Electrocardiographic Predictors for Early Risk Stratification: 30-Day Mortality in Older Adult Trauma Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. ECG Evaluation
2.3. Statistical Analysis
3. Results
4. Discussion
Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bourke, R.; Doody, P.; Pérez, S.; Moloney, D.; Lipsitz, L.A.; Kenny, R.A. Cardiovascular disorders and falls among older adults: A systematic review and meta-analysis. J. Gerontol. Ser. A 2024, 79, glad221. [Google Scholar] [CrossRef]
- Verma, S.; Garg, N.; Aggarwal, H.K.; Garg, P.; Verma, A.; Sagar, P. Prevalence and pattern of ECG abnormalities in patients with craniocerebral trauma. J. Dr. YSR Univ. Health Sci. 2023, 12, 115–119. [Google Scholar] [CrossRef]
- Chen, X.; Ma, Y.; Deng, Z.; Li, Q.; Liao, J.; Zheng, Q. Prediction of early postoperative major cardiac events and in-hospital mortality in elderly hip fracture patients: The role of different types of preoperative cardiac abnormalities on echocardiography report. Clin. Interv. Aging 2020, 15, 755–762. [Google Scholar] [CrossRef]
- Kavi, K.S.; Gall, N.P. Trauma and syncope: Looking beyond the injury. Trauma Surg. Acute Care Open 2023, 8, e001036. [Google Scholar] [CrossRef]
- Ceasovschih, A.; Sorodoc, V.; Covantsev, S.; Balta, A.; Uzokov, J.; Kaiser, S.E.; Almaghraby, A.; Lionte, C.; Statescu, C.; Sascau, R.A.; et al. Electrocardiogram Features in Non-Cardiac Diseases: From Mechanisms to Practical Aspects. J. Multidiscip. Healthc. 2024, 17, 1695–1719. [Google Scholar] [CrossRef] [PubMed]
- Purushothaman, S.; Salmani, D.; Prarthana, K.G.; Bandelkar, S.M.; Varghese, S. Study of ECG changes and its relation to mortality in cases of cerebrovascular accidents. J. Nat. Sci. Biol. Med. 2014, 5, 434–436. [Google Scholar] [CrossRef]
