Stroke in Transcatheter Aortic Valve Implantation (TAVI): A Comprehensive Review
Abstract
1. Introduction
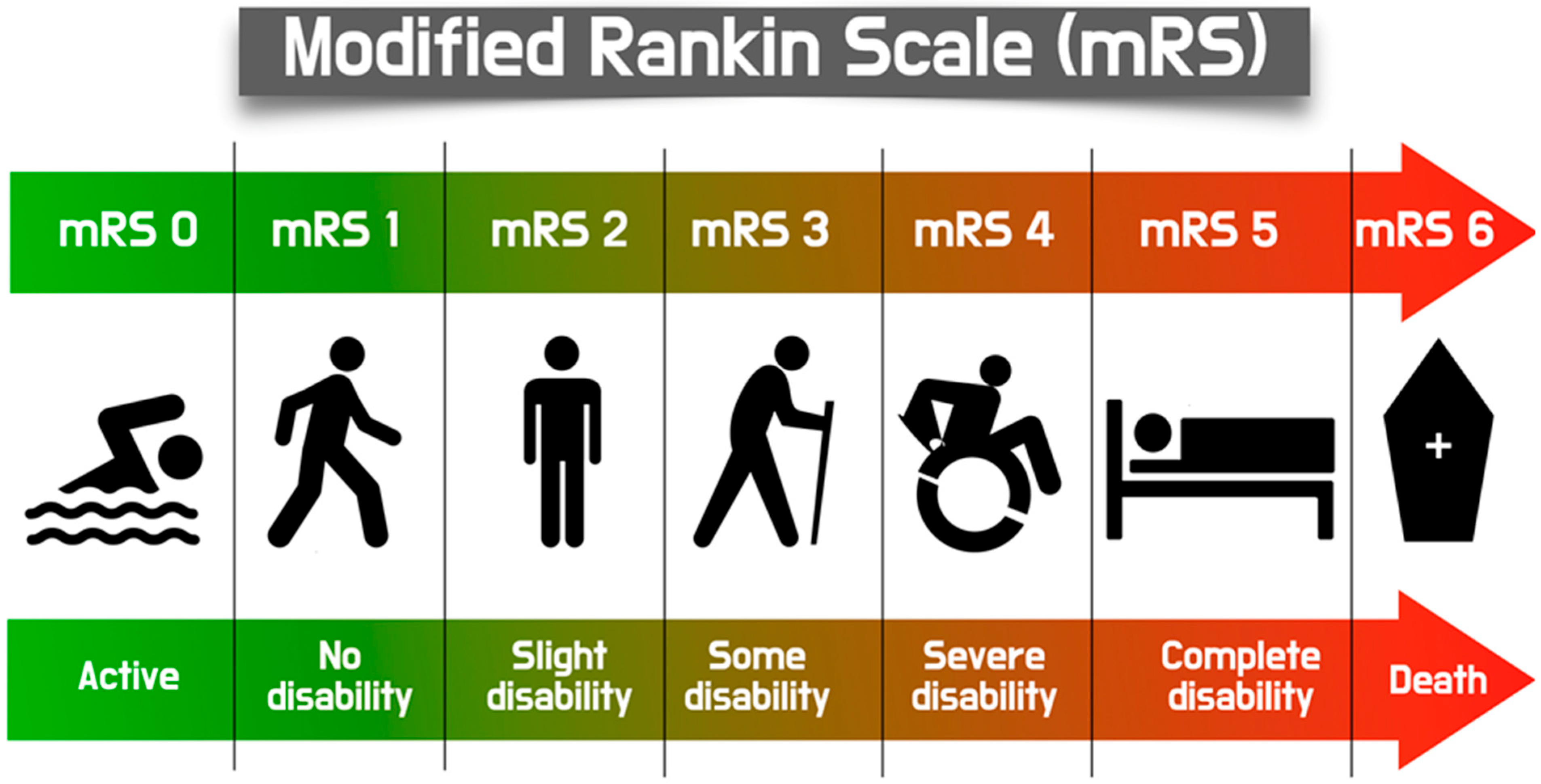
2. Definition of TAVI-Related Stroke
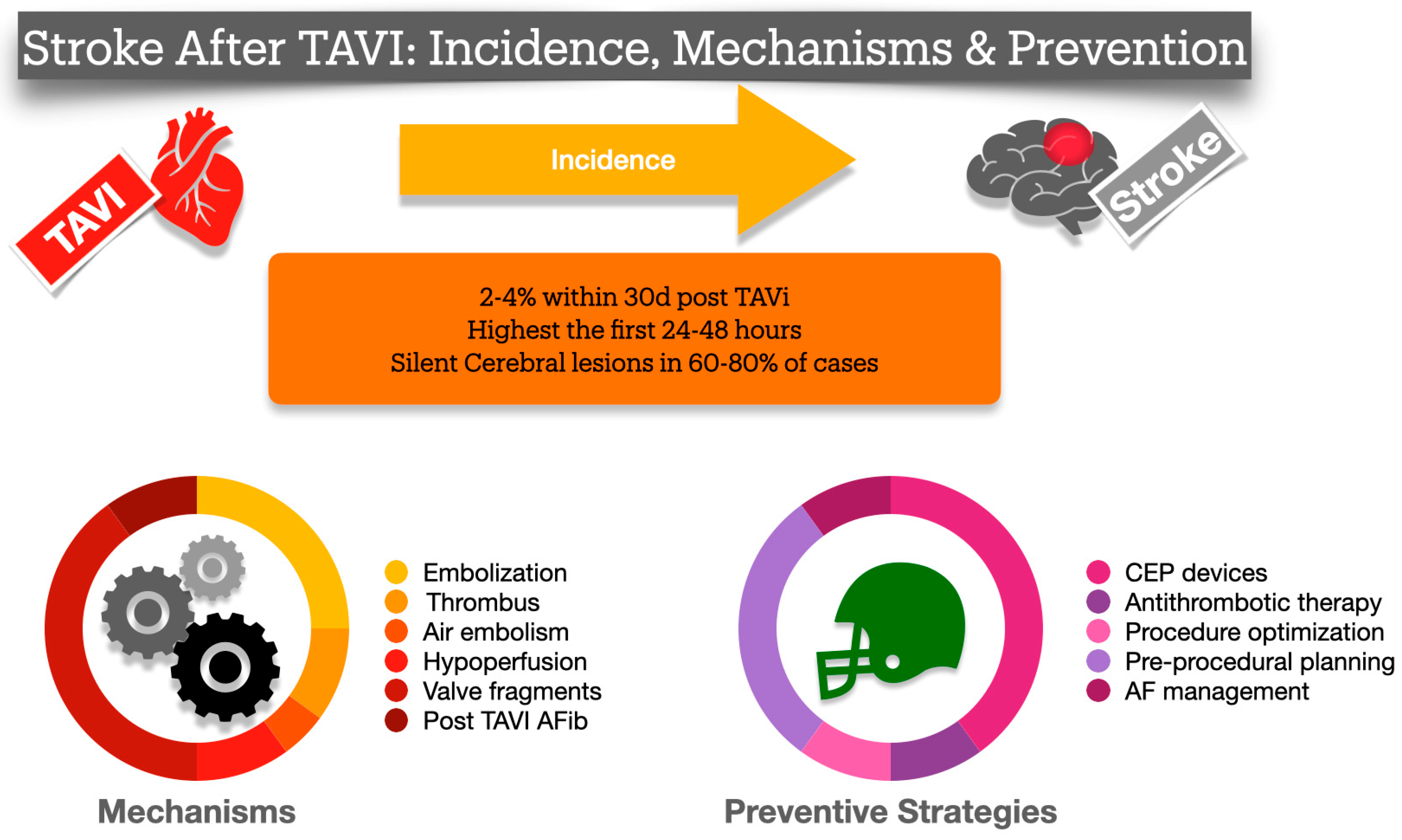
3. Incidence
4. Possible Mechanisms for Ischemic Stroke After TAVI
5. Predictors of Stroke After TAVI
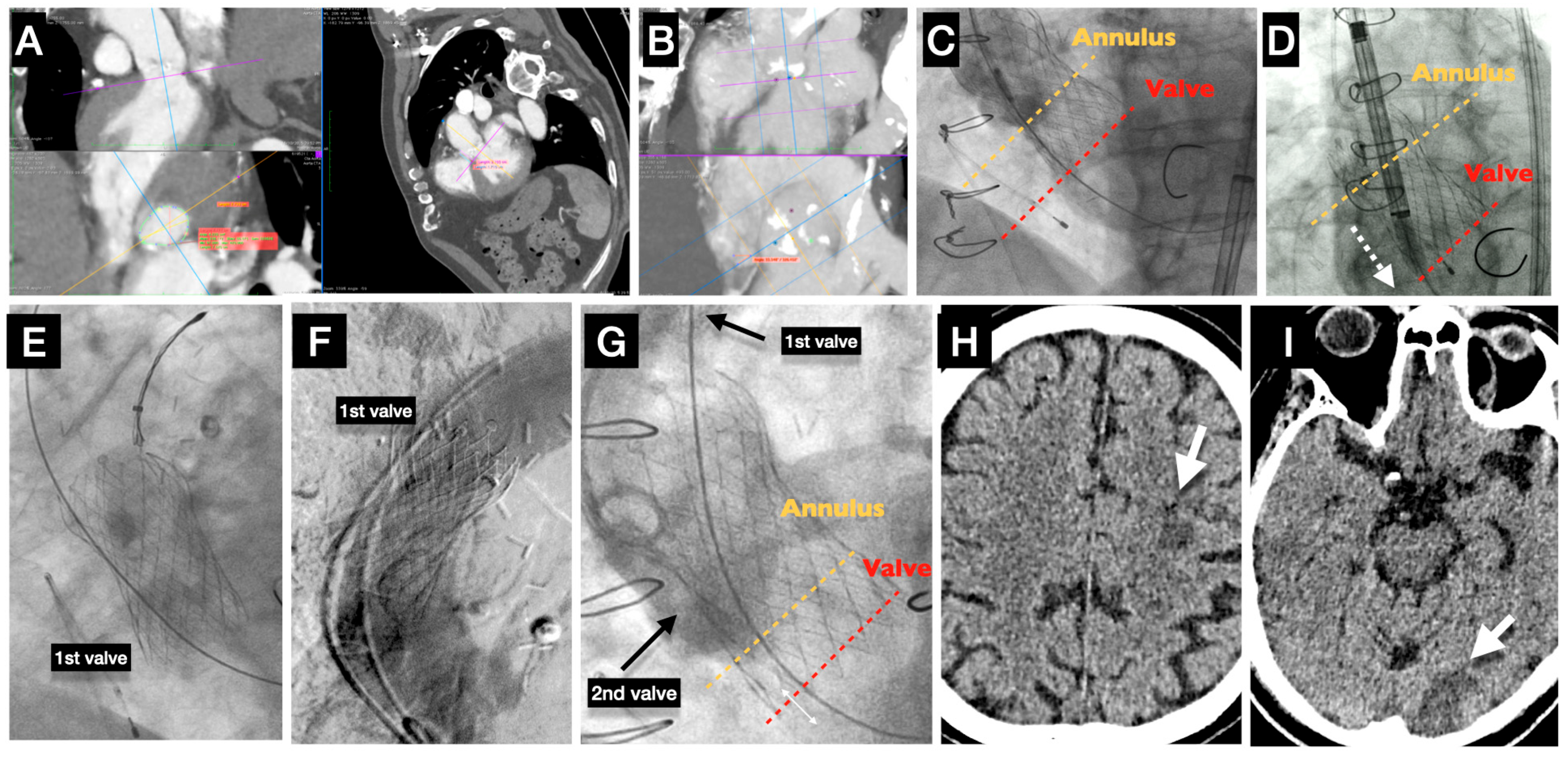
6. Pathophysiology and Technical Aspects Behind Stroke Causes (Figure 2)
7. Acute Stroke (Case Example)
8. Subacute/Late Stroke
9. Subclinical Leaflet Thrombosis (SLT)
10. Pharmaceutical Treatment for Stroke Prevention in TAVI
11. Endovascular Interventions in Acute Treatment of Stroke in TAVI Patients
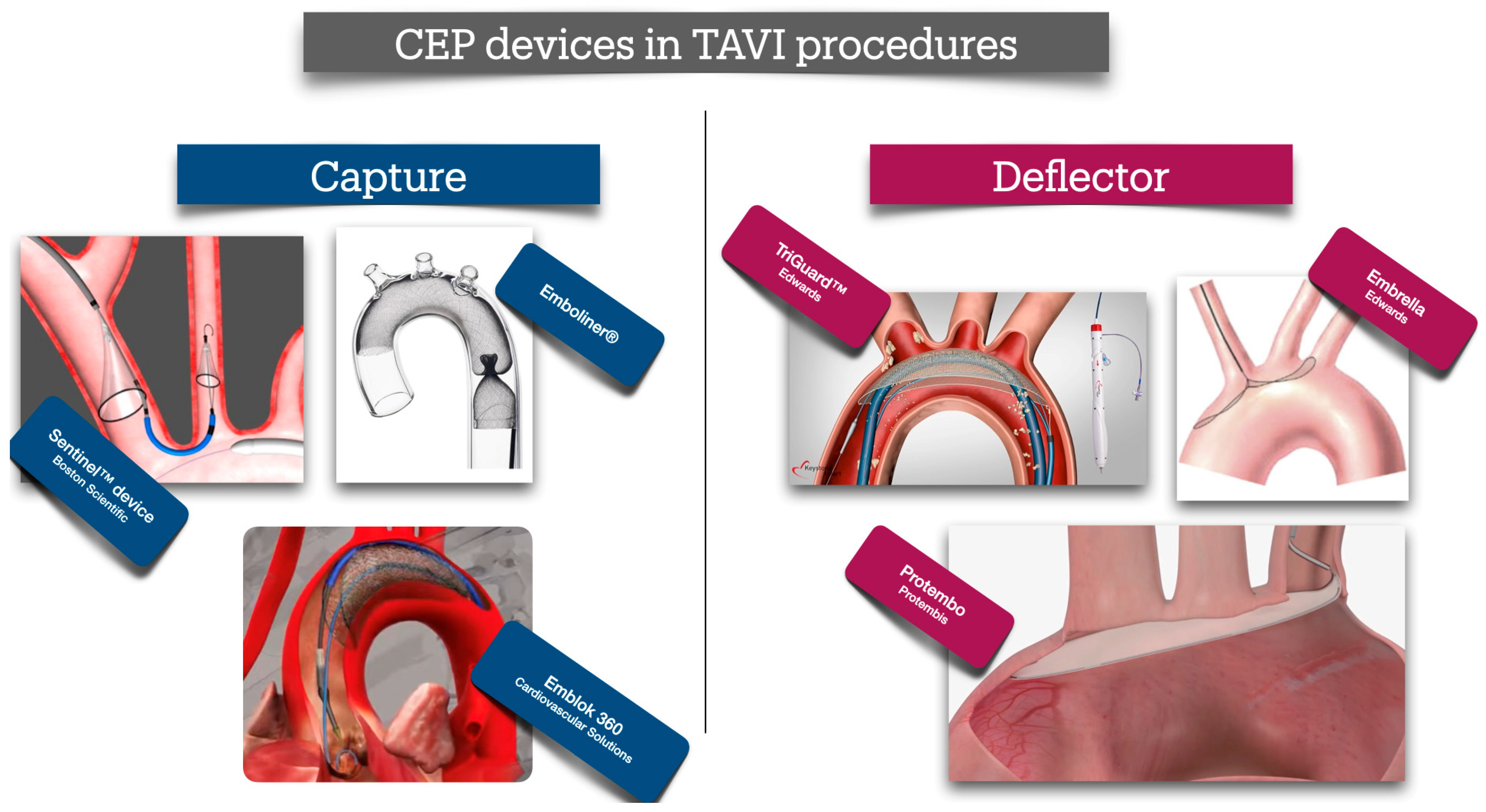
12. Devices to Reduce Periprocedural Stroke
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yadgir, S.; Johnson, C.O.; Aboyans, V.; Adebayo, O.M.; Adedoyin, R.A.; Afarideh, M.; Alahdab, F.; Alashi, A.; Alipour, V.; Arabloo, J.; et al. Global, Regional, and National Burden of Calcific Aortic Valve and Degenerative Mitral Valve Diseases, 1990–2017. Circulation 2020, 141, 1670–1680. [Google Scholar] [CrossRef]
- Leon, M.B.; Smith, C.R.; Mack, M.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 2010, 363, 1597–1607. [Google Scholar] [CrossRef]
- Holmes, D.R., Jr.; Mack, M.J.; Kaul, S.; Agnihotri, A.; Alexander, K.P.; Bailey, S.R.; Calhoon, J.H.; Carabello, B.A.; Desai, M.Y.; Edwards, F.H.; et al. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement: Developed in collaboration with the American Heart Association, American Society of Echocardiography, European Association for Cardio-Thoracic Surgery, Heart Failure Society of America, Mended Hearts, Society of Cardiovascular Anesthesiologists, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. Ann. Thorac. Surg. 2012, 93, 1340–1395. [Google Scholar] [CrossRef] [PubMed]
- Nombela-Franco, L.; Webb, J.G.; de Jaegere, P.P.; Toggweiler, S.; Nuis, R.J.; Dager, A.E.; Amat-Santos, I.J.; Cheung, A.; Ye, J.; Binder, R.K.; et al. Timing, predictive factors, and prognostic value of cerebrovascular events in a large cohort of patients undergoing transcatheter aortic valve implantation. Circulation 2012, 126, 3041–3053. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.M.; Shahian, D.M.; Filardo, G.; Ferraris, V.A.; Haan, C.K.; Rich, J.B.; Normand, S.L.; DeLong, E.R.; Shewan, C.M.; Dokholyan, R.S.; et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: Part 2—Isolated valve surgery. Ann. Thorac. Surg. 2009, 88 (Suppl. S1), S23–S42. [Google Scholar] [CrossRef]
- Messe, S.R.; Acker, M.A.; Kasner, S.E.; Fanning, M.; Giovannetti, T.; Ratcliffe, S.J.; Bilello, M.; Szeto, W.Y.; Bavaria, J.E.; Hargrove, W.C., 3rd; et al. Stroke after aortic valve surgery: Results from a prospective cohort. Circulation 2014, 129, 2253–2261. [Google Scholar] [CrossRef]
- Kappetein, A.P.; Head, S.J.; Genereux, P.; Piazza, N.; van Mieghem, N.M.; Blackstone, E.H.; Brott, T.G.; Cohen, D.J.; Cutlip, D.E.; van Es, G.A.; et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The Valve Academic Research Consortium-2 consensus document. J. Am. Coll. Cardiol. 2012, 60, 1438–1454. [Google Scholar] [CrossRef]
- Erdoes, G.; Basciani, R.; Huber, C.; Stortecky, S.; Wenaweser, P.; Windecker, S.; Carrel, T.; Eberle, B. Transcranial Doppler-detected cerebral embolic load during transcatheter aortic valve implantation. Eur. J. Cardiothorac. Surg. 2012, 41, 778–783. [Google Scholar] [CrossRef]
- Kahlert, P.; Al-Rashid, F.; Dottger, P.; Mori, K.; Plicht, B.; Wendt, D.; Bergmann, L.; Kottenberg, E.; Schlamann, M.; Mummel, P.; et al. Cerebral embolization during transcatheter aortic valve implantation: A transcranial Doppler study. Circulation 2012, 126, 1245–1255. [Google Scholar] [CrossRef]
