Nutritional Status Assessment Using the Patient-Generated Subjective Global Assessment (PG-SGA) in Individuals with Colorectal Cancer Undergoing Chemotherapy Regimens
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Data Sources
2.2. Demographic Variables
2.3. Treatment-Related Variables
2.4. Nutritional Status Assessment
2.5. Statistical Analysis
2.6. Ethical Considerations
3. Results
3.1. Demographic and Clinical Characteristics of the Cohort
3.2. Treatments Received and Associated Nutritional Risk
3.3. Nutritional Risk Stratification
3.4. Symptomatology and Profile by Chemotherapy Regimen
3.5. Analysis by Active Principle
4. Discussion
4.1. Prevalence and Prognostic Relevance of Malnutrition in Colorectal Cancer
4.2. Pathophysiology and Underlying Mechanisms
4.3. Influence of Age and Comorbidities
4.4. Nutritional Screening and Diagnostic Tools
4.5. Clinical and Strategic Implications
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CRC | Colorectal Cancer |
| PG-SGA | Patient-Generated Subjective Global Assessment |
| BMI | Body Mass Index |
| GLIM | Global Leadership Initiative on Malnutrition |
| MST | Malnutrition Screening Tool |
| MUST | Malnutrition Universal Screening Tool |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Baracos, V.; Bertz, H.; Bozzetti, F.; Calder, P.C.; Deutz, N.E.P.; Erickson, N.; Laviano, A.; Lisanti, M.P.; Lobo, D.N.; et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin. Nutr. 2017, 36, 1187–1196. [Google Scholar] [CrossRef]
- Caccialanza, R.; Cotogni, P.; Cereda, E.; Bossi, P.; Aprile, G.; Delrio, P.; Gnagnarella, P.; Mascheroni, A.; Monge, T.; Corradi, E.; et al. Nutritional Support in Cancer patients: Update of the Italian Intersociety Working Group practical recommendations. J. Cancer 2022, 13, 2705–2716. [Google Scholar] [CrossRef]
- Weigl, M.P.; Feurstein, B.; Clemens, P.; Attenberger, C.; Jäger, T.; Emmanuel, K.; Königsrainer, I.; Tschann, P. Alterations in Body Composition Lead to Changes in Postoperative Outcome and Oncologic Survival in Patients with Non-Metastatic Colon Cancer. J. Clin. Med. 2025, 14, 3438. [Google Scholar] [CrossRef] [PubMed]
- Baracos, V.E. Cancer-associated malnutrition. Eur. J. Clin. Nutr. 2018, 72, 1255–1259. [Google Scholar] [CrossRef]
- Muscaritoli, M.; the PreMiO Study Group; Lucia, S.; Farcomeni, A.; Lorusso, V.; Saracino, V.; Barone, C.; Plastino, F.; Gori, S.; Magarotto, R.; et al. Prevalence of malnutrition in patients at first medical oncology visit: The PreMiO study. Oncotarget 2017, 8, 79884. [Google Scholar] [CrossRef]