- Curfman, K.R.; Urias, D.S.; Simunich, T.J.; Dodson, B.D.; Morrissey, S.L. Benefit of continued noninvasive cardiac monitoring in geriatric trauma: A retrospective review of geriatric pelvis, hip, and femur fractures and analysis of cardiac events during immediate post-traumatic course. SAGE Open Med. 2021, 9, 20503121211047379. [Google Scholar] [CrossRef]
- Aurelian, S.M.; Pislaru, A.I.; Albisteanu, S.M.; Dragoescu, S.; Gidei, S.M.; Ilie, A.C.; Stefaniu, R.; Oancea, C.; Prada, A.G.; Alexa, I.D. Cardiovascular Pharmacotherapy and Falls in Old People: Risks and Prevention-An Observational Case-Control Study. J. Clin. Med. 2025, 14, 4570. [Google Scholar] [CrossRef] [PubMed]
- Marco, C.A.; Lynde, J.; Nelson, B.; Madden, J.; Schaefer, A.; Hardman, C.; McCarthy, M. Predictors of new-onset atrial fibrillation in geriatric trauma patients. JACEP Open 2020, 1, 102–106. [Google Scholar] [CrossRef]
- Furlan, J.; Verocai, F.; Palmares, X.; Fehlings, M. Electrocardiographic abnormalities in the early stage following traumatic spinal cord injury. Spinal Cord 2016, 54, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Lenstra, J.-J.; Kuznecova-Keppel Hesselink, L.; La Bastide-Van Gemert, S.; Jacobs, B.; Nijsten, M.W.N.; Van der Horst, I.C.C.; Van der Naalt, J. The association of early electrocardiographic abnormalities with brain injury severity and outcome in severe traumatic brain injury. Front. Neurol. 2021, 11, 597737. [Google Scholar] [CrossRef] [PubMed]
- Longhitano, Y.; Bottinelli, M.; Pappalardo, F.; Maj, G.; Audo, A.; Srejic, U.; Rasulo, F.A.; Zanza, C. Electrocardiogram alterations in non-traumatic brain injury: A systematic review. J. Clin. Monit. Comput. 2024, 38, 407–414. [Google Scholar] [CrossRef]
- Srinivasaiah, B.; Muthuchellappan, R.; Ganne Sesha, U.R. A prospective observational study of electrocardiographic and echocardiographic changes in traumatic brain injury—Effect of surgical decompression. Br. J. Neurosurg. 2024, 38, 1108–1113. [Google Scholar] [CrossRef] [PubMed]
