Endoscopic Outcomes and Inflammatory Marker Correlation in Adult Patients with Corrosive Substance Ingestion
Abstract
1. Introduction
2. Methods
2.1. Data Collection and Definitions
- Early complications: Those occurring within 14 days post-ingestion.
- Late complications: Those developing after 14 days.
- Total hospital stay: Combined duration of emergency department and intensive care unit (ICU) admission.
2.2. Injury Classification
- Group A (Mild injury): Grades 0, 1, and 2a.
- Group B (Severe injury): Grades 2b, 3a, 3b, and 4.
2.3. Data Extraction
- Demographic characteristics: Age, sex.
- Exposure details:
- ○
- Corrosive substance type (classified as alkali, acidic, neutral, or other).
- ○
- Intent (accidental vs. intentional).
- ○
- Ingestion-to-endoscopy interval.
- Clinical presentation:
- ○
- Admission symptoms.
- ○
- Physical examination findings.
- Outcome measures:
- ○
- ICU and total hospital stay duration.
- ○
- Systemic complications.
- ○
- Laboratory parameters (neutrophil-to-lymphocyte ratio [NLR], C-reactive protein [CRP]).
- Psychiatric comorbidities: Documented diagnoses. Psychiatric comorbidity was defined as a previously established psychiatric disorder documented in the patient’s medical records or confirmed by psychiatric consultation at the time of admission.
2.4. Substance Classification
- Alkali: Bleach and cleaning agents containing sodium hypochlorite, ammonium hydroxide, or sodium hydroxide.
- Acidic: Decalcifiers and cleaners with hydrochloric acid, ammonium sulfate, hydrogen peroxide, or acetic acid.
- Neutral: Thinners and potassium permanganate.
- Other: Substances with unknown composition.
- Performed directly by a board-certified gastroenterologist.
- Conducted by a gastroenterology fellow under the direct supervision of an attending gastroenterologist.
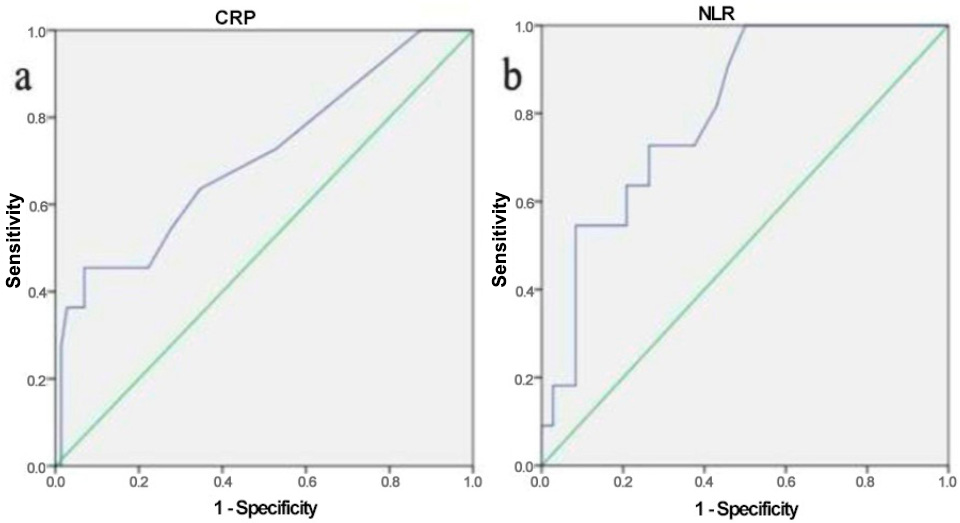
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Lusong, M.A.A.; Timbol, A.B.G.; Tuazon, D.J.S. Management of esophageal caustic injury. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, M.U.; Ali, M.; Ullah, K.; Aleem, A.; Khan, I.H. Clinico-epidemiological Characteristics of Corrosive Ingestion: A Cross-sectional Study at a Tertiary Care Hospital of Multan, South-Punjab Pakistan. Cureus 2018, 10, e2704. [Google Scholar] [CrossRef] [PubMed]
- Atiq, M.; Kibria, R.E.; Dang, S.; Patel, D.H.; Ali, S.A.; Beck, G.; Aduli, F. Corrosive injury to the GI tract in adults: A practical approach. Expert Rev. Gastroenterol. Hepatol. 2009, 3, 701–709. [Google Scholar] [CrossRef]
- Caganova, B.; Foltanova, T.; Puchon, E.; Ondriasova, E.; Plackova, S.; Fazekas, T.; Kuzelova, M. Caustic Ingestion in the Elderly: Influence of Age on Clinical Outcome. Molecules 2017, 22, 1726. [Google Scholar] [CrossRef]
