Clinical Impact of External Carotid Artery Remodeling Following Carotid Artery Stenting
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Characteristics
2.2. Equipment
2.3. Analyzed Data
2.4. Statistical Analysis
3. Results
3.1. Patients’ Demographics and General Characteristics
3.2. Periprocedural Characteristics
3.3. Correlations
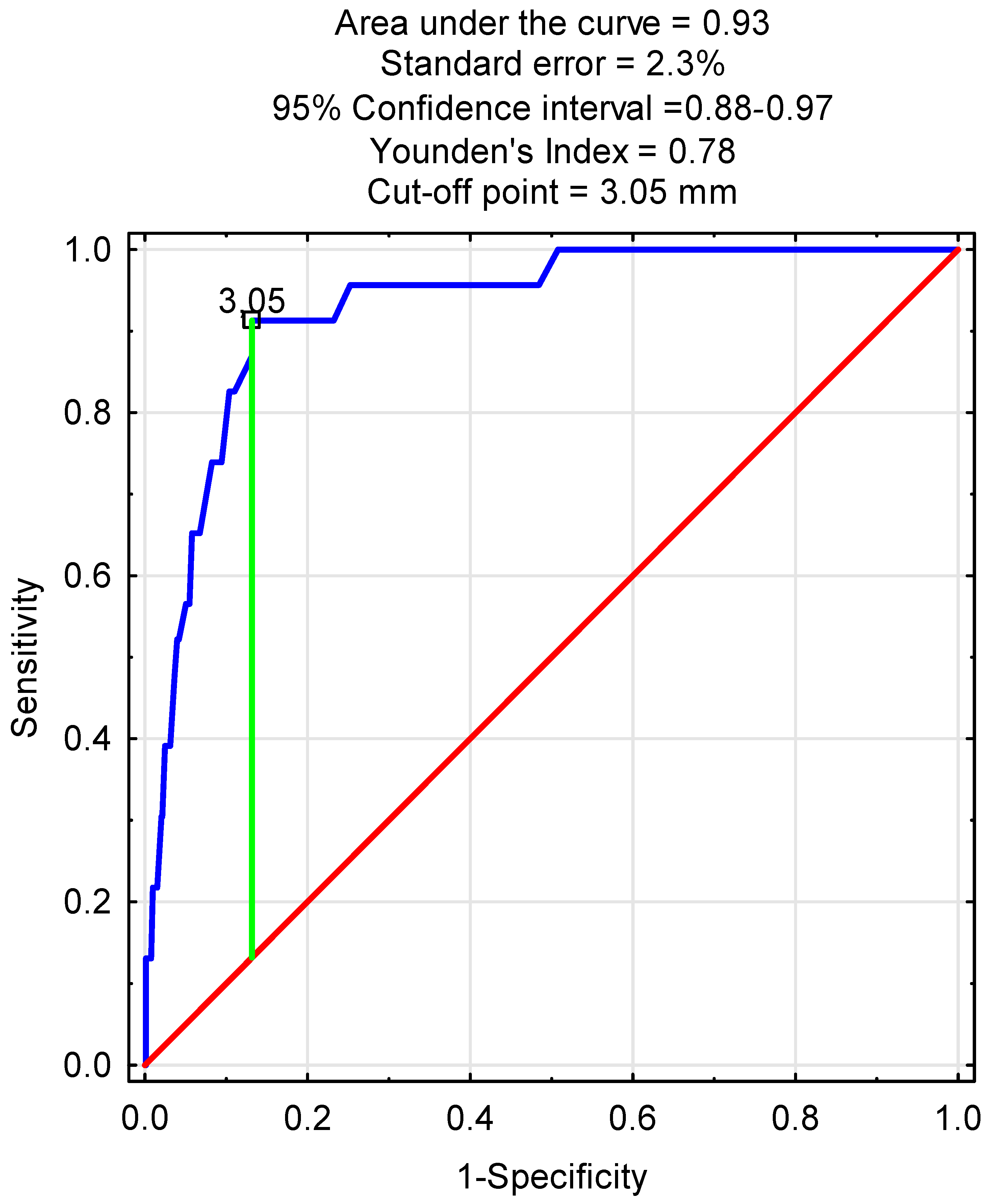
3.4. Predictive Factors for ECA Narrowing/Stenosis
3.5. Predictive Factors for ECA Occlusion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N. Engl. J. Med. 1991, 325, 445–453. [Google Scholar] [CrossRef]
- Yadav, J.S.; Wholey, M.H.; Kuntz, R.E.; Fayad, P.; Katzen, B.T.; Mishkel, G.J.; Bajwa, T.K.; Whitlow, P.; Strickman, N.E.; Jaff, M.R.; et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N. Engl. J. Med. 2004, 351, 1493–1501. [Google Scholar] [CrossRef]
- Setacci, C.; Chisci, E.; Setacci, F.; Iacoponi, F.; de Donato, G. Grading carotid artery stenosis using ultrasound criteria: A North American vs European perspective. Eur. J. Vasc. Endovasc. Surg. 2009, 37, 251–261. [Google Scholar] [CrossRef]
- Vos, J.A.; van den Berg, J.C. Carotid artery stenting: Current status and ongoing clinical trials. J. Cardiovasc. Surg. 2009, 50, 33–49. [Google Scholar]
- Elwertowski, M.; Leszczyński, J.; Kaszczewski, P.; Lamparski, K.; Yee Ho, S.S.; Gałązka, Z. The importance of blood flow volume in the brain-supplying arteries for the clinical management—The impact of collateral circulation. J. Ultrason. 2018, 18, 112–119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liebeskind, D.S. Collateral circulation. Stroke 2003, 34, 2279–2284. