E.; Hjort, J.; Rasmussen, J.; et al. Effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)—A multicentre, double-blind, randomised, placebo-controlled trial. Eur. J. Heart Fail. 2017, 19, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.-C.; Chen, M.-T.; Liu, C.-Y.; Chan, C.-Y.; Fang, Y.-W.; Liou, H.-H.; Tsai, M.-H. Glucagon-like peptide-1 receptor agonist therapy in patients with type 2 diabetes and advanced CKD: Kidney and cardiovascular outcomes in a real-world setting. Clin. Kidney J. 2025, 18, sfaf172. [Google Scholar] [CrossRef]
- Kristensen, S.L.; Rorth, R.; Jhund, P.S.; Docherty, K.F.; Sattar, N.; Preiss, D.; Kober, L.; Petrie, M.C.; McMurray, J.J.V. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019, 7, 776–785. [Google Scholar] [CrossRef]
- Zelniker, T.A.; Wiviott, S.D.; Raz, I.; Im, K.; Goodrich, E.L.; Furtado, R.H.M.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; et al. Comparison of the Effects of Glucagon-Like Peptide Receptor Agonists and Sodium-Glucose Cotransporter 2 Inhibitors for Prevention of Major Adverse Cardiovascular and Renal Outcomes in Type 2 Diabetes Mellitus. Circulation 2019, 139, 2022–2031. [Google Scholar] [CrossRef]
- Bethel, M.A.; Patel, R.A.; Merrill, P.; Lokhnygina, Y.; Buse, J.B.; Mentz, R.J.; Pagidipati, N.J.; Chan, J.C.; Gustavson, S.M.; Iqbal, N.; et al. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: A meta-analysis. Lancet Diabetes Endocrinol. 2018, 6, 105–113. [Google Scholar] [CrossRef]
- Marsico, F.; Paolillo, S.; Gargiulo, P.; Bruzzese, D.; Dell’Aversana, S.; Esposito, I.; Renga, F.; Esposito, L.; Marciano, C.; Dellegrottaglie, S.; et al. Effects of glucagon-like peptide-1 receptor agonists on major cardiovascular events in patients with Type 2 diabetes mellitus with or without established cardiovascular disease: A meta-analysis of randomized controlled trials. Eur. Heart J. 2020, 41, 3346–3358. [Google Scholar] [CrossRef]
- Lingvay, I.; Catarig, A.M.; Frias, J.P.; Kumar, H.; Lausvig, N.L.; le Roux, C.W.; Thielke, D.; Viljoen, A.; McCrimmon, R.J. Efficacy and safety of once-weekly semaglutide versus daily canagliflozin as add-on to metformin in patients with type 2 diabetes (SUSTAIN 8): A double-blind, phase 3b, randomised controlled trial. Lancet Diabetes Endocrinol. 2019, 7, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, J.; Wysham, C.; Frias, J.P.; Kaneko, S.; Lee, C.J.; Fernandez Lando, L.; Mao, H.; Cui, X.; Karanikas, C.A.; Thieu, V.T. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): A double-blind, randomised, phase 3 trial. Lancet 2021, 398, 143–155. [Google Scholar] [CrossRef]
- Baggio, L.L.; Drucker, D.J. Glucagon-like peptide-1 receptor co-agonists for treating metabolic disease. Mol. Metab. 2021, 46, 101090. [Google Scholar] [CrossRef]