- Mrazová, K.; Navrátil, T.; Pelclová, D. Consequences of ingestions of potentially corrosive cleaning products, One-Year Follow-Up. Int. J. Electrochem. Sci. 2012, 7, 1734–1748. [Google Scholar] [CrossRef]
- Faz, A.A.; Arsan, F.; Peyvandi, H.; Oroei, M.; Peyvandi, M.; Yousefi, M. Epidemiologic Features and Outcomes of Caustic Ingestions; a 10-Year Cross-Sectional Study. Emergency 2017, 5, e56. [Google Scholar]
- Haller, J.A., Jr.; Andrews, H.G.; White, J.J.; Tamer, M.A.; Cleveland, W.W. Pathophysiology and management of acute corrosive burns of the esophagus: Results of treatment in 285 children. J. Pediatr. Surg. 1971, 6, 578–584. [Google Scholar] [CrossRef]
- Cheng, H.-T.; Cheng, C.-L.; Lin, C.-H.; Tang, J.-H.; Chu, Y.-Y.; Liu, N.-J.; Chen, P.-C. Caustic ingestion in adults: The role of endoscopic classification in predicting outcome. BMC Gastroenterol. 2008, 8, 31. [Google Scholar] [CrossRef]
- Immaneni, S.; Ramamoorthy, M.; Rajeshwaran, V.A.; Murugesan, M.; Solomon, R.; Annasamy, C. Clinical, Epidemiological, Endoscopic Profile And Outcome Of Corrosive Injuries Of Gastrointestinal Tract—A tertiary care experience. Int. J. Sci. Res. 2019, 8, 11–13. [Google Scholar] [CrossRef]
- Radenkova-Saeva, J.; Loukova, A.; Tsekov, C. Caustic injury in Adults—A study for 3 year period. Acta Medica Bulg. 2016, 43, 39–44. [Google Scholar] [CrossRef][Green Version]
- Kaya, M.; Ozdemir, T.; Sayan, A.; Arıkan, A. The relationship between clinical findings and esophageal injury severity in children with corrosive agent ingestion. Ulus. Travma Acil Cerrahi Derg. 2010, 16, 537–540. [Google Scholar][Green Version]
- Chen, T.Y.; Ko, S.F.; Chuang, J.H.; Kuo, H.W.; Tiao, M.M. Predictors of esophageal stricture in children with unintentional ingestion of caustic agents. Chang Gung Med. J. 2003, 26, 233–239. [Google Scholar][Green Version]
- Havanond, C.; Havanond, P. Initial signs and symptoms as prognostic indicators of severe gastrointestinal tract injury due to corrosive ingestion. J. Emerg. Med. 2007, 33, 349–353. [Google Scholar] [CrossRef]
- Rigo, G.P.; Camellini, L.; Azzolini, F.; Guazzetti, S.; Bedogni, G.; Merighi, A.; Bellis, L.; Scarcelli, A.; Manenti, F. What is the utility of selected clinical and endoscopic parameters in predicting the risk of death after caustic ingestion? Endoscopy 2002, 34, 304–310. [Google Scholar] [CrossRef]
- Zargar, S.A.; Kochhar, R.; Mehta, S.; Mehta, S.K. The role of fiberoptic endoscopy in the management of corrosive ingestion and modified endoscopic classification of burns. Gastrointest. Endosc. 1991, 37, 165–169. [Google Scholar] [CrossRef]
- Chirica, M.; Bonavina, L.; Kelly, M.D.; Sarfati, E.; Cattan, P. Caustic ingestion. Lancet 2017, 389, 2041–2052. [Google Scholar] [CrossRef] [PubMed]
- Contini, S.; Scarpignato, C. Caustic injury of the upper gastrointestinal tract: A comprehensive review. World J. Gastroenterol. 2013, 19, 3918–3930. [Google Scholar] [CrossRef] [PubMed]