- Rostagno, C.; Polidori, G.; Ceccofiglio, A.; Cartei, A.; Boccaccini, A.; Peris, A.; Rubbieri, G.; Civinini, R.; Innocenti, M. Takotsubo Syndrome: Is this a Common Occurrence in Elderly Females after Hip Fracture? J. Crit. Care Med. 2020, 6, 146–151. [Google Scholar] [CrossRef]
- Khan, S.I.; Yonko, E.A.; Carter, E.M.; Dyer, D.; Sandhaus, R.A.; Raggio, C.L. Cardiopulmonary Status in Adults with Osteogenesis Imperfecta: Intrinsic Lung Disease May Contribute More Than Scoliosis. Clin. Orthop. Relat. Res. 2020, 478, 2833–2843. [Google Scholar] [CrossRef]
- Stewart, I.J.; Howard, J.T.; Amuan, M.E.; Kennedy, E.; Balke, J.E.; Poltavskiy, E.; Walker, L.E.; Haigney, M.; Pugh, M.J. Traumatic brain injury is associated with the subsequent risk of atrial fibrillation or atrial flutter. Heart Rhythm 2025, 22, 661–667. [Google Scholar] [CrossRef]
- Long, J.; Liu, X.; Li, S.; Yang, C.; Li, L.; Zhang, T.; Hu, R. A dynamic online nomogram predicting post-traumatic arrhythmias: A retrospective cohort study. Am. J. Emerg. Med. 2024, 84, 111–119. [Google Scholar] [CrossRef]
- Denfeld, Q.E.; Turrise, S.; MacLaughlin, E.J.; Chang, P.-S.; Clair, W.K.; Lewis, E.F.; Forman, D.E.; Goodlin, S.J. Preventing and Managing Falls in Adults with Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circ. Cardiovasc. Qual. Outcomes 2022, 15, e000108. [Google Scholar] [CrossRef]
- Hirsch, K.G.; Abella, B.S.; Amorim, E.; Bader, M.K.; Barletta, J.F.; Berg, K.; Callaway, C.W.; Friberg, H.; Gilmore, E.J.; Greer, D.M.; et al. Critical Care Management of Patients After Cardiac Arrest: A Scientific Statement from the American Heart Association and Neurocritical Care Society. Neurocrit. Care 2024, 40, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Zakkar, M.; Ascione, R.; James, A.F.; Angelini, G.D.; Suleiman, M.S. Inflammation, oxidative stress and postoperative atrial fibrillation in cardiac surgery. Pharmacol. Ther. 2015, 154, 13–20. [Google Scholar] [CrossRef]