- Knop, F.K.; Aroda, V.R.; Vale, R.D.D.; Holst-Hansen, T.; Laursen, P.N.; Rosenstock, J.; Rubino, D.M.; Garvey, W.T. Oral semaglutide 50 mg taken once per day in adults with overweight or obesity (OASIS 1): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023, 402, 705–719. [Google Scholar] [CrossRef]
- Rangaswami, J.; Bhalla, V.; Blair, J.E.A.; Chang, T.I.; Costa, S.; Krista, L.L.; Lerma, E.V.; Mezue, K.; Molitch, M.; Mullens, W.; et al. Cardiorenal Syndrome: Classification, Pathophysiology, Diagnosis, and Treatment Strategies: A Scientific Statement From the American Heart Association. Circulation 2019, 139, e840–e878. [Google Scholar] [CrossRef]
- Jia, G.; Hill, M.A.; Sowers, J.R. Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circ. Res. 2018, 122, 624–638. [Google Scholar] [CrossRef]
- Seferovic, P.M.; Petrie, M.C.; Filippatos, G.S.; Anker, S.D.; Rosano, G.; Bauersachs, J.; Paulus, W.J.; Komajda, M.; Cosentino, F.; de Boer, R.A.; et al. Type 2 diabetes mellitus and heart failure: A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 853–872. [Google Scholar] [CrossRef]
- Bugger, H.; Abel, E.D. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia 2014, 57, 660–671. [Google Scholar] [CrossRef]
- Noyan-Ashraf, M.H.; Momen, M.A.; Ban, K.; Sadi, A.M.; Zhou, Y.Q.; Riazi, A.M.; Baggio, L.L.; Henkelman, R.M.; Husain, M.; Drucker, D.J. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes 2009, 58, 975–983. [Google Scholar] [CrossRef]
- Nielsen, R.; Jorsal, A.; Iversen, P.; Tolbod, L.P.; Bouchelouche, K.; Sorensen, J.; Harms, H.J.; Flyvbjerg, A.; Tarnow, L.; Kistorp, C.; et al. Effect of liraglutide on myocardial glucose uptake and blood flow in stable chronic heart failure patients: A double-blind, randomized, placebo-controlled LIVE sub-study. J. Nucl. Cardiol. 2019, 26, 585–597. [Google Scholar] [CrossRef]
- Steven, S.; Oelze, M.; Hanf, A.; Kroller-Schon, S.; Kashani, F.; Roohani, S.; Welschof, P.; Kopp, M.; Godtel-Armbrust, U.; Xia, N.; et al. The SGLT2 inhibitor empagliflozin improves the primary diabetic complications in ZDF rats. Redox Biol. 2017, 13, 370–385. [Google Scholar] [CrossRef]
- Wei, H.; Bu, R.; Yang, Q.; Jia, J.; Li, T.; Wang, Q.; Chen, Y. Exendin-4 Protects against Hyperglycemia-Induced Cardiomyocyte Pyroptosis via the AMPK-TXNIP Pathway. J. Diabetes Res. 2019, 2019, 8905917. [Google Scholar] [CrossRef]
- Bizino, M.B.; Jazet, I.M.; Westenberg, J.J.M.; van Eyk, H.J.; Paiman, E.H.M.; Smit, J.W.A.; Lamb, H.J. Effect of liraglutide on cardiac function in patients with type 2 diabetes mellitus: Randomized placebo-controlled trial. Cardiovasc. Diabetol. 2019, 18, 55. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Mazer, C.D.; Yan, A.T.; Mason, T.; Garg, V.; Teoh, H.; Zuo, F.; Quan, A.; Farkouh, M.E.; Fitchett, D.H.; et al. Effect of Empagliflozin on Left Ventricular Mass in Patients with Type 2 Diabetes Mellitus and Coronary Artery Disease: The EMPA-HEART CardioLink-6 Randomized Clinical Trial. Circulation 2019, 140, 1693–1702. [Google Scholar] [CrossRef]