- Cabral, C.; Chirica, M.; de Chaisemartin, C.; Gornet, J.-M.; Munoz-Bongrand, N.; Halimi, B.; Cattan, P.; Sarfati, E. Caustic injuries of the upper digestive tract: A population observational study. Surg. Endosc. 2012, 26, 214–221. [Google Scholar] [CrossRef]
- Poley, J.-W.; Steyerberg, E.W.; Kuipers, E.J.; Dees, J.; Hartmans, R.; Tilanus, H.W.; Siersema, P.D. Ingestion of acid and alkaline agents: Outcome and prognostic value of early upper endoscopy. Gastrointest Endosc. 2004, 60, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Kate, V.; Ananthakrishnan, N.; Kalayarasan, R. Corrosive injury of esophagus and stomach. In Textbook of Surgical Gastroenterology; Jaypee Brothers Medical Publishers: Maharashtra, India, 2016; p. 194. [Google Scholar] [CrossRef]
- Lakshmi, C.P.; Vijayahari, R.; Kate, V.; Ananthakrishnan, N. A hospital-based epidemiological study of corrosive alimentary injuries with particular reference to the Indian experience. Natl. Med. J. India 2013, 26, 31–36. [Google Scholar]
- Mowry, J.B.; Spyker, D.A.; Cantilena, L.R.; McMillan, N.; Ford, M. 2013 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 31st Annual Report. Clin. Toxicol. 2014, 52, 1032–1283. [Google Scholar] [CrossRef]
- Acehan, S.; Satar, S.; Gulen, M.; Avci, A. Evaluation of corrosive poisoning in adult patients. Am. J. Emerg. Med. 2021, 39, 65–70. [Google Scholar] [CrossRef]
- Karaoğlu, A.; Özütemiz, Ö.; İlter, T.; Batur, Y.; Yönetçi, N.; Tekeşin, O.; Musoğlu, A.; Aydin, A.; Osmanoğlu, N.; Akarca, U.; et al. Akut korozif özofajit:108 olgunun değerlendirilmesi. Turk. J. Gastroenterol. 1998, 1, 55–60. [Google Scholar]
- Ain, Q.U.; Jamil, M.; Abu Safian, H.; Akhter, T.S.; Batool, S.; Arshad, M.; Jamal, A.M.; Iqbal, A.; Arsh, L.; Abbas, B. Assessing the Degree of Acute Esophageal Injury Secondary to Corrosive Intake: Insights from a Public Sector Hospitals of a Developing Country. Cureus 2020, 12, e10858. [Google Scholar] [CrossRef]
- Chirica, M.; Kelly, M.D.; Siboni, S.; Aiolfi, A.; Riva, C.G.; Asti, E.; Ferrari, D.; Leppäniemi, A.; Broek, R.P.G.T.; Brichon, P.Y.; et al. Esophageal emergencies: WSES guidelines. World J. Emerg. Surg. WJES 2019, 14, 26. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chibishev, A.; Simonovska, N.; Shikole, A. Post-corrosive injuries of upper gastrointestinal tract. Prilozi 2010, 31, 297–316. [Google Scholar]
- Chibishev, A.; Pereska, Z.; Chibisheva, V.; Simonovska, N. Corrosive poisonings in adults. Mater. Sociomed. 2012, 24, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Bremholm, L.; Winkel, R.; Born, P.; Suku, M.L. Akut øsofageal nekrose [Acute esophageal necrosis]. Ugeskr. Laeger. 2009, 171, 3282–3283. [Google Scholar] [PubMed]