- Abu-Assi, R.; Campbell, J.; Bacchi, S.; Gill, T.K.; George, D.; Chehade, M. Association between atrial fibrillation and hip fractures and the implications for hip fracture patients: A systematic review. ANZ J. Surg. 2020, 90, 448–453. [Google Scholar] [CrossRef]
- Wiseman, T.; Betihavas, V. The association between unexplained falls and cardiac arrhythmias: A scoping literature review. Aust. Crit. Care 2019, 32, 434–441. [Google Scholar] [CrossRef]
- Al-Khouja, F.; Paladugu, A.; Ruiz, A.; Prentice, K.; Kirby, K.; Santos, J.; Rockne, W.; Nahmias, J. Evaluating the need for prolonged telemetry monitoring in patients with isolated sternal fractures. J. Surg. Res. 2022, 280, 320–325. [Google Scholar] [CrossRef]
- Agnihotri, K.; Pothineni, N.; Charilaou, P.; Vaidya, V.R.; Thakkar, B.; Goyal, V.; Kadavath, S.; Patel, N.; Badheka, A.; Noseworthy, P. Impact of atrial fibrillation on outcomes with motor vehicle accidents. Int. J. Cardiol. 2018, 250, 128–132. [Google Scholar] [CrossRef]
- Sztulman, L.; Ritter, A.; de Rosa, R.; Pfeiffer, V.; Leppik, L.; Busse, L.C.; Kontaxi, E.; Stormann, P.; Verboket, R.; Adam, E.; et al. Cardiac damage after polytrauma: The role of systematic transthoracic echocardiography—A pilot study. World J. Emerg. Surg. 2025, 20, 21. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Krishnamoorthy, V.; Vavilala, M.S.; Miller, J.; Minic, Z.; Ohnuma, T.; Laskowitz, D.; Goldstein, B.A.; Ulloa, L.; Sheng, H.; et al. Data analysis protocol for early autonomic dysfunction characterization after severe traumatic brain injury. Front. Neurol. 2024, 15, 1484986. [Google Scholar] [CrossRef] [PubMed]
