Value of Continuous Hemofiltration in Patients with Severe Acute Pancreatitis at Onset: Single Centre Experience on 48 Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Clinical Setting
2.2. Patient Selection Criteria
2.3. Preoperative Assessment and Surgical Procedure
2.4. Hemofiltration with oXiris Filter
- Filter: oXiris (AN69 membrane modified with polyethyleneimine coating and heparinised), with adsorption capacity for endotoxins and cytokines.
- Mode: continuous convective hemofiltration (pre-dilution).
- Blood flow rate: ≥75 mL/min (standard 180 mL/min).
- Ultrafiltration dose: 35 mL/kg/h, delivered with balanced replacement solution (PrismaSol) in pre-dilution.
- Anticoagulation: low-molecular-weight heparin continuous infusion (5–10 U/kg/h) or citrate.
- Filter replacement: every 24 h or earlier in the event of circuit clotting.
- Treatment duration: minimum 72 h, extended until haemodynamic stabilisation and reduction in inflammatory markers (mean 5 days, range 3–7 days).
2.5. Outcome Measures and Data Collection
- Demographic and clinical data: age, sex, aetiology of pancreatitis, and comorbidities.
- Inflammatory markers: leukocyte count, serum C-reactive protein (CRP), procalcitonin (PCT), TNF-α and IL-6 measured in serum, peritoneal lavage fluid, and CVVH ultrafiltrate.
- Haemodynamic parameters: heart rate, mean arterial pressure (MAP), lactate levels, pH, and base excess.
- Organ function indices: serum creatinine, PaO2/FiO2 ratio, intra-abdominal pressure.
- Intraoperative microbiological cultures from intra-abdominal collections.
- Prognostic scores: APACHE II and SOFA, calculated daily.
- Adverse events: hypotension, filter clotting, electrolyte disturbances.
2.6. Statistical Analysis
2.7. Ethical Considerations
3. Results
3.1. Population and Clinical Characteristics
3.2. Primary and Secondary Outcomes
3.2.1. Tolerability and Survival
3.2.2. Hospital Stay
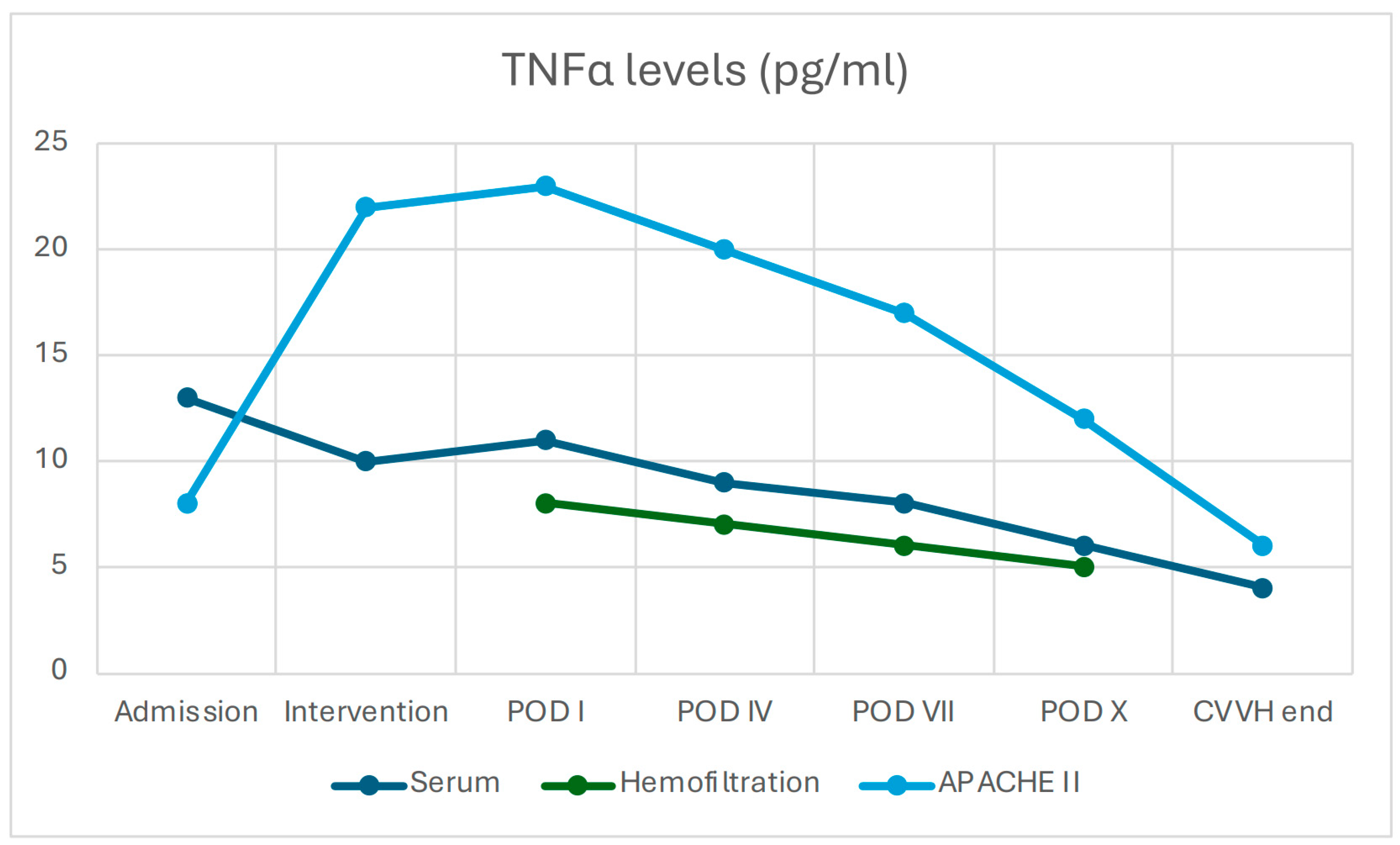
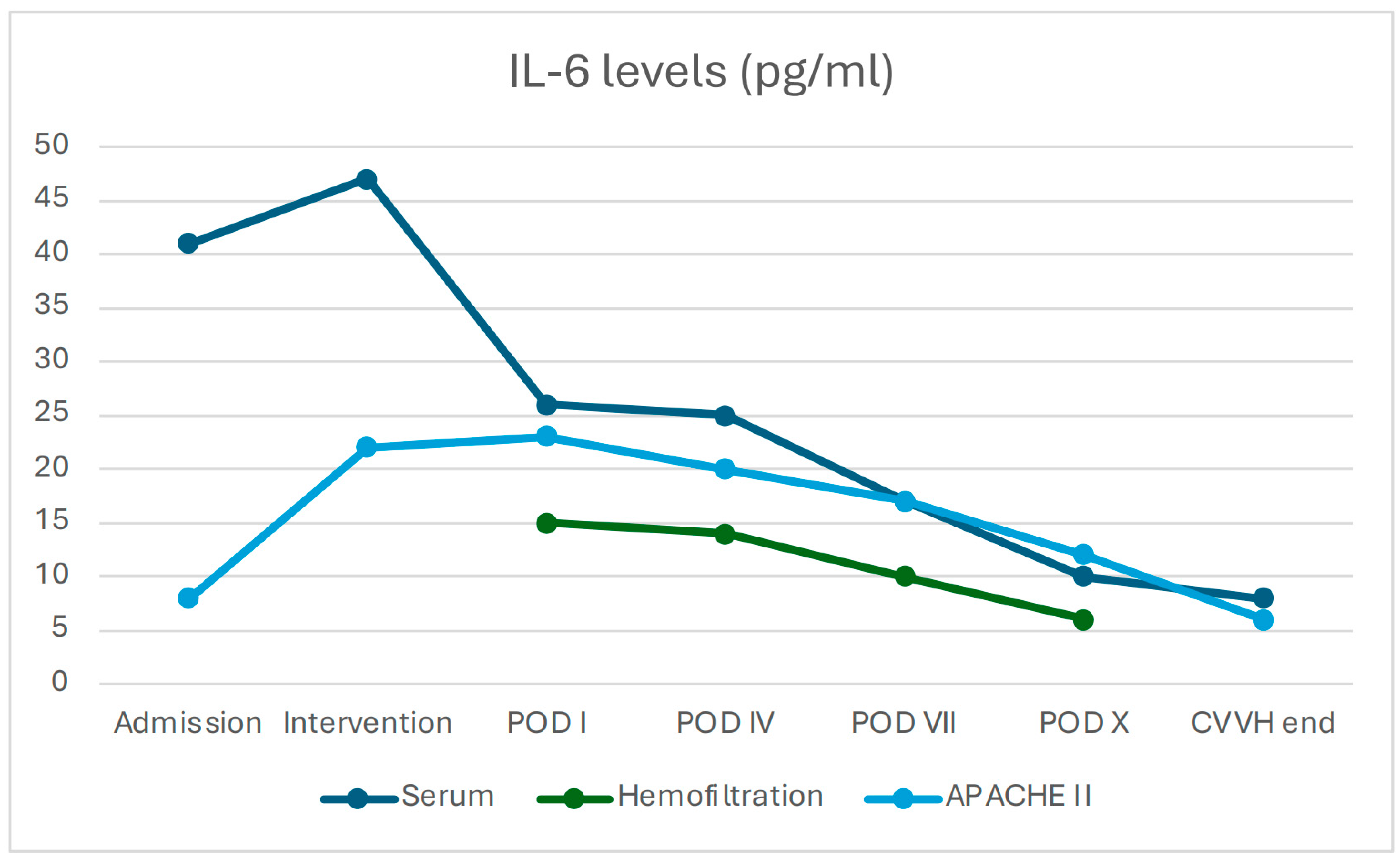
3.2.3. Changes in Inflammatory Biomarkers and Clinical Scores
3.2.4. Filter Performance
3.2.5. Prognostic Scores