- French, D.; Sundaresan, S. Caustic esophageal injury. In Shackelford’s Surgery of the Alimentary Tract, 8th ed.; Elsevier: Philadelphia, PA, USA, 2019; pp. 515–526. [Google Scholar]
- Wax, P.M.; Yarema, M. Corrosives. In Haddad and Winchester’s Clinical Management of Poisoning and Drug Overdose, 4th ed.; Shannon, M.W., Borron, S.W., Burns, M.J., Eds.; Saunders/Elsevier: Philadelphia, PA, USA, 2007; pp. 1407–1414. [Google Scholar]
- Baskın, D.; Urgancı, N.; Abbasoğlu, L.; Alkım, C.; Yalçın, M.; Karadağ, Ç.; Sever, N. A standardised protocol for the acute management of corrosive ingestion in children. Pediatr Surg Int. 2004, 20, 824–828. [Google Scholar] [CrossRef]
- Kay, M.; Wyllie, R. Caustic ingestions in children. Curr. Opin. Pediatr. 2009, 21, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.S.; Burns, M.M.; Gosselin, S. Ingestion of Caustic Substances. N. Engl. J. Med. 2020, 382, 1739–1748. [Google Scholar] [CrossRef]
- Hall, A.H.; Jacquemin, D.; Henny, D.; Mathieu, L.; Josset, P.; Meyer, B. Corrosive substances ingestion: A review. Crit. Rev. Toxicol. 2019, 49, 637–669. [Google Scholar] [CrossRef]
- Ramasamy, K.; Gumaste, V.V. Corrosive ingestion in adults. J. Clin. Gastroenterol. 2003, 37, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Kirsh, M.M.; Ritter, F. Caustic ingestion and subsequent damage to the oropharyngeal and digestive passages. Ann Thorac. Surg. 1976, 21, 74–82. [Google Scholar] [CrossRef]
- Balta, S.; Celik, T.; Mikhailidis, D.P.; Ozturk, C.; Demirkol, S.; Aparci, M.; Iyisoy, A. The Relation Between Atherosclerosis and the Neutrophil-Lymphocyte Ratio. Clin. Appl. Thromb Hemost. 2016, 22, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Isaac, V.; Wu, C.Y.; Huang, C.T.; Baune, B.T.; Tseng, C.L.; McLachlan, C.S. Elevated neutrophil to lymphocyte ratio predicts mortality in medical inpatients with multiple chronic conditions. Medicine 2016, 95, e3832, Erratum in Medicine 2016, 95, e0916. [Google Scholar] [CrossRef] [PubMed]
- Dundar, Z.D.; Ergin, M.; Koylu, R.; Ozer, R.; Cander, B.; Gunaydin, Y.K. Neutrophil-lymphocyte ratio in patients with pesticide poisoning. J. Emerg. Med. 2014, 47, 286–293. [Google Scholar] [CrossRef]
- Liu, F.; Jiang, M.Z.; Shu, X.L.; Zhang, X.P. Role of oxidative stress in the pathogenesis of esophageal mucosal injury in children with reflux esophagitis. Chin. J. Contemp. Pediatr. 2009, 11, 425–428. [Google Scholar]
- Ranjbar, A.; Solhi, H.; Mashayekhi, F.J.; Susanabdi, A.; Rezaie, A.; Abdollahi, M. Oxidative stress in acute human poisoning with organophosphorus insecticides; A case control study. Environ. Toxicol. Pharmacol. 2005, 20, 88–91. [Google Scholar] [CrossRef]
- Kiyan, G.; Aktas, S.; Ozel, K.; Isbilen, E.; Kotiloglu, E.; Dagli, T.E. Effects of hyperbaric oxygen therapy on caustic esophageal injury in rats. J. Pediatr. Surg. 2004, 39, 1188–1193. [Google Scholar] [CrossRef]