- Lansky, A.J.; Grubman, D.; Dwyer, M.G., 3rd; Zivadinov, R.; Parise, H.; Moses, J.W.; Shah, T.; Pietras, C.; Tirziu, D.; Gambone, L.; et al. Clinical Significance of Diffusion-Weighted Brain MRI Lesions After TAVR: Results of a Patient-Level Pooled Analysis. J. Am. Coll. Cardiol. 2024, 84, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Eggebrecht, H.; Schmermund, A.; Voigtlander, T.; Kahlert, P.; Erbel, R.; Mehta, R.H. Risk of stroke after transcatheter aortic valve implantation (TAVI): A meta-analysis of 10,037 published patients. EuroIntervention 2012, 8, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Vlastra, W.; Jimenez-Quevedo, P.; Tchetche, D.; Chandrasekhar, J.; de Brito, F.S., Jr.; Barbanti, M.; Kornowski, R.; Latib, A.; D’Onofrio, A.; Ribichini, F.; et al. Predictors, Incidence, and Outcomes of Patients Undergoing Transfemoral Transcatheter Aortic Valve Implantation Complicated by Stroke. Circ. Cardiovasc. Interv. 2019, 12, e007546. [Google Scholar] [CrossRef]
- Okuno, T.; Alaour, B.; Heg, D.; Tueller, D.; Pilgrim, T.; Muller, O.; Noble, S.; Jeger, R.; Reuthebuch, O.; Toggweiler, S.; et al. Long-Term Risk of Stroke After Transcatheter Aortic Valve Replacement: Insights from the SwissTAVI Registry. JACC Cardiovasc. Interv. 2023, 16, 2986–2996. [Google Scholar] [CrossRef]
- Almarzooq, Z.I.; Kazi, D.S.; Wang, Y.; Chung, M.; Tian, W.; Strom, J.B.; Baron, S.J.; Yeh, R.W. Outcomes of stroke events during transcatheter aortic valve implantation. EuroIntervention 2022, 18, e335–e344. [Google Scholar] [CrossRef]
- Omran, H.; Schmidt, H.; Hackenbroch, M.; Illien, S.; Bernhardt, P.; von der Recke, G.; Fimmers, R.; Flacke, S.; Layer, G.; Pohl, C.; et al. Silent and apparent cerebral embolism after retrograde catheterisation of the aortic valve in valvular stenosis: A prospective, randomised study. Lancet 2003, 361, 1241–1246. [Google Scholar] [CrossRef]
- Rodes-Cabau, J.; Dumont, E.; Boone, R.H.; Larose, E.; Bagur, R.; Gurvitch, R.; Bedard, F.; Doyle, D.; De Larochelliere, R.; Jayasuria, C.; et al. Cerebral embolism following transcatheter aortic valve implantation: Comparison of transfemoral and transapical approaches. J. Am. Coll. Cardiol. 2011, 57, 18–28. [Google Scholar] [CrossRef]
- Katayama, T.; Yokoyama, N.; Watanabe, Y.; Takahashi, S.; Hioki, H.; Kawasugi, K.; Kozuma, K. Blood Coagulation Changes With or Without Direct Oral Anticoagulant Therapy Following Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2021, 147, 88–93. [Google Scholar] [CrossRef]
- Mourikis, P.; Dannenberg, L.; Zako, S.; Helten, C.; M’Pembele, R.; Richter, H.; Hohlfeld, T.; Jung, C.; Zeus, T.; Kelm, M.; et al. Impact of Transcatheter Aortic Valve Implantation on Thrombin Generation and Platelet Function. Thromb. Haemost. 2021, 121, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, A.O.; Gherasim, L.; Vinereanu, D. Risk of Stroke After Transcatheter Aortic Valve Implantation: Epidemiology, Mechanism, and Management. Am. J. Ther. 2021, 28, e560–e572. [Google Scholar] [CrossRef]
- Seppelt, P.C.; De Rosa, R.; Mas-Peiro, S.; Zeiher, A.M.; Vasa-Nicotera, M. Early hemodynamic changes after transcatheter aortic valve implantation in patients with severe aortic stenosis measured by invasive pressure volume loop analysis. Cardiovasc. Interv. Ther. 2022, 37, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Athappan, G.; Gajulapalli, R.D.; Sengodan, P.; Bhardwaj, A.; Ellis, S.G.; Svensson, L.; Tuzcu, E.M.; Kapadia, S.R. Influence of transcatheter aortic valve replacement strategy and valve design on stroke after transcatheter aortic valve replacement: A meta-analysis and systematic review of literature. J. Am. Coll. Cardiol. 2014, 63, 2101–2110. [Google Scholar] [CrossRef]
- Seppelt, P.C.; Mas-Peiro, S.; De Rosa, R.; Dimitriasis, Z.; Zeiher, A.M.; Vasa-Nicotera, M. Thirty-day incidence of stroke after transfemoral transcatheter aortic valve implantation: Meta-analysis and mixt-treatment comparison of self-expandable versus balloon-expandable valve prostheses. Clin. Res. Cardiol. 2021, 110, 640–648. [Google Scholar] [CrossRef]
- Nanjudappa, A.; Bhagat, A.; Bates, M.C. A rare cause of stroke after transcatheter aortic valve replacement: Retained foreign body. Catheter. Cardiovasc. Interv. 2019, 94, 870–873. [Google Scholar] [CrossRef]