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.M.; Scott, M. Collateral circulation of the external carotid artery and the internal carotid artery through the ophthalmic artery in cases of internal carotid artery thrombosis; report of five cases. Radiology 1955, 65, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Łyko-Morawska, D.; Serafin, M.; Szostek, J.; Mąka, M.; Kania, I.; Kuczmik, W. Fate of External Carotid Artery Following Carotid Artery Stenting for Internal Carotid Artery near Occlusion. Biomedicines 2025, 13, 303. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; Itum, D.S.; Preiss, J.; Duwayri, Y.; Veeraswamy, R.K.; Salam, A.; Dodson, T.F.; Brewster, L.P. Carotid artery stenting has increased risk of external carotid artery occlusion compared with carotid endarterectomy. J. Vasc. Surg. 2015, 61, 119–124. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Woo, E.Y.; Karmacharya, J.; Velazquez, O.C.; Carpenter, J.P.; Skelly, C.L.; Fairman, R.M. Differential effects of carotid artery stenting versus carotid endarterectomy on external carotid artery patency. J. Endovasc. Ther. 2007, 14, 208–213. [Google Scholar] [CrossRef] [PubMed]
- de Borst, G.J.; Vos, J.A.; Reichmann, B.; Hellings, W.E.; de Vries, J.P.P.M.; Suttorp, M.J.; Moll, F.L.; Ackerstaff, R.G.A. The Fate of the External Carotid Artery after Carotid Artery Stenting. A Follow-up Study with Duplex Ultrasonography. Eur. J. Vasc. Endovasc. Surg. 2007, 33, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Fearn, S.J.; Picton, A.J.; Mortimer, A.J.; Parry, A.D.; McCollum, C.N. The Contribution of the External Carotid Artery to Cerebral Perfusion in Carotid Disease. J. Vasc. Surg. 2000, 31, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Diethrich, E.B.; Liddicoat, J.E.; McCutchen, J.J.; De Bakey, M.E. Surgical Significance of the External Carotid Artery in the Treatment of Cerebrovascular Insufficiency. J. Cardiovasc. Surg. 1968, 9, 213–223. [Google Scholar]