- Guven, A.; Demirbag, S.; Uysal, B.; Topal, T.; Erdogan, E.; Korkmaz, A.; Ozturk, H. Effect of 3-amino benzamide, a poly(adenosine diphosphate-ribose) polymerase inhibitor, in experimental caustic esophageal burn. J. Pediatr. Surg. 2008, 43, 1474–1479. [Google Scholar] [CrossRef] [PubMed]
- Uyar, S.; Kok, M. Neutrophil to lymphocyte ratio as a predictor of endoscopic damage in caustic injuries. J. Clin. Toxicol. 2017, 7, 349. [Google Scholar] [CrossRef]
- Mathews, N.V.; Premkumar, K.; Ramamoorthy, M.; Chezhian, A.; Venkateswaran, A.; Shubha, I. Neutrophil to lymphocyte ratio, a novel bedside predictor of endoscopic damage in corrosive gi injuries. Int. J. Sci. Res. 2020, 9, 11–13. [Google Scholar] [CrossRef]
| Mean ± SD Median (Range) | n (%) | |
|---|---|---|
| Age (years) | 41.5 ± 17.3 40 (17–84) | |
| Hospitalization Duration | ||
| ICU stay (days) | 1.8 ± 0.6 0 (0–25) | |
| Total hospital stay (days) | 2.9 ± 4.7 1 (1–28) | |
| Laboratory Markers | ||
| CRP (mg/L) | 5.7 ± 11 2 (0–58) | |
| NLR | 3.5 ± 4.5 2.3 (1.1–36) | |
| Gender (female) | 46 (55.4) | |
| Psychiatric comorbidity | 15 (18.1) | |
| Ingestion Characteristics Intent; | ||
| Accidental [f/m] | 62 (74.7) [35/27] | |
| Suicidal [f/m] | 21 (25.3) [11/10] | |
| Corrosive substance: | ||
| Bleach | 34 (41) | |
| Decalcifier | 10 (12.1) | |
| Muriatic acid | 9 (10.8) | |
| Thinner | 4 (4.8) | |
| Detergent | 3 (3.6) | |
| Disinfectant | 2 (2.4) | |
| Other a | 21 (25.3) | |
| Substance type | ||
| Alkali | 37 (44.6) | |
| Acid | 20 (24.1) | |
| Neutral | 8 (9.6) | |
| Other | 18 (21.7) | |
| Amount of substance ingested | ||
| 0–50 cc | 53 (63.9) | |
| 50–100 cc | 24 (28.9) | |
| Above 100 cc | 6 (7.2) | |
| Clinical presentation | ||
| Symptoms at presentation | ||
| No symptoms | 33 (39.8) | |
| Sore throat | 18 (21.7) | |
| Abdominal pain | 4 (4.8) | |
| Retrosternal burning | 4 (4.8) | |
| Vomiting | 3 (3.6) | |
| Chest pain | 3 (3.6) | |
| More than one symptom | 14 (16.9) | |
| Other b | 4 (4.8) | |
| Physical examination signs | ||
| Normal | 50 (60.2) | |
| Oropharyngeal hyperemia | 22 (26.5) | |
| Epigastric tenderness | 2 (2.4) | |
| Roughness in lung sounds | 1 (1.2) | |
| More than one sign | 8 (9.7) | |
| Time of endoscopy | ||
| 0–24 h | 77 (92.8) | |
| 24–48 h | 5 (6) | |
| Over 48 h | 1 (1.2) | |
| Outcome | ||
| Discharged from the emergency unit | 65 (78.3) | |
| Hospitalization | 17 (20.5) | |
| Discharged voluntarily | 1 (1.2) | |
| Early complications | ||
| None | 79 (95.2) | |
| Perforation | 2 (2.4) | |
| Bleeding | 2 (2.4) | |
| Late complications | ||
| None | 40 (48.2) | |
| Esophageal stricture | 2 (2.4) | |
| Gastric stricture | 3 (3.6) | |
| Unknown | 38 (45.8) |
| Characteristic | n (%) | Additional Details |
|---|---|---|
| Organ involvement | ||
| None | 37 (44.6) | |
| Esophagus | 9 (10.8) | |
| Stomach | 15 (18.1) | |