- Ryan, T.; Grindal, A.; Jinah, R.; Um, K.J.; Vadakken, M.E.; Pandey, A.; Jaffer, I.H.; Healey, J.S.; Belley-Cote, E.P.; McIntyre, W.F. New-Onset Atrial Fibrillation After Transcatheter Aortic Valve Replacement: A Systematic Review and Meta-Analysis. JACC Cardiovasc. Interv. 2022, 15, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Linke, A.; Hollriegel, R.; Walther, T.; Schierle, K.; Wittekind, C.; Ender, J.; Mohr, F.W.; Schuler, G. Ingrowths of a percutaneously implanted aortic valve prosthesis (corevalve) in a patient with severe aortic stenosis. Circ. Cardiovasc. Interv. 2008, 1, 155–158. [Google Scholar] [CrossRef][Green Version]
- Tay, E.L.; Gurvitch, R.; Wijesinghe, N.; Nietlispach, F.; Wood, D.; Cheung, A.; Ye, J.; Lichtenstein, S.V.; Carere, R.; Thompson, C.; et al. A high-risk period for cerebrovascular events exists after transcatheter aortic valve implantation. JACC Cardiovasc. Interv. 2011, 4, 1290–1297. [Google Scholar] [CrossRef]
- Foley, M.; Hall, K.; Howard, J.P.; Ahmad, Y.; Gandhi, M.; Mahboobani, S.; Okafor, J.; Rahman, H.; Hadjiloizou, N.; Ruparelia, N.; et al. Aortic Valve Calcium Score Is Associated with Acute Stroke in Transcatheter Aortic Valve Replacement Patients. J. Soc. Cardiovasc. Angiogr. Interv. 2022, 1, 100349. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Dai, H.; Sheng, W.; Zheng, R.; Fan, J.; Yidilisi, A.; Aihemaiti, A.; Liu, Q.; Chen, J.; He, Y.; et al. Evaluation of aortic arch calcification to predict prognosis after transcatheter aortic valve replacement. Sci. Rep. 2025, 15, 6396. [Google Scholar] [CrossRef] [PubMed]
- Bayar, N.; Erkal, Z.; Koklu, E.; Guven, R.; Arslan, S. Increased Intima-Media Thickness of the Ascending Aorta May Predict Neurological Complications Associated with TAVI. J. Stroke Cerebrovasc. Dis. 2021, 30, 105665. [Google Scholar] [CrossRef]
- Huded, C.P.; Tuzcu, E.M.; Krishnaswamy, A.; Mick, S.L.; Kleiman, N.S.; Svensson, L.G.; Carroll, J.; Thourani, V.H.; Kirtane, A.J.; Manandhar, P.; et al. Association Between Transcatheter Aortic Valve Replacement and Early Postprocedural Stroke. JAMA 2019, 321, 2306–2315. [Google Scholar] [CrossRef]
- Jimenez Diaz, V.A.; Tello-Montoliu, A.; Moreno, R.; Cruz Gonzalez, I.; Baz Alonso, J.A.; Romaguera, R.; Molina Navarro, E.; Juan Salvadores, P.; Paredes Galan, E.; De Miguel Castro, A.; et al. Assessment of Platelet REACtivity After Transcatheter Aortic Valve Replacement: The REAC-TAVI Trial. JACC Cardiovasc. Interv. 2019, 12, 22–32. [Google Scholar] [CrossRef]
- Hatoum, H.; Gooden, S.C.M.; Sathananthan, J.; Sellers, S.; Kutting, M.; Marx, P.; Lilly, S.M.; Ihdayhid, A.R.; Thourani, V.H.; Dasi, L.P. Neosinus and Sinus Flow After Self-Expanding and Balloon-Expandable Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2021, 14, 2657–2666. [Google Scholar] [CrossRef]
- Taube, L.; Sugiura, A.; Hartmann, A.; Schaefer, C.; Hamiko, M.; Zimmer, S.; Nickenig, G.; Schahab, N. Carotid Artery Stenosis as a Predictor for Stroke Following Transcatheter Aortic Valve Implantation. Angiology 2024, 76, 33197241239687. [Google Scholar] [CrossRef] [PubMed]
- Seppelt, P.C.; Mas-Peiro, S.; De Rosa, R.; Murray, I.M.; Arsalan, M.; Holzer, L.; Lotz, G.; Meybohm, P.; Zacharowski, K.; Walther, T.; et al. Dynamics of cerebral oxygenation during rapid ventricular pacing and its impact on outcome in transfemoral transcatheter aortic valve implantation. Catheter. Cardiovasc. Interv. 2021, 97, E146–E153. [Google Scholar] [CrossRef]
- Tchetche, D.; Farah, B.; Misuraca, L.; Pierri, A.; Vahdat, O.; Lereun, C.; Dumonteil, N.; Modine, T.; Laskar, M.; Eltchaninoff, H.; et al. Cerebrovascular events post-transcatheter aortic valve replacement in a large cohort of patients: A FRANCE-2 registry substudy. JACC Cardiovasc. Interv. 2014, 7, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.D.; Vemulapalli, S.; Dai, D.; Matsouaka, R.; Blackstone, E.; Edwards, F.; Masoudi, F.A.; Mack, M.; Peterson, E.D.; Holmes, D.; et al. Procedural Experience for Transcatheter Aortic Valve Replacement and Relation to Outcomes: The STS/ACC TVT Registry. J. Am. Coll. Cardiol. 2017, 70, 29–41. [Google Scholar] [CrossRef]