- Li, C.J.; Lv, L.; Li, H.; Yu, D.M. Cardiac fibrosis and dysfunction in experimental diabetic cardiomyopathy are ameliorated by alpha-lipoic acid. Cardiovasc. Diabetol. 2012, 11, 73. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Korantzopoulos, P.; Letsas, K.P.; Tse, G.; Gong, M.; Meng, L.; Li, G.; Liu, T. Thiazolidinedione use and atrial fibrillation in diabetic patients: A meta-analysis. BMC Cardiovasc. Disord. 2017, 17, 96. [Google Scholar] [CrossRef]
- Yin, M.; Sillje, H.H.; Meissner, M.; van Gilst, W.H.; de Boer, R.A. Early and late effects of the DPP-4 inhibitor vildagliptin in a rat model of post-myocardial infarction heart failure. Cardiovasc. Diabetol. 2011, 10, 85. [Google Scholar] [CrossRef]
- Udell, J.A.; Cavender, M.A.; Bhatt, D.L.; Chatterjee, S.; Farkouh, M.E.; Scirica, B.M. Glucose-lowering drugs or strategies and cardiovascular outcomes in patients with or at risk for type 2 diabetes: A meta-analysis of randomised controlled trials. Lancet Diabetes Endocrinol. 2015, 3, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, J.M.; Nuffer, W.; Smith, B.A. GLP-1 receptor agonists: An updated review of head-to-head clinical studies. Ther. Adv. Endocrinol. Metab. 2021, 12, 2042018821997320. [Google Scholar] [CrossRef] [PubMed]
- Htike, Z.Z.; Zaccardi, F.; Papamargaritis, D.; Webb, D.R.; Khunti, K.; Davies, M.J. Efficacy and safety of glucagon-like peptide-1 receptor agonists in type 2 diabetes: A systematic review and mixed-treatment comparison analysis. Diabetes Obes. Metab. 2017, 19, 524–536. [Google Scholar] [CrossRef]
- Monami, M.; Nreu, B.; Scatena, A.; Cresci, B.; Andreozzi, F.; Sesti, G.; Mannucci, E. Safety issues with glucagon-like peptide-1 receptor agonists (pancreatitis, pancreatic cancer and cholelithiasis): Data from randomized controlled trials. Diabetes Obes. Metab. 2017, 19, 1233–1241. [Google Scholar] [CrossRef]
- He, L.; Wang, J.; Ping, F.; Yang, N.; Huang, J.; Li, Y.; Xu, L.; Li, W.; Zhang, H. Association of Glucagon-Like Peptide-1 Receptor Agonist Use with Risk of Gallbladder and Biliary Diseases: A Systematic Review and Meta-analysis of Randomized Clinical Trials. JAMA Intern. Med. 2022, 182, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Vilsboll, T.; Bain, S.C.; Leiter, L.A.; Lingvay, I.; Matthews, D.; Simo, R.; Helmark, I.C.; Wijayasinghe, N.; Larsen, M. Semaglutide, reduction in glycated haemoglobin and the risk of diabetic retinopathy. Diabetes Obes. Metab. 2018, 20, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Yudkin, J.S.; Kehlenbrink, S.; Davies, J.I.; Wild, S.H.; Lipska, K.J.; Sussman, J.B.; Beran, D. Estimation of global insulin use for type 2 diabetes, 2018–2030: A microsimulation analysis. Lancet Diabetes Endocrinol. 2019, 7, 25–33. [Google Scholar] [CrossRef]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Economic Burden of Cardiovascular Disease in Type 2 Diabetes: A Systematic Review. Value Health 2018, 21, 881–890. [Google Scholar] [CrossRef]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 receptor agonists in the treatment of type 2 diabetes—State-of-the-art. Mol. Metab. 2021, 46, 101102. [Google Scholar] [CrossRef]