- Ediga, P.K.; Saradhi, M.V.; Alugolu, R.; Maddury, J. Correlation of head injury with ECG and echo changes. Surg. Neurol. Int. 2024, 15, 296. [Google Scholar] [CrossRef] [PubMed]
- Lele, A.V.; Liu, J.; Kunapaisal, T.; Chaikittisilpa, N.; Kiatchai, T.; Meno, M.K.; Assad, O.R.; Pham, J.; Fong, C.T.; Walters, A.M.; et al. Early Cardiac Evaluation, Abnormal Test Results, and Associations with Outcomes in Patients with Acute Brain Injury Admitted to a Neurocritical Care Unit. J. Clin. Med. 2024, 13, 2526. [Google Scholar] [CrossRef]
- Al-Azayzih, A.; Al-Qerem, W.; Al-Azzam, S.; Muflih, S.; Al-Husein, B.A.; Kharaba, Z.; Kanaan, R.J.; Rahhal, D. Prevalence of Medication Associated with QTc Prolongation Used Among Critically Ill Patients. Vasc. Health Risk Manag. 2024, 20, 27–37. [Google Scholar] [CrossRef]
- Tirupati, S.; Gulati, S. Electrocardiographic abnormalities and psychotropic polypharmacy in schizophrenia and schizoaffective disorders. Australas. Psychiatry 2022, 30, 243–246. [Google Scholar] [CrossRef]
- Drewas, L.; Ghadir, H.; Neef, R.; Delank, K.S.; Wolf, U. Individual Pharmacotherapy Management (IPM)—I: A group-matched retrospective controlled clinical study on prevention of complicating delirium in the elderly trauma patients and identification of associated factors. BMC Geriatr. 2022, 22, 29. [Google Scholar] [CrossRef] [PubMed]
- Ng, P.C.; Schimmel, J.; Wang, G.S. Lacosamide Overdose: A Case of QRS Prolongation and Seizure. J. Emerg. Med. 2019, 56, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Nahid, M.; Musse, M.; Chen, L.; Hilmer, S.N.; Zullo, A.; Kwak, M.J.; Lachs, M.; Levitan, E.B.; Safford, M.M.; et al. Prescribing patterns of fall risk-increasing drugs in older adults hospitalized for heart failure. BMC Cardiovasc. Disord. 2023, 23, 372. [Google Scholar] [CrossRef]