3.2.6. Safety Profile
4. Discussion
4.1. Pathophysiological Mechanisms Involved
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SAP | Severe acute pancreatitis |
| MODS | Multiple Organ Dysfunction Syndrome |
| SIRS | Systemic Inflammatory Response Syndrome |
| CVVH | Continuous Veno-Venous Hemofiltration |
| HVHF | High-Volume Hemofiltration |
| CRRT | Continuous Renal Replacement Therapy |
| AN69 | Acrylonitrile–metallyl sulfonate sodium copolymer 69 |
| PEI | Polyethyleneimine |
| TMP | Transmembrane Pressure |
| APACHE II | Acute Physiology and Chronic Health Evaluation II |
| SOFA | Sequential Organ Failure Assessment |
| ICU | Intensive Care Unit |
| POD | Post-Operative Day |
| CVC | Central Venous Catheter |
| COPD | Chronic Obstructive Pulmonary Disease |
| CKD | Chronic Kidney Disease |
| DM | Diabetes Mellitus |
| ALL | Acute Lymphoblastic Leukaemia |
| CRP | C-Reactive Protein |
| PCT | Procalcitonin |
| MAP | Mean Arterial Pressure |
| ARDS | Acute Respiratory Distress Syndrome |
References
- Leppäniemi, A.; Tolonen, M.; Tarasconi, A.; Segivua-Lohse, H.; Gamberini, E.; Kirkpatrick, A.W.; Ball, C.G.; Sartelli, M.; Wolbrink, D.; van Goor, H.; et al. 2019 WSES guidelines for the management of severe acute pancreatitis. World J. Emerg. Surg. 2019, 14, 27. [Google Scholar] [CrossRef]
- Pupelis, G.; Plaudis, H.; Grigane, A.; Zeiza, K.; Purmalis, G. Continuous veno-venous haemofiltration in the treatment of severe acute pancreatitis: 6-year experience. HPB 2007, 9, 295–301. [Google Scholar] [CrossRef]
- Hu, Y.; Xiong, W.; Li, C.; Cui, Y. Continuous blood purification for severe acute pancreatitis: A systematic review and meta-analysis. Medicine 2019, 98, e14873. [Google Scholar] [CrossRef]
- Zerem, E. Treatment of severe acute pancreatitis and its complications. World J. Gastroenterol. 2014, 20, 13879–13892. [Google Scholar] [CrossRef]
- Xu, J.; Yang, H.; Tian, X. Effects of early hemofiltration on organ function and intra-abdominal pressure in severe acute pancreatitis patients with abdominal compartment syndrome. Clin. Nephrol. 2019, 91, 237–243. [Google Scholar] [CrossRef]