- Kikuchi, S.; Trimaille, A.; Carmona, A.; Truong, D.P.; Matsushita, K.; Marchandot, B.; Granier, A.; Reydel, A.; Vu, M.C.; Zheng, F.; et al. Protruding and Ulcerated Aortic Atheromas as Predictors of Periprocedural Ischemic Stroke Post-Transcatheter Aortic Valve Replacement. JACC Asia 2025, 5, 258–269. [Google Scholar] [CrossRef]
- Ranasinghe, M.P.; Peter, K.; McFadyen, J.D. Thromboembolic and Bleeding Complications in Transcatheter Aortic Valve Implantation: Insights on Mechanisms, Prophylaxis and Therapy. J. Clin. Med. 2019, 8, 280. [Google Scholar] [CrossRef]
- Lenders, G.D.; Paelinck, B.P.; Wouters, K.; Claeys, M.J.; Rodrigus, I.E.; Van Herck, P.L.; Vrints, C.J.; Bosmans, J.M. Transesophageal echocardiography for cardiac thromboembolic risk assessment in patients with severe, symptomatic aortic valve stenosis referred for potential transcatheter aortic valve implantation. Am. J. Cardiol. 2013, 111, 1470–1474. [Google Scholar] [CrossRef]
- Tarantini, G.; Mojoli, M.; Urena, M.; Vahanian, A. Atrial fibrillation in patients undergoing transcatheter aortic valve implantation: Epidemiology, timing, predictors, and outcome. Eur. Heart J. 2017, 38, 1285–1293. [Google Scholar] [CrossRef]
- Amat-Santos, I.J.; Rodes-Cabau, J.; Urena, M.; DeLarochelliere, R.; Doyle, D.; Bagur, R.; Villeneuve, J.; Cote, M.; Nombela-Franco, L.; Philippon, F.; et al. Incidence, predictive factors, and prognostic value of new-onset atrial fibrillation following transcatheter aortic valve implantation. J. Am. Coll. Cardiol. 2012, 59, 178–188. [Google Scholar] [CrossRef]
- Sannino, A.; Gargiulo, G.; Schiattarella, G.G.; Perrino, C.; Stabile, E.; Losi, M.A.; Galderisi, M.; Izzo, R.; de Simone, G.; Trimarco, B.; et al. A meta-analysis of the impact of pre-existing and new-onset atrial fibrillation on clinical outcomes in patients undergoing transcatheter aortic valve implantation. EuroIntervention 2016, 12, e1047–e1056. [Google Scholar] [CrossRef]
- Vavuranakis, M.; Kolokathis, A.M.; Vrachatis, D.A.; Kalogeras, K.; Magkoutis, N.A.; Fradi, S.; Ghostine, S.; Karamanou, M.; Tousoulis, D. Atrial Fibrillation During or After TAVI: Incidence, Implications and Therapeutical Considerations. Curr. Pharm. Des. 2016, 22, 1896–1903. [Google Scholar] [CrossRef]
- Yoon, Y.H.; Ahn, J.M.; Kang, D.Y.; Ko, E.; Lee, P.H.; Lee, S.W.; Kim, H.J.; Kim, J.B.; Choo, S.J.; Park, D.W.; et al. Incidence, Predictors, Management, and Clinical Significance of New-Onset Atrial Fibrillation After Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2019, 123, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- van Nieuwkerk, A.C.; Aarts, H.M.; Hemelrijk, K.I.; Urbano Carrillo, C.; Tchetche, D.; de Brito, F.S., Jr.; Barbanti, M.; Kornowski, R.; Latib, A.; D’Onofrio, A.; et al. Cerebrovascular Events in Patients Undergoing Transfemoral Transcatheter Aortic Valve Implantation: A Pooled Patient-Level Study. J. Am. Heart Assoc. 2024, 13, e032901. [Google Scholar] [CrossRef]
- van Bergeijk, K.H.; van Ginkel, D.J.; Brouwer, J.; Nijenhuis, V.J.; van der Werf, H.W.; van den Heuvel, A.F.M.; Voors, A.A.; Wykrzykowska, J.J.; Ten Berg, J.M. Sex Differences in Outcomes After Transcatheter Aortic Valve Replacement: A POPular TAVI Subanalysis. JACC Cardiovasc. Interv. 2023, 16, 1095–1102. [Google Scholar] [CrossRef]
- Makkar, R.R.; Fontana, G.; Jilaihawi, H.; Chakravarty, T.; Kofoed, K.F.; De Backer, O.; Asch, F.M.; Ruiz, C.E.; Olsen, N.T.; Trento, A.; et al. Possible Subclinical Leaflet Thrombosis in Bioprosthetic Aortic Valves. N. Engl. J. Med. 2015, 373, 2015–2024. [Google Scholar] [CrossRef]
- Chakravarty, T.; Sondergaard, L.; Friedman, J.; De Backer, O.; Berman, D.; Kofoed, K.F.; Jilaihawi, H.; Shiota, T.; Abramowitz, Y.; Jorgensen, T.H.; et al. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: An observational study. Lancet 2017, 389, 2383–2392. [Google Scholar] [CrossRef] [PubMed]