- Zaskey, M.; Seely, K.; Hansen, M.; Burns, A.; Burns, J. Outcomes after stairway falls in a rural Appalachian trauma center. Surgery 2023, 174, 626–630. [Google Scholar] [CrossRef] [PubMed]
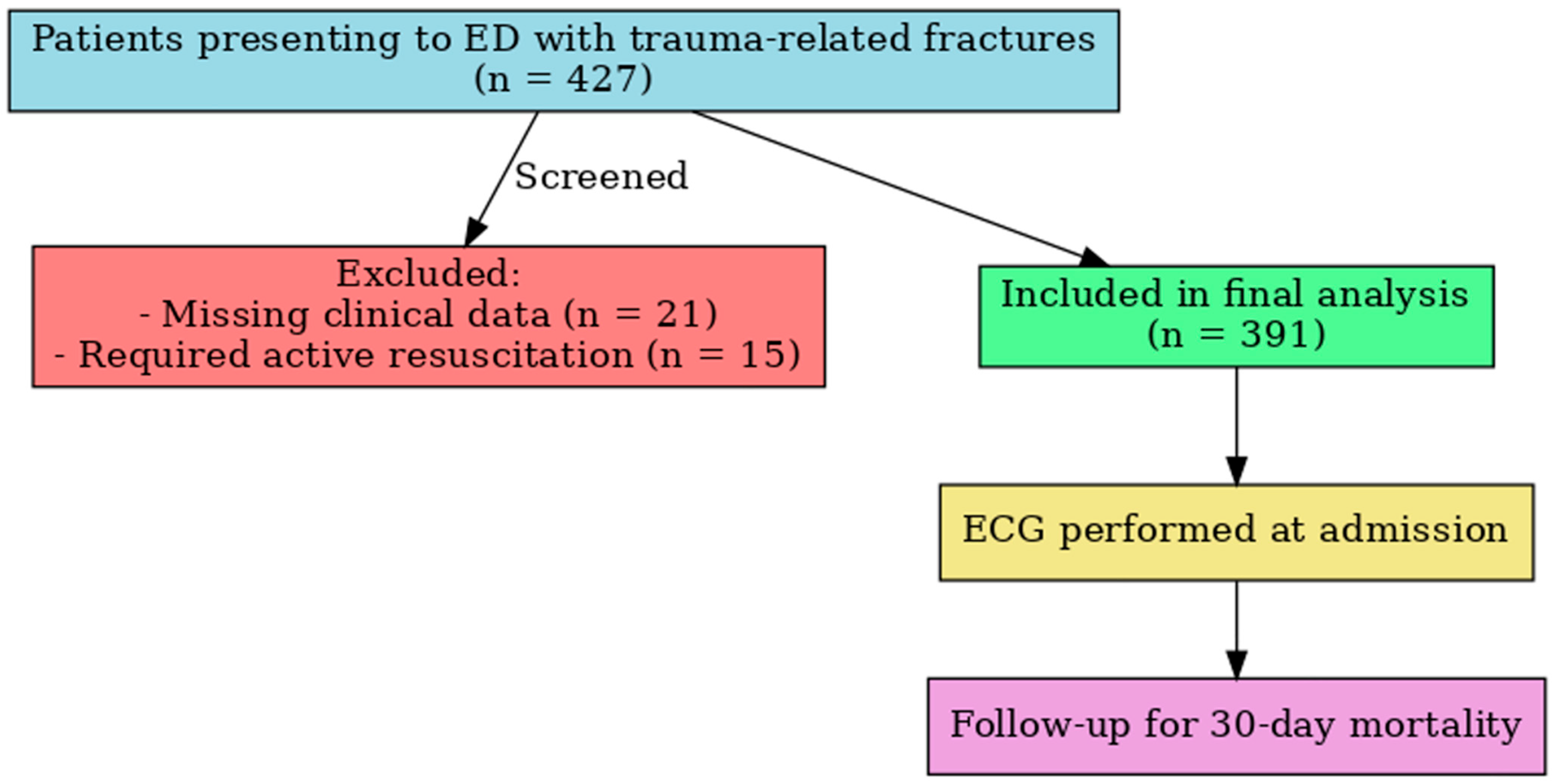
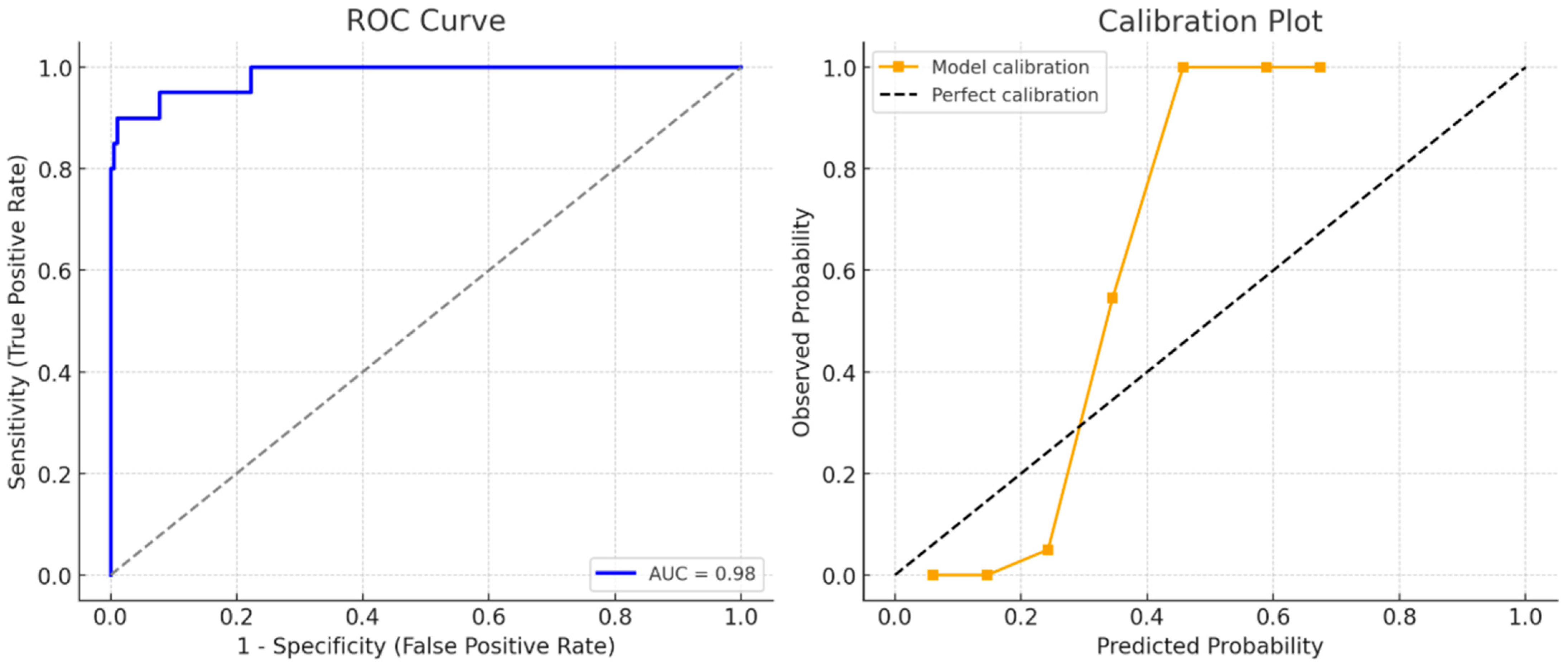
| Characteristic | n (%) or Mean ± SD/Median (IQR) |
|---|---|
| Gender | |
| Male | 195 (49.9%) |
| Female | 196 (50.1%) |
| Polypharmacy | 253 (64.7%) |
| Comorbidities | |
| Diabetes Mellitus | 135 (34.5%) |
| Hypertension | 219 (56.0%) |
| Cerebrovascular Disease | 72 (18.4%) |
| Coronary Artery Disease | 78 (19.9%) |
| Electrocardiographic Findings | |
| Normal Sinus Rhythm | 123 (31.5%) |
| Bundle Branch Block | 60 (15.3%) |
| T Wave Inversion | 36 (9.2%) |
| First-degree AV Block | 14 (3.6%) |
| Sinus Tachycardia | 80 (20.5%) |
| Atrial Fibrillation | 37 (9.5%) |
| Sinus Arrhythmia | 15 (3.8%) |
| Pacemaker Rhythm | 8 (2.0%) |
| Extrasystole | 11 (2.8%) |
| Sinus Bradycardia | 7 (1.8%) |
| Trauma Localization | |
| Head | 81 (20.7%) |
| Thorax | 45 (11.5%) |
| Upper Extremity | 86 (22.0%) |
| Lower Extremity | 61 (15.6%) |
| Pelvis | 52 (13.3%) |
| Thoracic Spine | 31 (7.9%) |
| Lumbar Spine | 35 (9.0%) |
| ED Disposition | |
| Discharged | 234 (59.8%) |
| Admitted to Ward | 118 (30.2%) |
| Admitted to ICU | 39 (10.0%) |
| Mechanism of Injury | |
| Low-energy fall | 281 (71.9%) |
| Fall from stairs | 61 (15.6%) |
| Fall from bed | 31 (7.9%) |
| High-energy fall | 18 (4.6%) |
| 30-day Mortality | 20 (5.1%) |
| Vital Signs | |
| Age (years) | 73.9 ± 6.7/73 (11) |
| Systolic BP (mmHg) | 128.9 ± 22.9/126 (32) |
| Diastolic BP (mmHg) | 72.3 ± 15.3/69 (18) |
| Heart Rate (bpm) | 88.5 ± 17.8/88 (22) |
| SpO2 (%) | 95.3 ± 3.1/95 (4) |
| Characteristic | Survivors (n = 371) | Non-Survivors (n = 20) | p-Value † |