- Bozzetti, F. Nutritional support of the oncology patient. Crit. Rev. Oncol. Hematol. 2013, 87, 172–200. [Google Scholar] [CrossRef]
- Stein, A.; Voigt, W.; Jordan, K. Chemotherapy-induced diarrhea: Pathophysiology, frequency and guideline-based management. Ther. Adv. Med. Oncol. 2010, 2, 51–63. [Google Scholar] [CrossRef]
- Abushullaih, S.; Saad, E.D.; Munsell, M.; Hoff, P.M. Incidence and severity of hand-foot syndrome in colorectal cancer patients treated with capecitabine: A single-institution experience. Cancer Investig. 2002, 20, 3–10. [Google Scholar] [CrossRef]
- Benson, A.B.; Ajani, J.A.; Catalano, R.B.; Engelking, C.; Kornblau, S.M.; Martenson, J.A.; McCallum, R.; Mitchell, E.P.; O’DOrisio, T.M.; Vokes, E.E.; et al. Recommended guidelines for the treatment of cancer treatment-induced diarrhea. J. Clin. Oncol. 2004, 22, 2918–2926. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Capra, S.; Ferguson, M. Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur. J. Clin. Nutr. 2002, 56, 779–785. [Google Scholar] [CrossRef]
- Isenring, E.A.; Capra, S.; Bauer, J.D. Nutrition intervention is beneficial in oncology outpatients receiving radiotherapy to the gastrointestinal or head and neck area. Br. J. Cancer 2004, 91, 447. [Google Scholar] [CrossRef]
- Carriço, M.; Guerreiro, C.S.; Parreira, A. The validity of the Patient-Generated Subjective Global Assessment Short-form© in cancer patients undergoing chemotherapy. Clin. Nutr. ESPEN 2021, 43, 296–301. [Google Scholar] [CrossRef]
- Huong, L.T.; Phuong, D.T.; Anh, D.K.; Toi, P.L.; Anh, N.L.T.; Le Huy, T.; Linh, N.T. Nutritional intervention improves nutrition outcomes in stomach and colon cancer patients receiving chemotherapy: Finding from a quasi-experiment in Vietnam. Healthcare 2021, 9, 843. [Google Scholar] [CrossRef] [PubMed]
- Ngoc Anh, L.T.; Kien, T.G.; Tuan, N.V.; Tuong, T.T.A.; Ko, J.; Dan, P.T.; Cho, J.; Tap, N.V. Malnutrition in Colorectal Cancer Patients: Association with the Lack of Eating Motivation and Inappropriate Diet. Asian Pac. J. Cancer Prev. 2025, 26, 1661. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.; Salari, N.; Darvishi, N.; Siahkamari, Z.; Rahmani, A.; Shohaimi, S.; Faghihi, S.H.; Mohammadi, M. Prevalence of severe malnutrition in cancer patients: A systematic review and meta-analysis. J. Health Popul. Nutr. 2025, 44, 252. [Google Scholar] [CrossRef]