- Xu, D.S.; Abruzzo, T.A.; Albuquerque, F.C.; Dabus, G.; Eskandari, M.K.; Guterman, L.R.; Hage, Z.A.; Hurley, M.C.; Hanel, R.A.; Levy, E.I.; et al. External carotid artery stenting to treat patients with symptomatic ipsilateral internal carotid artery occlusion: A multicenter case series. Neurosurgery 2010, 67, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Liu, X.; Zhang, Y.; Zhang, N.; Ye, X.; Deng, X. Hemodynamic Impact of Stenting on Carotid Bifurcation: A Potential Role of the Stented Segment and External Carotid Artery. Comput. Math. Methods Med. 2021, 2021, 7604532. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Willfort-Ehringer, A.; Ahmadi, R.; Gruber, D.; Gschwandtner, M.E.; Haumer, A.; Heinz, G.; Lang, W.; Ehringer, H. Effect of Carotid Artery Stenting on the External Carotid Artery. J. Vasc. Surg. 2003, 38, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Casey, K.; Zhou, W.; Tedesco, M.M.; Al-Khatib, W.K.; Hernandez-Boussard, T.; Bech, F. Fate of the external carotid artery following carotid interventions. Int. J. Angiol. 2009, 18, 173–176. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reid, D.B.; Irshad, K.; Miller, S.; Reid, A.W.; Reid, W.; Diethrich, E.B. Endovascular Significance of the External Carotid Artery in the Treatment of Cerebrovascular Insufficiency. J. Endovasc. Ther. 2004, 11, 727–733. [Google Scholar] [CrossRef]
- Dong, H.; Jiang, X.; Zou, Y.; Chen, Y.; He, J.; Deng, Y.; Xu, B.; Gao, R. External carotid artery stenting in patients with ipsilateral internal carotid artery occlusion: Peri-operative and 12-month follow-up. Catheter. Cardiovasc. Interv. 2021, 97 (Suppl. S2), 982–987. [Google Scholar] [CrossRef]
- Rajani, R.R. How much should we know about the external carotid artery? J. Vasc. Surg. 2020, 72, 958. [Google Scholar] [CrossRef]