- Apor, A.; Bartykowszki, A.; Szilveszter, B.; Varga, A.; Suhai, F.I.; Manouras, A.; Molnar, L.; Jermendy, A.L.; Panajotu, A.; Turani, M.F.; et al. Subclinical leaflet thrombosis after transcatheter aortic valve implantation is associated with silent brain injury on brain magnetic resonance imaging. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 1584–1595. [Google Scholar] [CrossRef]
- Sannino, A.; Hahn, R.T.; Leipsic, J.; Mack, M.J.; Grayburn, P.A. Meta-analysis of Incidence, Predictors and Consequences of Clinical and Subclinical Bioprosthetic Leaflet Thrombosis After Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2020, 132, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Rheude, T.; Pellegrini, C.; Stortecky, S.; Marwan, M.; Xhepa, E.; Ammon, F.; Pilgrim, T.; Mayr, N.P.; Husser, O.; Achenbach, S.; et al. Meta-Analysis of Bioprosthetic Valve Thrombosis After Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2021, 138, 92–99. [Google Scholar] [CrossRef]
- Rodes-Cabau, J.; Masson, J.B.; Welsh, R.C.; Garcia Del Blanco, B.; Pelletier, M.; Webb, J.G.; Al-Qoofi, F.; Genereux, P.; Maluenda, G.; Thoenes, M.; et al. Aspirin Versus Aspirin Plus Clopidogrel as Antithrombotic Treatment Following Transcatheter Aortic Valve Replacement with a Balloon-Expandable Valve: The ARTE (Aspirin Versus Aspirin + Clopidogrel Following Transcatheter Aortic Valve Implantation) Randomized Clinical Trial. JACC Cardiovasc. Interv. 2017, 10, 1357–1365. [Google Scholar] [CrossRef]
- Dangas, G.D.; Tijssen, J.G.P.; Wohrle, J.; Sondergaard, L.; Gilard, M.; Mollmann, H.; Makkar, R.R.; Herrmann, H.C.; Giustino, G.; Baldus, S.; et al. A Controlled Trial of Rivaroxaban after Transcatheter Aortic-Valve Replacement. N. Engl. J. Med. 2020, 382, 120–129. [Google Scholar] [CrossRef]
- Nijenhuis, V.J.; Brouwer, J.; Delewi, R.; Hermanides, R.S.; Holvoet, W.; Dubois, C.L.F.; Frambach, P.; De Bruyne, B.; van Houwelingen, G.K.; Van Der Heyden, J.A.S.; et al. Anticoagulation with or without Clopidogrel after Transcatheter Aortic-Valve Implantation. N. Engl. J. Med. 2020, 382, 1696–1707. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.P.; Van Belle, E.; Thiele, H.; Berti, S.; Lhermusier, T.; Manigold, T.; Neumann, F.J.; Gilard, M.; Attias, D.; Beygui, F.; et al. Apixaban vs. standard of care after transcatheter aortic valve implantation: The ATLANTIS trial. Eur. Heart J. 2022, 43, 2783–2797. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, J.; Nijenhuis, V.J.; Delewi, R.; Hermanides, R.S.; Holvoet, W.; Dubois, C.L.F.; Frambach, P.; De Bruyne, B.; van Houwelingen, G.K.; Van Der Heyden, J.A.S.; et al. Aspirin with or without Clopidogrel after Transcatheter Aortic-Valve Implantation. N. Engl. J. Med. 2020, 383, 1447–1457. [Google Scholar] [CrossRef] [PubMed]
- Bogyi, M.; Schernthaner, R.E.; Loewe, C.; Gager, G.M.; Dizdarevic, A.M.; Kronberger, C.; Postula, M.; Legutko, J.; Velagapudi, P.; Hengstenberg, C.; et al. Subclinical Leaflet Thrombosis After Transcatheter Aortic Valve Replacement: A Meta-Analysis. JACC Cardiovasc. Interv. 2021, 14, 2643–2656. [Google Scholar] [CrossRef]
- Badhiwala, J.H.; Nassiri, F.; Alhazzani, W.; Selim, M.H.; Farrokhyar, F.; Spears, J.; Kulkarni, A.V.; Singh, S.; Alqahtani, A.; Rochwerg, B.; et al. Endovascular Thrombectomy for Acute Ischemic Stroke: A Meta-analysis. JAMA 2015, 314, 1832–1843. [Google Scholar] [CrossRef]
- Levi, A.; Linder, M.; Seiffert, M.; Witberg, G.; Pilgrim, T.; Tomii, D.; Talmor-Barkan, Y.; Van Mieghem, N.M.; Adrichem, R.; Codner, P.; et al. Management and Outcome of Acute Ischemic Stroke Complicating Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2022, 15, 1808–1819. [Google Scholar] [CrossRef]
- Guisado-Alonso, D.; Martinez-Domeno, A.; Prats-Sanchez, L.; Delgado-Mederos, R.; Camps-Renom, P.; Abilleira, S.; de la Ossa, N.P.; Ramos-Pachon, A.; Cardona, P.; Rodriguez-Campello, A.; et al. Reasons for Not Performing Mechanical Thrombectomy: A Population-Based Study of Stroke Codes. Stroke 2021, 52, 2746–2753. [Google Scholar] [CrossRef]