- Akbarali, H.I.; Muchhala, K.H.; Jessup, D.K.; Cheatham, S. Chemotherapy induced gastrointestinal toxicities. Adv. Cancer Res. 2022, 155, 131–166. [Google Scholar]
- Baracos, V.E.; Martin, L.; Korc, M.; Guttridge, D.C.; Fearon, K.C.H. Cancer-associated cachexia. Nat. Rev. Dis. Primers 2018, 18, 17105. [Google Scholar] [CrossRef]
- Vergara-Fernandez, O.; Trejo-Avila, M.; Salgado-Nesme, N. Sarcopenia in patients with colorectal cancer: A comprehensive review. World J. Clin. Cases 2020, 8, 1188. [Google Scholar] [CrossRef]
- Zhang, J.; Quan, Y.; Wang, X.; Wei, X.; Shen, X.; Li, X.; Liang, T. Global epidemiological characteristics of malnutrition in cancer patients: A comprehensive meta-analysis and systematic review. BMC Cancer 2025, 25, 1191. [Google Scholar] [CrossRef] [PubMed]
- Isenring, E.; Cross, G.; Daniels, L.; Kellett, E.; Koczwara, B. Validity of the malnutrition screening tool as an effective predictor of nutritional risk in oncology outpatients receiving chemotherapy. Support. Care Cancer 2006, 14, 1152–1156. [Google Scholar] [CrossRef]
- Beichmann, B.; Henriksen, C.; Paur, I.; Paulsen, M.M. Barriers and facilitators of improved nutritional support for patients newly diagnosed with cancer: A pre-implementation study. BMC Health Serv. Res. 2024, 24, 815. [Google Scholar] [CrossRef]
- Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; Crivelli, A.; Evans, D.; Gramlich, L.; Fuchs-Tarlovsky, V.; Keller, H.; Llido, L.; et al. GLIM criteria for the diagnosis of malnutrition-A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar]
- Theilla, M.; Rattanachaiwong, S.; Kagan, I.; Rigler, M.; Bendavid, I.; Singer, P. Validation of GLIM malnutrition criteria for diagnosis of malnutrition in ICU patients: An observational study. Clin. Nutr. 2021, 40, 3578–3584. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef] [PubMed]



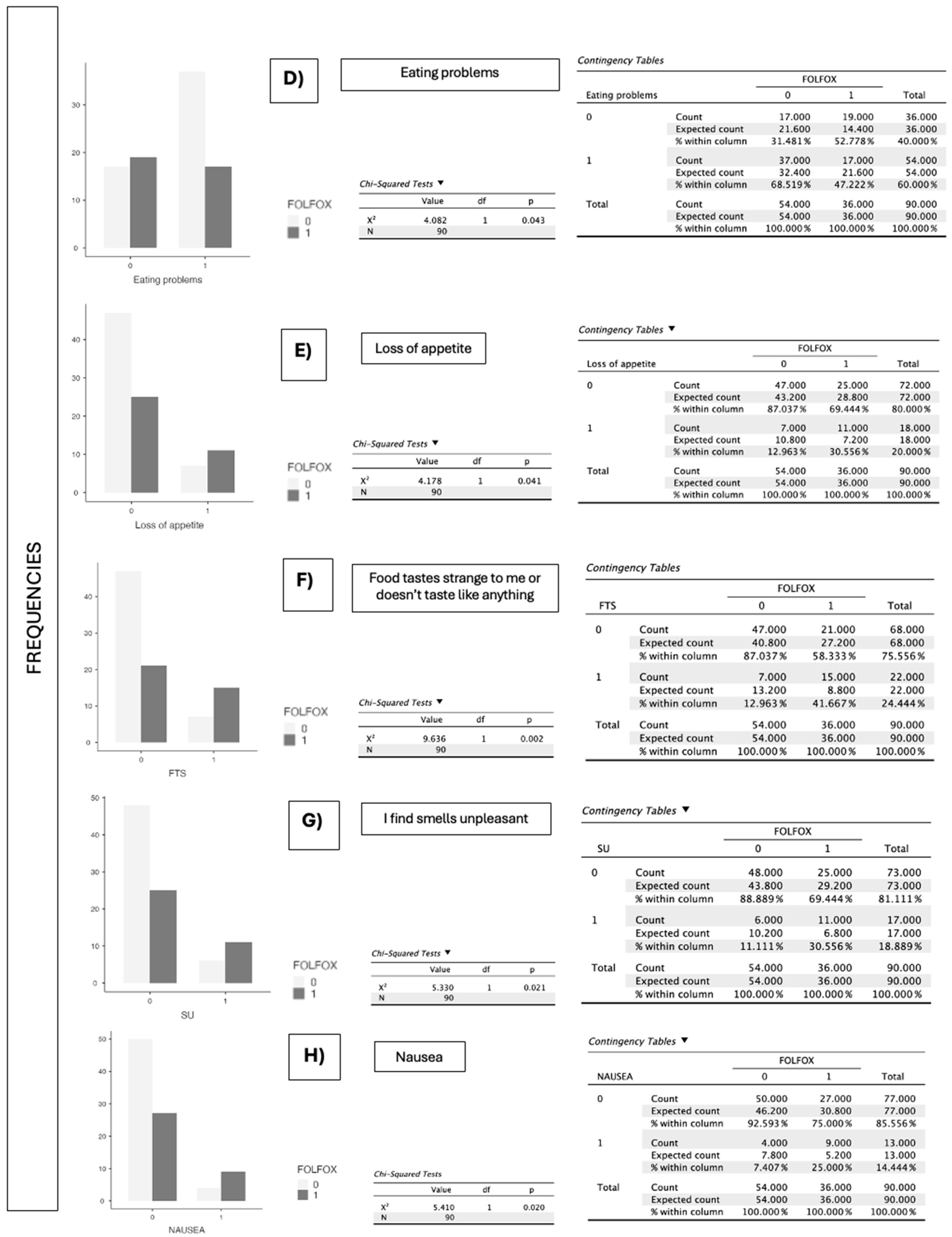
| Symptoms the Patient Has Experienced That Have Prevented Them from Eating Enough over the Past 15 Days (PG-SGA) | FOLFOX (p-Value) | XELOX (p-Value) |
|---|---|---|
| Eating problems | 0.043 | 0.931 |
| Loss of appetite (3 pts.) | 0.041 | 0.751 |
| Constipation (1 pts.) | 0.573 | 0.782 |
| Canker sores (2 pts.) | 0.749 | 0.229 |
| Food tastes strange to me or doesn’t taste like anything (1 pts.) | 0.002 | 0.533 |
| I find smells unpleasant (1 pts.) | 0.021 | 0.297 |
| Pain (3 pts.) | 0.153 | 0.114 |
| Nausea (1 pts.) | 0.020 | 0.521 |
| Vomits (3 pts.) | 0.709 | 0.719 |
| Diarrhea (3 pts.) | 0.150 | 0.009 |
| Dry mouth (1 pts.) | 0.482 | 0.038 |