| Duodenum | 0 (0) | |
| Esophagus + stomach | 15 (18.1) | |
| Stomach + duodenum | 1 (1.2) | |
| Esophagus + duodenum | 1 (1.2) | |
| Esophagus + stomach + duodenum | 5 (6) | |
| Grade of esophageal injury (Zargar Grade) | ||
| Grade 0 | 52 (62.7) | Normal mucosa |
| Grade 1 | 10 (12) | Mucosal edema/hyperemia |
| Grade 2a | 14 (16.9) | Superficial ulcers |
| Grade 2b | 4 (4.8) | Deep focal ulcers |
| Grade 3a | 3 (3.6) | Circumferential deep ulcers |
| Grade 3b-4 | 0 (0) | Necrosis/perforation |
| Grade of gastric injury (Zargar Grade) | ||
| Grade 0 | 47 (56.6) | |
| Grade 1 | 21 (25.3) | |
| Grade 2a | 5 (6) | |
| Grade 2b | 2 (2.4) | |
| Grade 3a | 7 (8.5) | |
| Grade 3b-4 | 1 (1.2) | |
| Grade 4 | 0 (0) | |
| Grade of duodenal injury (Zargar Grade) | ||
| Grade 0 | 76 (91.6) | |
| Grade 1 | 2 (2.4) | |
| Grade 2a | 3 (3.6) | |
| Grade 2b | 2 (2.4) | |
| Grade 3a-4 | 0 (0) |
| Alkali (n = 37) | Acidic (n = 20) | Neutral (n = 8) | Other (n = 18) | p-Value | |
|---|---|---|---|---|---|
| Hospitalization Duration [Median (range)] | |||||
| Total stay (days) | 1 (1–21) | 1.5 (1–16) | 1 (1–2) | 1 (1–28) | 0.009 |
| ICU stay (days) | 0 (0–20) | 0 (0–16) | 0 (0–25) | 0 (0–2) | 0.006 |
| Clinical Presentation [n (%)] | |||||
| Asymptomatic | 16 (43.2) | 5 (25) | 3 (37.5) | 9 (50) | 0.317 |
| Symptomatic (1 symptom) | 16 (43.2) | 10 (50) | 2 (25) | 8 (44.4) | |
| Symptomatic (>1 symptom) | 5 (13.5) | 5 (25) | 3 (37.5) | 1 (5.6) | |
| Examination sign [n (%)] | |||||
| None | 23 (62.2) | 8 (40) | 5 (62.5) | 14 (77.8) | 0.229 |
| One sign | 11 (26.7) | 8 (40) | 3 (37.5) | 3 (16.7) | |
| More than one sign | 3 (8.1) | 4 (20) | 0 (0) | 1 (5.6) | |
| Injury Severity [n (%)] | |||||
| Group A (Mild) | 36 (97.3) | 12 (60) | 7 (87.5) | 17 (94.4) | 0.002 |
| Group B (Severe) | 1 (2.7) | 8 (40) | 1 (12.5) | 1 (5.6) | |
| Early complication [n (%)] | |||||
| No | 37 (100) | 16 (80) | 8 (0) | 18 (100) | 0.007 |
| Yes | 0 (0) | 4 (20) | 0 (0) | 0 (0) | |
| Late complication [n (%)] | |||||
| No | 21 (56.8) | 9 (22.5) | 3 (37.5) | 7 (38.9) | 0.063 |
| Yes | 0 (0) | 4 (20) | 0 (0) | 1 (5.6) | |
| Unknown | 16 (43.2) | 7 (35) | 5 (62.5) | 10 (55.6) | |
| Outcomes [n (%)] | |||||
| Discharged | 35 (94.6) | 11 (55) | 13 (72.2) | 7 (87.5) | 0.003 |
| Hospitalization | 2 (5.4) | 9 (45) | 5 (27.8) | 1 (12.5) | |
| Treatment modality [n (%)] | |||||
| Surgical | 0 (0) | 1 (5) | 0 (0) | 0 (0) | 0.410 |
| Conservative | 37 (100) | 19 (95) | 8 (100) | 18 (0) | |
| Organ involvement [n (%)] | |||||
| None | 19 (51.4) | 5 (25) | 4 (50) | 9 (50) | 0.310 |
| Esophagus | 3 (8.1) | 4 (20) | 1 (12.5) | 1 (5.6) | |
| Stomach | 9 (24.3) | 2 (10) | 2 (25) | 2 (11.1) | |
| Esophagus-stomach | 3 (8.1) | 6 (30) | 1 (12.5) | 5 (27.8) | |
| Stomach-duodenum | 1 (2.7) | 0 (0) | 0 (0) | 0 (0) | |