- Palmer, S.C.; Tendal, B.; Mustafa, R.A.; Vandvik, P.O.; Li, S.; Hao, Q.; Tunnicliffe, D.; Ruospo, M.; Natale, P.; Saglimbene, V.; et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: Systematic review and network meta-analysis of randomised controlled trials. BMJ 2021, 372, m4573. [Google Scholar] [CrossRef] [PubMed]
- Dave, C.V.; Kim, S.C.; Goldfine, A.B.; Glynn, R.J.; Tong, A.; Patorno, E. Risk of Cardiovascular Outcomes in Patients with Type 2 Diabetes After Addition of SGLT2 Inhibitors Versus Sulfonylureas to Baseline GLP-1RA Therapy. Circulation 2021, 143, 770–779. [Google Scholar] [CrossRef]
- Schnurr, T.M.; Jakupovic, H.; Carrasquilla, G.D.; Angquist, L.; Grarup, N.; Sorensen, T.I.A.; Tjonneland, A.; Overvad, K.; Pedersen, O.; Hansen, T.; et al. Obesity, unfavourable lifestyle and genetic risk of type 2 diabetes: A case-cohort study. Diabetologia 2020, 63, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]

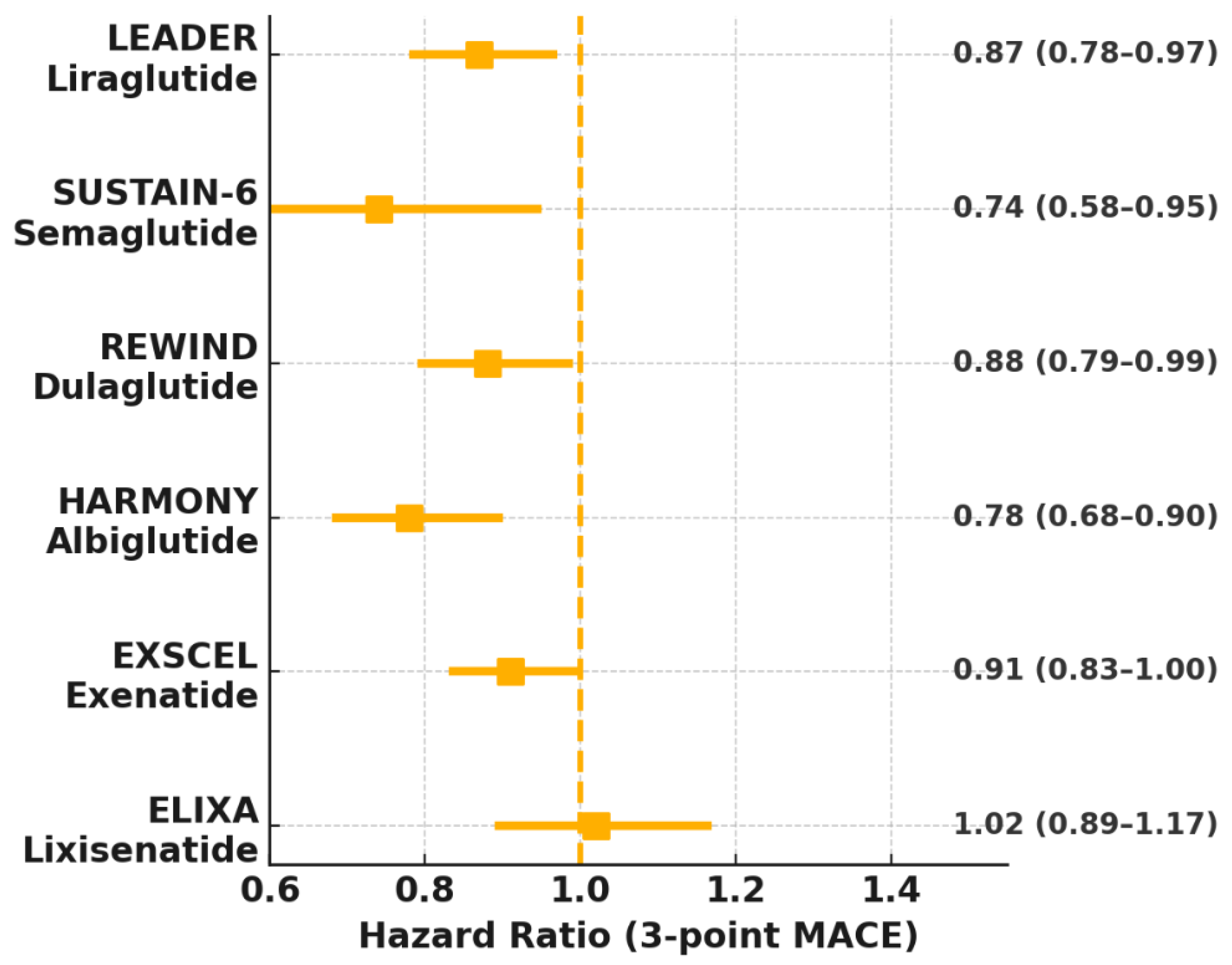
| Trial (Year) | Agent/Dose | Study Population (N) | Primary Outcome (3-Point MACE Unless Stated) | Key CV/HF Findings | Key Ref. |
|---|---|---|---|---|---|
| LEADER (2016) | Liraglutide 1.8 mg qd | 9340 T2DM + high CV risk | ↓ MACE 13% (HR 0.87) | ↓ CV death 22%, ↓ all-cause mortality 15% | [6] |
| SUSTAIN-6 (2016) | Semaglutide 0.5/1 mg qw | 3297 T2DM | ↓ MACE 26% (HR 0.74) | ↓ stroke 39% | [7] |
| EXSCEL (2017) | Exenatide 2 mg qw | 14,752 T2DM | NS 9% MACE↓ (HR 0.91) | ↓ all-cause mortality in sub-groups | [17] |
| HARMONY (2018) | Albiglutide 30–50 mg qw | 9463 T2DM + ASCVD | ↓ MACE 22% (HR 0.78) | Driven by MI ↓ | [18] |
| REWIND (2019) | Dulaglutide 1.5 mg qw | 9901 T2DM (31% ASCVD) | ↓ MACE 12% (HR 0.88) | Consistent across MI/stroke | [8] |
| AMPLITUDE-O (2021) | Efpeglenatide 4–6 mg qw | 4076 T2DM | ↓ MACE 27% (HR 0.73) | ↓ stroke & HF events | [19] |
| ELIXA (2015) | Lixisenatide 20 µg qd | 6068 T2DM + recent ACS | Neutral (HR 1.02) | Heterogeneous class effect | [20] |
| STEP-HFpEF (2023) | Semaglutide 2.4 mg qw | 529 obese HFpEF (±T2DM) | KCCQ +16.6 pts† | ↓ HF hospitalisation (HR 0.79) | [11] |
| SELECT (2023) | Semaglutide 2.4 mg qw | 17,604 obese, non-DM + ASCVD | ↓ MACE 20% (HR 0.80) | Benefit in primary prevention | [12] |
| SURPASS-CVOT | Tirzepatide 10/15 mg qw | 13,299 T2DM + high CV risk | Non-inferior vs dulaglutide; (HR 0.92) | Secondary analysis → significant-cause death ↓ | [21] |
| SOUL (2025) | Oral semaglutide | 9650 T2DM + ASCVD/CKD | ↓ MACE 14% (HR 0.86) | Secondary analysis → | [22] |
| Mechanistic Domain | Major Actions | Representative Evidence | Key Ref. |
|---|---|---|---|
| Anti-atherogenic | ↓ LDL-C & TG, plaque stabilization, ↓ VSMC proliferation | Liraglutide & semaglutide attenuated atherosclerosis in ApoE/LDLR-KO mice | [24,25] |