| Variable | n (%), Median (Range), IQR |
|---|---|
| Age (years) | 70 (45–93), IQR 9 |
| Gender | |
| Male | 623 (64.7%) |
| Female | 340 (35.3%) |
| Current cigarette smoking | 392 (40.7%) |
| Presence of comorbidities (yes) | 940 (97.6%) |
| Arterial hypertension | 899 (93.4%) |
| Dyslipidemia | 833 (86.5%) |
| Diabetes mellitus | 305 (31.7%) |
| Symptomatic ICA stenosis | 372 (38.6%) |
| Stroke | 300 (31.2%) |
| TIA | 85 (8.8%) |
| Other symptoms | |
| Dizziness | 192 (19.9%) |
| Headache | 86 (8.9%) |
| Syncope | 64 (6.7%) |
| Tinnitus | 25 (2.56%) |
| Variable | n (%), Mean/Median (Range) SD/IQR |
|---|---|
| Preoperative ICA diameter (mm) | 2.8 (0.4–9.2), IQR 1.6 |
| Postoperative ICA diameter (mm) | 6.6 (2.7–11) IQR 1.7 |
| Difference in post- and preoperative ICA diameters (mm) | 3.6 (0–8.6), IQR 1.9 |
| Side of the procedure | |
| Left ICA | 518 (53.8%) |
| Right ICA | 445 (46.2%) |
| Stent used | |
| 1st generation | 404 (41.9%) |
| 2nd generation | 559 (58.1%) |
| Stent length | |
| 60 mm | 1 (0.1%) |
| 50 mm | 1 (0.1%) |
| 40 mm | 652 (67.7%) |
| 30 mm | 309 (32.1%) |
| Stent width | |
| 6 mm | 2 (0.2%) |
| 7 mm | 285 (29.6%) |
| 8 mm | 435 (45.2%) |
| 9 mm | 177 (18.4%) |
| 10 mm | 64 (6.7%) |
| Covering of ECA orifice by a carotid stent | |
| Yes | 928 (96.4%) |
| No | 35 (3.6%) |
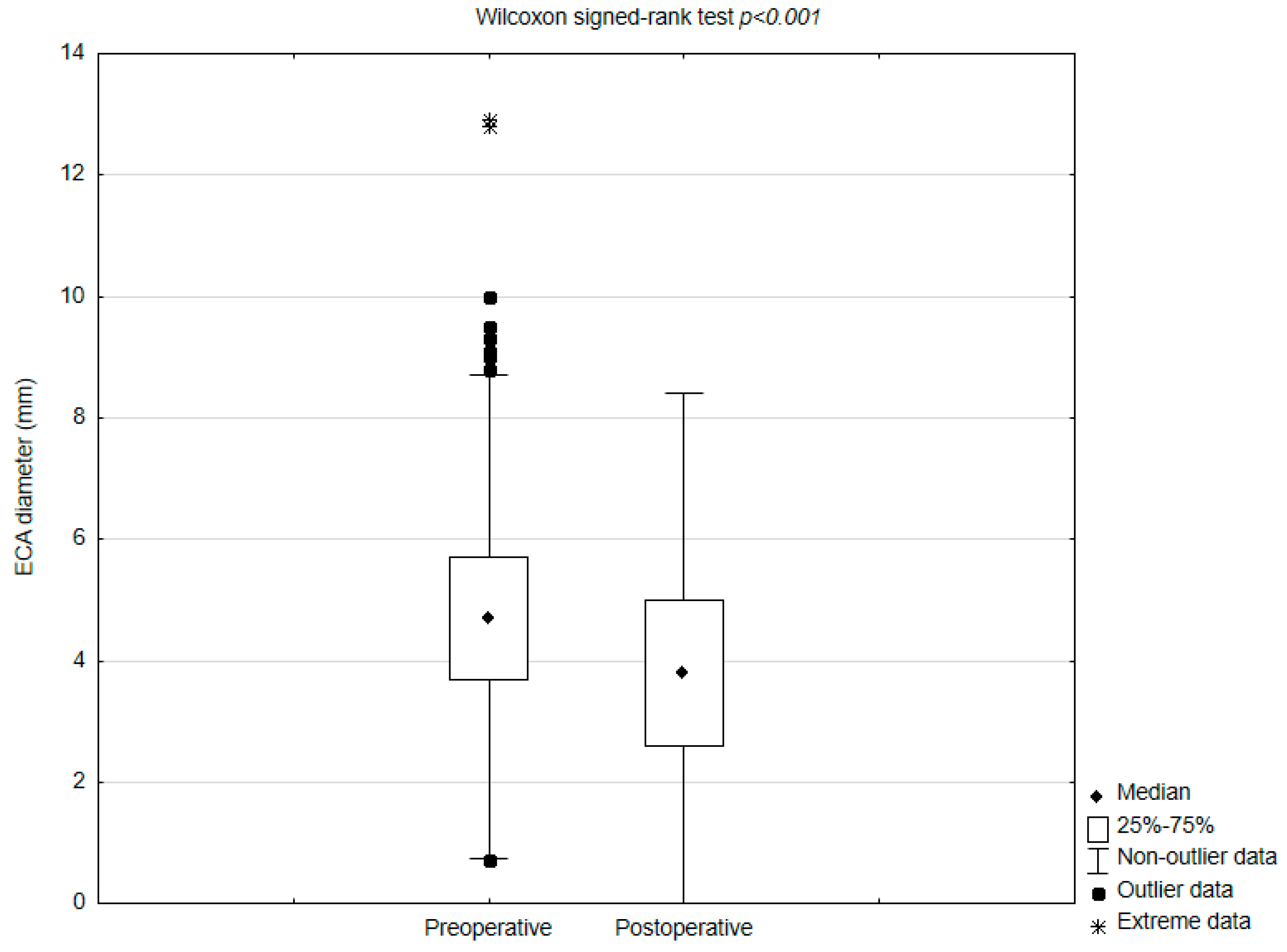
| ECA diameter before CAS (mm) | 4.7 (0.7–12.9), IQR 2 |
| ECA diameter after CAS (mm) | 3.8 (0–9.5), IQR 2.4 |
| Difference between pre- and postoperative ECA diameters (mm) | 0.8 (−2.7–6.7), IQR 1.7 |
| ECA fate after CAS (n = 928) | |
| Decrease in the diameter | 666 (71.7%) |
| ECA occlusion | 23 (2.5%) |
| No change in the diameter | 200 (21.6%) |
| Increase in the diameter | 62 (6.7%) |