- Coughlan, J.J.; Fleck, R.; O’Connor, C.; Crean, P. Mechanical thrombectomy of embolised native aortic valve post-TAVI. BMJ Case Rep. 2017, 2017, bcr2016218787. [Google Scholar] [CrossRef]
- D’Anna, L.; Demir, O.; Banerjee, S.; Malik, I. Intravenous Thrombolysis and Mechanical Thrombectomy in Patients with Stroke after TAVI: A Report of Two Cases. J. Stroke Cerebrovasc. Dis. 2019, 28, 104277. [Google Scholar] [CrossRef] [PubMed]
- Hammond-Haley, M.; Almohtadi, A.; Gonnah, A.R.; Raha, O.; Khokhar, A.; Hartley, A.; Khawaja, S.; Hadjiloizou, N.; Ruparelia, N.; Mikhail, G.; et al. Management of Acute Ischemic Stroke Following Transcatheter Aortic Valve Implantation: A Systematic Review and Multidisciplinary Treatment Recommendations. J. Clin. Med. 2024, 13, 7437. [Google Scholar] [CrossRef]
- Stabile, E.; Giugliano, G.; Cremonesi, A.; Bosiers, M.; Reimers, B.; Setacci, C.; Cao, P.; Schmidt, A.; Sievert, H.; Peeters, P.; et al. Impact on outcome of different types of carotid stent: Results from the European Registry of Carotid Artery Stenting. EuroIntervention 2016, 12, e265–e270. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, D.; Fan, J.; Dai, H.; Zhu, G.; Chen, J.; Guo, Y.; Yidilisi, A.; Zhu, Q.; He, Y.; et al. Cerebral Ischemic Lesions after Transcatheter Aortic Valve Implantation in Patients with Non-Calcific Aortic Stenosis. J. Clin. Med. 2022, 11, 6502. [Google Scholar] [CrossRef]
- Kapadia, S.R.; Makkar, R.; Leon, M.; Abdel-Wahab, M.; Waggoner, T.; Massberg, S.; Rottbauer, W.; Horr, S.; Sondergaard, L.; Karha, J.; et al. Cerebral Embolic Protection during Transcatheter Aortic-Valve Replacement. N. Engl. J. Med. 2022, 387, 1253–1263. [Google Scholar] [CrossRef]
- Butala, N.M.; Makkar, R.; Secemsky, E.A.; Gallup, D.; Marquis-Gravel, G.; Kosinski, A.S.; Vemulapalli, S.; Valle, J.A.; Bradley, S.M.; Chakravarty, T.; et al. Cerebral Embolic Protection and Outcomes of Transcatheter Aortic Valve Replacement: Results from the Transcatheter Valve Therapy Registry. Circulation 2021, 143, 2229–2240. [Google Scholar] [CrossRef] [PubMed]
- Kharbanda, R.K.; Kennedy, J.; Jamal, Z.; Dodd, M.; Evans, R.; Bal, K.K.; Perkins, A.D.; Blackman, D.J.; Hildick-Smith, D.; Banning, A.P.; et al. Routine Cerebral Embolic Protection during Transcatheter Aortic-Valve Implantation. N. Engl. J. Med. 2025, 392, 2403–2412. [Google Scholar] [CrossRef]
- Latib, A.; Mangieri, A.; Vezzulli, P.; Spagnolo, P.; Sardanelli, F.; Fellegara, G.; Pagnesi, M.; Giannini, F.; Falini, A.; Gorla, R.; et al. First-in-Man Study Evaluating the Emblok Embolic Protection System During Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2020, 13, 860–868. [Google Scholar] [CrossRef]
- Grubman, D.; Ahmad, Y.; Leipsic, J.A.; Blanke, P.; Pasupati, S.; Webster, M.; Nazif, T.M.; Parise, H.; Lansky, A.J. Predictors of Cerebral Embolic Debris During Transcatheter Aortic Valve Replacement: The SafePass 2 First-in-Human Study. Am. J. Cardiol. 2023, 207, 28–34. [Google Scholar] [CrossRef]
- Jagielak, D.; Targonski, R.; Frerker, C.; Abdel-Wahab, M.; Wilde, J.; Werner, N.; Lauterbach, M.; Leick, J.; Grygier, M.; Misterski, M.; et al. Safety and performance of a novel cerebral embolic protection device for transcatheter aortic valve implantation: The PROTEMBO C Trial. EuroIntervention 2022, 18, 590–597. [Google Scholar] [CrossRef]
- Fezzi, S.; Jagielak, D.; Targonski, R.; Schmidt, T.; Frerker, C.; Witkowski, A.R.; Lauterbach, M.; Leick, J.; Erglis, A.; Narbute, I.; et al. Final report of the PROTEMBO C Trial: A prospective evaluation of a novel cerebral protection device during TAVI. EuroIntervention 2024, 20, e264–e267. [Google Scholar] [CrossRef]
- Carpenter, J.P.; Carpenter, J.T.; Tellez, A.; Webb, J.G.; Yi, G.H.; Granada, J.F. A percutaneous aortic device for cerebral embolic protection during cardiovascular intervention. J. Vasc. Surg. 2011, 54, 174–181.e1. [Google Scholar] [CrossRef][Green Version]