| Endothelial/Vascular | ↑ eNOS-NO, improved FMD, ↓ SBP 2–5 mmHg, antithrombotic | Exenatide ↑ FMD 2–3% (human data); liraglutide eNOS activation (in vitro data) | [26,27] |
| Anti-inflammatory | ↓ TNF-α, IL-6; NLRP3 inhibition; macrophage M2 shift | Semaglutide ↓ hs-CRP 20–30% (human data); exenatide ↓ NLRP3 (in vitro data) | [28,29] |
| Direct cardioprotection | ↓ Ischemia–reperfusion injury, anti-apoptotic, anti-fibrotic | Exenatide ↓ infarct 20–25% in porcine models; semaglutide ↑ Akt (in vitro data) | [30,31] |
| Metabolic/Weight | 5–15% weight loss; improved insulin sensitivity | STEP trials: semaglutide 12–15% weight loss (human data) | [32,33] |
| Neuro-/Cerebro-vascular | BBB protection, ↑ CBF, ↑ BDNF; ↓ ROS | Exenatide ↓ BBB permeability (animal data); liraglutide ↑ neurogenesis (animal data) | [13,34] |
| Agent/Platform | Mechanism | Development Status | Key Efficacy Signals | Key Ref. |
|---|---|---|---|---|
| Tirzepatide | Dual GLP-1/GIP | Approved; CVOT (SURPASS-CVOT) | ↓ MACE (see Table 1); HbA1c −2.0–2.5%, weight −15–20% | [21,52] |
| CagriSema (cagrilintide + semaglutide) | Amylin analog + GLP-1 | Phase 2 obesity & T2DM trials | ↓ Weight loss; favourable glycaemia & lipids | [53,54] |
| Retatrutide (LY 3437943) | Triple GLP-1/GIP/Glucagon | Phase 2 (obesity 2023); CV studies in design | Up to 24% weight loss; ↑ energy expenditure, lipid lowering; pre-clinical CV benefit | [55] |
| Feature | Liraglutide (LEADER) | Semaglutide (SUSTAIN-6) | Dulaglutide (REWIND) |
|---|---|---|---|
| Dosing Frequency | Once Daily | Once Weekly | Once Weekly |
| Primary MACE Reduction | 13% | 26% | 12% |
| Primary Driver of Benefit | CV Death Reduction | Stroke Reduction | Broadly Consistent |
| All-Cause Mortality | Significant Reduction (15%) | Neutral | Significant Reduction (in some analyses) |
| Patient Population | High-Risk T2DM | High-Risk T2DM | Broader-Risk T2DM |
| Typical Weight Loss | Modest (~2–3 kg) | Moderate (~4–6 kg) | Modest (~2–3 kg) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.-M. GLP-1 Agonists in Cardiovascular Diseases: Mechanisms, Clinical Evidence, and Emerging Therapies. J. Clin. Med. 2025, 14, 6758. https://doi.org/10.3390/jcm14196758
Yang H-M. GLP-1 Agonists in Cardiovascular Diseases: Mechanisms, Clinical Evidence, and Emerging Therapies. Journal of Clinical Medicine. 2025; 14(19):6758. https://doi.org/10.3390/jcm14196758
Chicago/Turabian StyleYang, Han-Mo. 2025. "GLP-1 Agonists in Cardiovascular Diseases: Mechanisms, Clinical Evidence, and Emerging Therapies" Journal of Clinical Medicine 14, no. 19: 6758. https://doi.org/10.3390/jcm14196758
APA StyleYang, H.-M. (2025). GLP-1 Agonists in Cardiovascular Diseases: Mechanisms, Clinical Evidence, and Emerging Therapies. Journal of Clinical Medicine, 14(19), 6758. https://doi.org/10.3390/jcm14196758