| Patients’ clinical outcome | |
| Follow-up time (months) | 22.57 (0.03–80.2) SD 21.16 |
| Ipsilateral Stroke | 22 (2.3%) |
| 30-day stroke | 9 (0.9%) |
| Follow-up stroke | 13 (1.4%) |
| TIA | 19 (2%) |
| 30-day TIA | 14 (1.5%) |
| Follow-up TIA | 5 (0.5%) |
| MI | 29 (3%) |
| 30-day MI | 1 (0.1%) |
| Follow-up MI | 28 (2.9%) |
| In-stent restenosis | 26 (2.7%) |
| Mortality | 14 (1.5%) |
| 30-day mortality | 7 (0.7%) |
| Follow-up mortality | 7 (0.7%) |
| Correlations | R | p |
|---|---|---|
| Preoperative ICA/ECA diameter | 0.1 | |
| Preoperative ECA diameter/postoperative ICA diameter | 0.12 | <0.001 |
| Preoperative ICA diameter/postoperative ECA diameter | 0.13 | <0.001 |
| Postoperative ICA/ECA diameter | 0.17 | <0.001 |
| Preoperative ICA diameter/difference between post and -preoperative ECA diameter | −0.07 | 0.04 |
| Postoperative ICA diameter/difference between post and -preoperative ECA diameter | −0.07 | 0.03 |
| Preoperative ECA diameter/difference between post and -preoperative ECA diameter | 0.22 | <0.001 |
| Postoperative ECA diameter/difference between post and -preoperative ECA diameter | −0.44 | <0.001 |
| Univariate Analysis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|
| Variable | R2 | b | SE | p | b | SE | p |
| Predilatation | 0.004 | 0.19 | 0.09 | 0.04 | 0.1 | 0.09 | 0.26 |
| 2nd-generation stents | 0.006 | 0.19 | 0.08 | 0.02 | 0.17 | 0.08 | 0.02 |
| Stent width | 0.002 | 0.05 | 0.05 | 0.24 | |||
| Stent length | 0.001 | −0.01 | 0.01 | 0.29 | |||
| Preoperative ICA diameter | 0.005 | −0.07 | 0.03 | 0.03 | −0.10 | 0.03 | 0.001 |
| Preoperative ECA diameter | 0.097 | 0.22 | 0.02 | <0.001 | 0.23 | 0.023 | <0.001 |
| Univariate Analysis | |||
|---|---|---|---|
| Variable | OR | 95% CI | p |
| Predilatation | 0.38 | ||