| Esophagus-duodenum | 0 (0) | 0 (0) | 0 (0) | 1 (5,6) | |
| Esophagus-stomach-duodenum | 2 (5.4) | 3 (15) | 0 (0) | 0 (0) |
| Group A | Group B | p-Value | |
|---|---|---|---|
| Age [Median (range)] | 39 (17–84) | 41 (21–56) | 0.773 |
| Hospitalization Duration [Median (range)] | |||
| Total stay (days) | 1 (1–28) | 8 (2–16) | <0.001 |
| ICU stay (days) | 0 (0–1) | 6 (2–16) | <0.001 |
| Gender [n (%)] | |||
| Female | 43 (59.7) | 3 (27.3) | 0.055 |
| Male | 29 (40.3) | 8 (72.7) | |
| Ingestion Characteristics Intent | |||
| Accidental | 56 (77.8) | 6 (54.5) | 0.135 |
| Suicidal | 16 (22.2) | 5 (45.5) | |
| Amount of substance ingested | |||
| 0–50 cc | 48 (66.7) | 5 (45.5) | 0.280 |
| 50–100 cc | 20 (27.8) | 4 (36.4) | |
| >100 cc | 4 (5.6) | 2 (18.2) | |
| Early complication | |||
| No | 72 (100) | 7 (63.6) | <0.001 |
| Yes | 0 (0) | 4 (36.4) | |
| Late complication | |||
| No | 37 (51.4) | 3 (27.3) | <0.001 |
| Yes | 1 (1.4) | 4 (36.4) | |
| Unknown | 34 (47.2) | 4 (36.4) | |
| Outcome | |||
| Discharge | 66 (91.7) | 0 (0) | <0.001 |
| Hospitalization | 6 (8.3) | 11 (100) | |
| Treatment modality | |||
| Surgical | 0 (0) | 1 (9.1) | 0.133 |
| Conservative | 72 (100) | 10 (90.9) | |
| Organ involvement | |||
| None | 37 (51.4) | 0 (0) | <0.001 |
| Esophagus | 9 (12.5) | 0 (0) | |
| Stomach | 14 (19.4) | 1 (9.1) | |
| Esophagus-stomach | 9 (12.5) | 6 (54.5) | |
| Stomach-duodenum | 1 (1.4) | 0 (0) | |
| Esophagus-duodenum | 1 (1.4) | 0 (0) | |
| Esophagus-stomach-duodenum | 1 (1.4) | 4 (36.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aslanov, S.; Senkaya, A.; Unal, N.G.; Karahanlı, C.; Kurt, I.; Celik, F.; Uysal, A.; Sarıkaya, O.F.; Ozutemiz, A.O. Endoscopic Outcomes and Inflammatory Marker Correlation in Adult Patients with Corrosive Substance Ingestion. J. Clin. Med. 2025, 14, 6663. https://doi.org/10.3390/jcm14186663
Aslanov S, Senkaya A, Unal NG, Karahanlı C, Kurt I, Celik F, Uysal A, Sarıkaya OF, Ozutemiz AO. Endoscopic Outcomes and Inflammatory Marker Correlation in Adult Patients with Corrosive Substance Ingestion. Journal of Clinical Medicine. 2025; 14(18):6663. https://doi.org/10.3390/jcm14186663
Chicago/Turabian StyleAslanov, Seymur, Ali Senkaya, Nalan Gulsen Unal, Cengiz Karahanlı, Idris Kurt, Ferit Celik, Alper Uysal, Ozan Fatih Sarıkaya, and Ahmet Omer Ozutemiz. 2025. "Endoscopic Outcomes and Inflammatory Marker Correlation in Adult Patients with Corrosive Substance Ingestion" Journal of Clinical Medicine 14, no. 18: 6663. https://doi.org/10.3390/jcm14186663
APA StyleAslanov, S., Senkaya, A., Unal, N. G., Karahanlı, C., Kurt, I., Celik, F., Uysal, A., Sarıkaya, O. F., & Ozutemiz, A. O. (2025). Endoscopic Outcomes and Inflammatory Marker Correlation in Adult Patients with Corrosive Substance Ingestion. Journal of Clinical Medicine, 14(18), 6663. https://doi.org/10.3390/jcm14186663