| Dysphagia (2 pts.) | 0.475 | 0.077 |
| I feel full after eating only a little (1 pts.) | 0.337 | 0.271 |
| Symptoms the Patient Has Experienced That Have Prevented Them from Eating Enough over the Past 15 Days (PG-SGA) | OXALIPLATIN (p-Value) | 5-FLUOROURACIL/LEUCOVORIN (p-Value) |
|---|---|---|
| Eating problems | 0.206 | 0.037 |
| Loss of appetite (3 pts.) | 0.034 | 0.019 |
| Constipation (1 pts.) | 0.917 | 0.456 |
| Canker sores (2 pts.) | 0.276 | 0.517 |
| Food tastes strange to me or doesn’t taste like anything (1 pts.) | 0.112 | <0.001 |
| I find smells unpleasant (1 pts.) | 0.035 | 0.037 |
| Pain (3 pts.) | 0.921 | 0.392 |
| Nausea (1 pts.) | 0.172 | 0.006 |
| Vomits (3 pts.) | 1 | 0.275 |
| Diarrhea (3 pts.) | 0.219 | 0.267 |
| Dry mouth (1 pts.) | 0.243 | 0.727 |
| Dysphagia (2 pts.) | 0.106 | 0.485 |
| I feel full after eating only a little (1 pts.) | 0.156 | 0.244 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Diestro, L.E.; Macias-Montero, R.; Ramalho-Galhanas, A.I.; Aguiar-Frias, A.M.; Paniagua-Vivas, M.S.; Guerrero-Martín, J. Nutritional Status Assessment Using the Patient-Generated Subjective Global Assessment (PG-SGA) in Individuals with Colorectal Cancer Undergoing Chemotherapy Regimens. J. Clin. Med. 2025, 14, 6664. https://doi.org/10.3390/jcm14186664
Sánchez-Diestro LE, Macias-Montero R, Ramalho-Galhanas AI, Aguiar-Frias AM, Paniagua-Vivas MS, Guerrero-Martín J. Nutritional Status Assessment Using the Patient-Generated Subjective Global Assessment (PG-SGA) in Individuals with Colorectal Cancer Undergoing Chemotherapy Regimens. Journal of Clinical Medicine. 2025; 14(18):6664. https://doi.org/10.3390/jcm14186664
Chicago/Turabian StyleSánchez-Diestro, Luis Enrique, Raquel Macias-Montero, Ana Isabel Ramalho-Galhanas, Ana Maria Aguiar-Frias, María Sandra Paniagua-Vivas, and Jorge Guerrero-Martín. 2025. "Nutritional Status Assessment Using the Patient-Generated Subjective Global Assessment (PG-SGA) in Individuals with Colorectal Cancer Undergoing Chemotherapy Regimens" Journal of Clinical Medicine 14, no. 18: 6664. https://doi.org/10.3390/jcm14186664
APA StyleSánchez-Diestro, L. E., Macias-Montero, R., Ramalho-Galhanas, A. I., Aguiar-Frias, A. M., Paniagua-Vivas, M. S., & Guerrero-Martín, J. (2025). Nutritional Status Assessment Using the Patient-Generated Subjective Global Assessment (PG-SGA) in Individuals with Colorectal Cancer Undergoing Chemotherapy Regimens. Journal of Clinical Medicine, 14(18), 6664. https://doi.org/10.3390/jcm14186664