| Yes | 1.50 | 0.61–3.7 | |
| No | 1 | ||
| Stent generation | 0.51 | ||
| 1st generation | 1.34 | 0.56–3.18 | |
| 2nd generation | 1 | ||
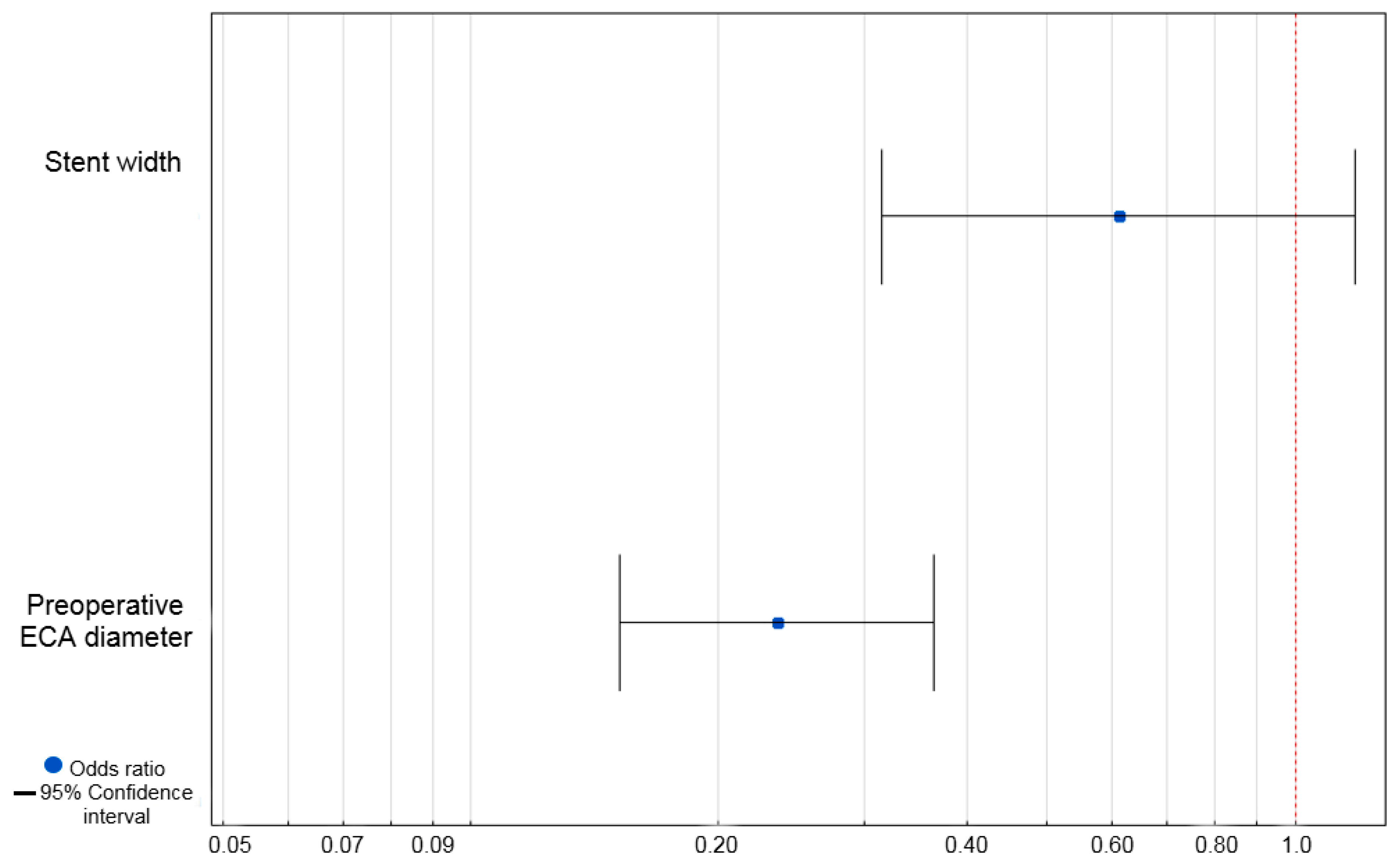
| Stent width | 0.56 | 0.32–0.98 | 0.04 |
| Stent length | 1.03 | 0.94–1.12 | 0.579 |
| Preoperative ICA diameter | 0.99 | 0.69–1.40 | 0.93 |
| Preoperative ECA diameter | 0.24 | 0.16–0.36 | <0.001 |
| Variable | ECA Occlusion (n = 23) | No ECA Occlusion (n = 940) | p |
|---|---|---|---|
| Ipsilateral stroke | 2 (8.7%) | 20 (2.1%) | 0.04 |
| TIA | 2 (8.7%) | 17 (1.8%) | 0.01 |
| MI | 1 (4.4%) | 27 (2.9%) | 0.68 |
| In-stent restenosis | 4 (17.4%) | 23 (2.5%) | <0.001 |
| Death | 1 (4.4%) | 13 (1.4%) | 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łyko-Morawska, D.; Serafin, M.; Szostek, J.; Mąka, M.; Kania, I.; Kuczmik, W. Clinical Impact of External Carotid Artery Remodeling Following Carotid Artery Stenting. J. Clin. Med. 2025, 14, 6682. https://doi.org/10.3390/jcm14186682
Łyko-Morawska D, Serafin M, Szostek J, Mąka M, Kania I, Kuczmik W. Clinical Impact of External Carotid Artery Remodeling Following Carotid Artery Stenting. Journal of Clinical Medicine. 2025; 14(18):6682. https://doi.org/10.3390/jcm14186682
Chicago/Turabian StyleŁyko-Morawska, Dorota, Michał Serafin, Julia Szostek, Magdalena Mąka, Iga Kania, and Wacław Kuczmik. 2025. "Clinical Impact of External Carotid Artery Remodeling Following Carotid Artery Stenting" Journal of Clinical Medicine 14, no. 18: 6682. https://doi.org/10.3390/jcm14186682
APA StyleŁyko-Morawska, D., Serafin, M., Szostek, J., Mąka, M., Kania, I., & Kuczmik, W. (2025). Clinical Impact of External Carotid Artery Remodeling Following Carotid Artery Stenting. Journal of Clinical Medicine, 14(18), 6682. https://doi.org/10.3390/jcm14186682