|---|---|---|---|
| n (%) or Mean ± SD/Median (IQR) | |||
| Gender | |||
| Male | 184 (49.6) | 11 (55) | 0.638 |
| Female | 187 (50.4) | 9 (45) | |
| Polypharmacy | 233 (62.8%) | 20 (100%) | 0.001 ** |
| Diabetes Mellitus | 127 (34.2%) | 8 (40.0%) | 0.597 |
| Hypertension | 203 (54.7%) | 16 (80.0%) | 0.026 * |
| Cerebrovascular Disease | 61 (16.4%) | 11 (55.0%) | <0.001 ** |
| Coronary Artery Disease | 70 (18.9%) | 8 (40.0%) | 0.021 * |
| Chronic Kidney Disease | 49 (13.2%) | 2 (10.0%) | 0.678 |
| Vital Signs | p-value †† | ||
| Age (years) | 73.9 ± 6.8/73 (11) | 73.9 ± 6.3/74 (13) | 0.909 |
| Systolic BP (mmHg) | 128.8 ± 22.9/126 (32) | 132.4 ± 23.3/127 (37) | 0.522 |
| Diastolic BP (mmHg) | 72.0 ± 15.3/69 (18) | 77.2 ± 15.5/72 (19) | 0.177 |
| Heart Rate | 88.2 ± 17.9/87 (23) | 94.6 ± 15.2/95 (25) | 0.055 |
| SpO2 (%) | 95.3 ± 3.1/95 (4) | 95.5 ± 3.3/96 (4) | 0.627 |
| Survivors (n = 371) | Non-Survivors (n = 20) | p-Value | |
|---|---|---|---|
| N (%) | |||
| Electrocardiographic Findings | |||
| Normal Sinus Rhythm | 121 (32.6) | 2 (10) | 0.034 * |
| Bundle Branch Block | 58 (15.6) | 2 (10) | 0.496 |
| T Wave Inversion | 34 (9.2) | 2 (10) | 0.900 |
| First-degree AV Block | 13 (3.5) | 1 (5) | 0.726 |
| Sinus Tachycardia | 74 (19.9) | 6 (30) | 0.278 |
| Atrial Fibrillation | 30 (8.1) | 7 (35) | 0.001 ** |
| Sinus Arrhythmia | 15 (4) | - | 0.359 |
| Pacemaker Rhythm | 8 (2.2) | - | 0.507 |
| Extrasystole | 11 (3) | - | 0.435 |
| Sinus Bradycardia | 7 (1.9) | - | 0.535 |
| Trauma Localization | |||
| Head | 67 (18.1) | 14 (70) | <0.001 ** |
| Thorax | 45 (12.1) | - | 0.098 |
| Upper Extremity | 84 (22.6) | 2 (10) | 0.184 |
| Lower Extremity | 61 (16.4) | - | 0.048 * |
| Pelvis | 50 (13.5) | 2 (10) | 0.656 |
| Thoracic Spine | 31 (8.4) | - | 0.178 |
| Lumbar Spine | 33 (8.9) | 2 (10) | 0.866 |