- Rodes-Cabau, J.; Kahlert, P.; Neumann, F.J.; Schymik, G.; Webb, J.G.; Amarenco, P.; Brott, T.; Garami, Z.; Gerosa, G.; Lefevre, T.; et al. Feasibility and exploratory efficacy evaluation of the Embrella Embolic Deflector system for the prevention of cerebral emboli in patients undergoing transcatheter aortic valve replacement: The PROTAVI-C pilot study. JACC Cardiovasc. Interv. 2014, 7, 1146–1155. [Google Scholar] [CrossRef]
- Samim, M.; Agostoni, P.; Hendrikse, J.; Budde, R.P.; Nijhoff, F.; Kluin, J.; Ramjankhan, F.; Doevendans, P.A.; Stella, P.R. Embrella embolic deflection device for cerebral protection during transcatheter aortic valve replacement. J. Thorac. Cardiovasc. Surg. 2015, 149, 799–805.e1-2. [Google Scholar] [CrossRef][Green Version]
- Baumbach, A.; Mullen, M.; Brickman, A.M.; Aggarwal, S.K.; Pietras, C.G.; Forrest, J.K.; Hildick-Smith, D.; Meller, S.M.; Gambone, L.; den Heijer, P.; et al. Safety and performance of a novel embolic deflection device in patients undergoing transcatheter aortic valve replacement: Results from the DEFLECT I study. EuroIntervention 2015, 11, 75–84. [Google Scholar] [CrossRef]
- Samim, M.; van der Worp, B.; Agostoni, P.; Hendrikse, J.; Budde, R.P.; Nijhoff, F.; Ramjankhan, F.; Doevendans, P.A.; Stella, P.R. TriGuardTM HDH embolic deflection device for cerebral protection during transcatheter aortic valve replacement. Catheter. Cardiovasc. Interv. 2017, 89, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Lansky, A.J.; Makkar, R.; Nazif, T.; Messe, S.; Forrest, J.; Sharma, R.; Schofer, J.; Linke, A.; Brown, D.; Dhoble, A.; et al. A randomized evaluation of the TriGuard HDH cerebral embolic protection device to Reduce the Impact of Cerebral Embolic LEsions after TransCatheter Aortic Valve ImplanTation: The REFLECT I trial. Eur. Heart J. 2021, 42, 2670–2679. [Google Scholar] [CrossRef]
- Heuts, S.; Gabrio, A.; Veenstra, L.; Maesen, B.; Kats, S.; Maessen, J.G.; Walton, A.S.; Nanayakkara, S.; Lansky, A.J.; van ‘t Hof, A.W.J.; et al. Stroke reduction by cerebral embolic protection devices in transcatheter aortic valve implantation: A systematic review and Bayesian meta-analysis. Heart 2024, 110, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Warraich, N.; Sa, M.P.; Jacquemyn, X.; Kuno, T.; Serna-Gallegos, D.; Sultan, I. Cerebral Embolic Protection Devices in Transcatheter Aortic Valve Implantation: Meta-Analysis with Trial Sequential Analysis. J. Am. Heart Assoc. 2025, 14, e038869. [Google Scholar] [CrossRef]
- Armoundas, A.A.; Narayan, S.M.; Arnett, D.K.; Spector-Bagdady, K.; Bennett, D.A.; Celi, L.A.; Friedman, P.A.; Gollob, M.H.; Hall, J.L.; Kwitek, A.E.; et al. Use of Artificial Intelligence in Improving Outcomes in Heart Disease: A Scientific Statement from the American Heart Association. Circulation 2024, 149, e1028–e1050. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, R.M.; Hernandez, E.J.; Tristani-Firouzi, M.; Yandell, M.; Steinberg, B.A. Explainable artificial intelligence for stroke risk stratification in atrial fibrillation. Eur. Heart J.-Digit. Health 2025, 6, 317–325. [Google Scholar] [CrossRef] [PubMed]
| Early Stroke |
|
| Late Stroke |
|
| Device Name | Manufacturer | Type | Deployment Access | Cerebral Coverage | Key Characteristics |
|---|---|---|---|---|---|
| Sentinel CPS | Boston Scientific, St. Paul, MN, USA | Filter | Right radial artery (6 Fr sheath) | Brachiocephalic and left carotid arteries (partial; ~90% cerebral flow, excludes left vertebral artery) | Dual-filter system; captures debris; FDA-approved (2017); high technical success (94.5%); no significant stroke reduction in PROTECTED TAVR trial (RR 0.88, p = 0.566). |
| TriGuard 3 | Keystone Heart, Caesarea, Israel | Deflector | Femoral artery (9 Fr sheath) | All major cerebral arteries (innominate, left carotid, left subclavian) | Nitinol mesh (130-µm pores); deflects debris to descending aorta; improved cognitive outcomes in DEFLECT III; ongoing REFLECT trial (NCT02536196). |