| Mechanism of Injury | |||
| Low-energy fall | 277 (74.7) | 4 (20) | <0.001 ** |
| Fall from stairs | 53 (14.3) | 8 (40) | 0.002 ** |
| Fall from bed | 27 (7.3) | 4 (20) | 0.040 * |
| High-energy fall | 14 (3.8) | 4 (20) | 0.001 ** |
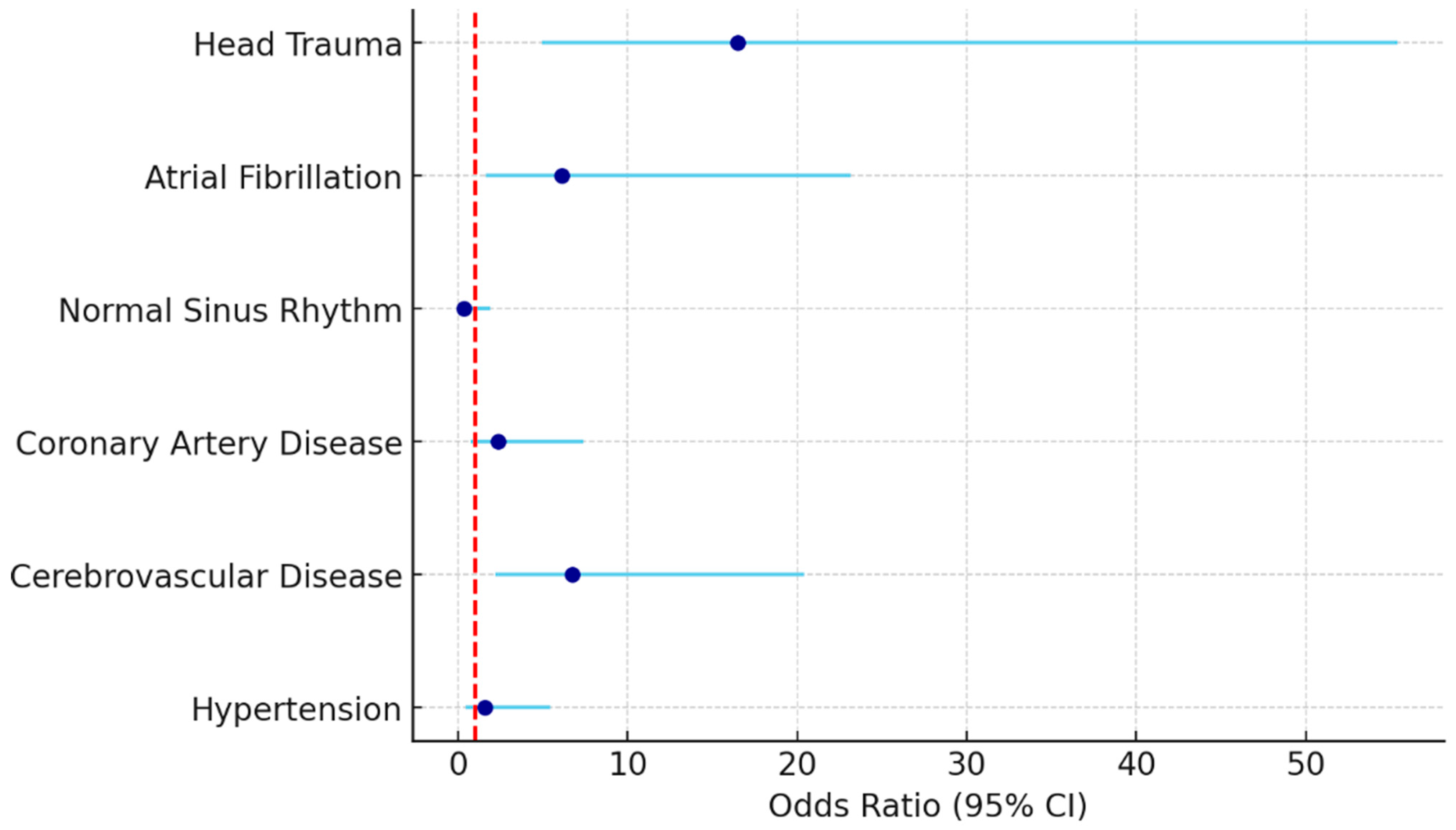
| Variables | p-Value | OR | %95 CI | |
|---|---|---|---|---|
| Lower | Upper | |||
| Hypertension | 0.478 | 1.568 | 0.453 | 5.434 |
| Cerebrovascular Disease | 0.001 | 6.725 | 2.219 | 20.385 |
| Coronary Artery Disease | 0.141 | 2.359 | 0.752 | 7.406 |
| Normal Sinus Rhythm | 0.237 | 0.370 | 0.071 | 1.921 |
| Atrial Fibrillation | 0.008 | 6.112 | 1.612 | 23.176 |
| Head Trauma | <0.001 | 16.514 | 4.925 | 55.367 |
| Intercept | <0.001 | 0.115 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozdemir, S.; Oktay, M.M.; Tiftikci, I.; Altinsoy, K.E. Electrocardiographic Predictors for Early Risk Stratification: 30-Day Mortality in Older Adult Trauma Patients. J. Clin. Med. 2025, 14, 6659. https://doi.org/10.3390/jcm14186659
Ozdemir S, Oktay MM, Tiftikci I, Altinsoy KE. Electrocardiographic Predictors for Early Risk Stratification: 30-Day Mortality in Older Adult Trauma Patients. Journal of Clinical Medicine. 2025; 14(18):6659. https://doi.org/10.3390/jcm14186659
Chicago/Turabian StyleOzdemir, Sedat, Mehmet Murat Oktay, Iffet Tiftikci, and Kazim Ersin Altinsoy. 2025. "Electrocardiographic Predictors for Early Risk Stratification: 30-Day Mortality in Older Adult Trauma Patients" Journal of Clinical Medicine 14, no. 18: 6659. https://doi.org/10.3390/jcm14186659
APA StyleOzdemir, S., Oktay, M. M., Tiftikci, I., & Altinsoy, K. E. (2025). Electrocardiographic Predictors for Early Risk Stratification: 30-Day Mortality in Older Adult Trauma Patients. Journal of Clinical Medicine, 14(18), 6659. https://doi.org/10.3390/jcm14186659