| Embrella | Edwards Lifesciences, Irvine, CA, US | Deflector | Right radial artery (6 Fr sheath) | Brachiocephalic and left carotid arteries | Polyurethane membrane; deflects debris; higher lesion rates on MRI in some studies; not widely adopted. |
| Emboliner | Emboline, Inc., Santa Cruz, CA, USA | Filter | Femoral artery (9 Fr sheath) | Full cerebral (all supra-aortic branches) and non-cerebral vessels | Cylindrical nitinol mesh; captures 5× more debris (>150 µm) than Sentinel in SafePass 2; TAVR-focused, no CAS data; investigational. |
| ProtEmbo | Protembis GmbH, Aachen, Germany | Deflector | Left radial artery (6 Fr sheath) | All major cerebral arteries | Low-profile heparin-coated mesh; deflects debris; ongoing PROTEMBO C trial (NCT04205916); investigational. |
| Device | Study Design | Basic Results | Clinical Meaning |
|---|---|---|---|
| Sentinel CPS | Randomized controlled trial (n = 363 TAVI patients) | 94.5% technical success; reduced new cerebral lesion volume on MRI (p = 0.03); no significant stroke reduction at 30 days (5.6% vs. 9.1%, p = 0.25); debris captured in 99% of filters. | Reduced only the large strokes. Currently no indication for routine usage |
| TriGuard 3 | Randomized controlled trial (n = 258 TAVI patients) | 85.7% technical success; improved cognitive outcomes (p = 0.04); reduced total lesion volume on MRI (34% less, p = 0.057); no significant stroke reduction (8.3% vs. 11.4%, p = 0.48). | Unproven clinical benefit for stroke reduction. No indication for routine usage. |
| Embrella | Prospective pilot study (n = 52 TAVI patients) | 97.5% technical success; debris deflected in all cases; higher new lesion rates on MRI vs. controls (p = 0.02); no stroke reduction (10% vs. 11%, p = 0.89); limited adoption. | No randomized data. Decreased the size, yet increasing the number of lesions. Limit the new large lesions. No clinical benefit. No evidence for beneficial clinical use. |
| Emboliner | Prospective, non-randomized study (n = 24 TAVI patients) | 100% technical success; debris captured in 100% of cases (5× more particles >150 µm than Sentinel); no stroke rates reported; investigational, promising for full cerebral protection. | Easy to deploy, efficient to capture debris. No evidence for clinical application. |
| ProtEmbo | Prospective feasibility study (n = 30 TAVI patients) | 100% technical success; debris deflected in all cases; reduced lesion volume on MRI (p = 0.06); no stroke rates reported; ongoing PROTEMBO C trial | Successful deployment. Reduced lesion size. No clinical data on reducing clinically evident stroke complication |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikas, D.N.; Lakkas, L.; Nikopoulos, S.; Tsamis, K.; Sakellariou, X.; Florentin, M.; Papanagiotou, P.; Naka, K.K.; Ntaios, G.; Michalis, L. Stroke in Transcatheter Aortic Valve Implantation (TAVI): A Comprehensive Review. J. Clin. Med. 2025, 14, 6754. https://doi.org/10.3390/jcm14196754
Nikas DN, Lakkas L, Nikopoulos S, Tsamis K, Sakellariou X, Florentin M, Papanagiotou P, Naka KK, Ntaios G, Michalis L. Stroke in Transcatheter Aortic Valve Implantation (TAVI): A Comprehensive Review. Journal of Clinical Medicine. 2025; 14(19):6754. https://doi.org/10.3390/jcm14196754
Chicago/Turabian StyleNikas, Dimitrios N., Lampros Lakkas, Sotirios Nikopoulos, Konstantinos Tsamis, Xenofon Sakellariou, Matilda Florentin, Panagiotis Papanagiotou, Katerina K. Naka, George Ntaios, and Lampros Michalis. 2025. "Stroke in Transcatheter Aortic Valve Implantation (TAVI): A Comprehensive Review" Journal of Clinical Medicine 14, no. 19: 6754. https://doi.org/10.3390/jcm14196754
APA StyleNikas, D. N., Lakkas, L., Nikopoulos, S., Tsamis, K., Sakellariou, X., Florentin, M., Papanagiotou, P., Naka, K. K., Ntaios, G., & Michalis, L. (2025). Stroke in Transcatheter Aortic Valve Implantation (TAVI): A Comprehensive Review. Journal of Clinical Medicine, 14(19), 6754. https://doi.org/10.3390/jcm14196754