- Petrov, M.S.; Shanbhag, S.; Chakraborty, M.; Phillips, A.R.; Windsor, J.A. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology 2010, 139, 813–820. [Google Scholar] [CrossRef]
- Li, Y.; Sun, P.; Chang, K.; Yang, M.; Deng, N.; Chen, S.; Su, B. Effect of continuous renal replacement therapy with the oXiris hemofilter on critically ill patients: A narrative review. J. Clin. Med. 2022, 11, 6719. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; He, Y.; Guo, Q.; Zhao, Y.; He, J.; Chen, Y.; Chen, W.; Zhou, Y.; Peng, Z.; Deng, K.; et al. Continuous renal replacement therapy with the adsorptive oXiris filter may be associated with the lower 28-day mortality in sepsis: A systematic review and meta-analysis. Crit. Care 2023, 27, 54. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, C.; Ouyang, L.; Peng, Y.; Zhong, D.; Xiang, X.; Li, J. Application of oXiris-continuous hemofiltration adsorption in patients with sepsis and septic shock: A single-centre experience in China. Front. Public Health 2022, 10, 1012998. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Cao, T. CRRT with the oXiris filter attenuates IL-6 in a patient with severe COVID-19. J. Am. Soc. Nephrol. 2021, 32, 10S1103C. [Google Scholar] [CrossRef]
- Wong, E.; Ong, V.; Remani, D.; Wong, W.; Haroon, S.; Lau, T.; Nyeo, H.-Q.; Mukhopadhyay, A.; Tan, B.-H.; Chua, H.R. Filter life and safety of heparin-grafted membrane for continuous renal replacement therapy: A randomized controlled trial. Semin. Dial. 2021, 34, 300–308. [Google Scholar] [CrossRef]
- Broman, M.E.; Hansson, F.; Vincent, J.L.; Bodelsson, M. Endotoxin and cytokine reducing properties of the oXiris membrane in patients with septic shock: A randomized crossover double-blind study. PLoS ONE 2019, 14, e0220444. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, M.J.; Lin, X.; Jiang, J.H.; Zhuo, H.C. Comparative efficacy of two hemopurification filters for treating intra-abdominal sepsis: A retrospective study. Chin. J. Traumatol. 2025, 28, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Chen, Z.; Li, P.; Tang, X.; Marshall, M.R.; Zhang, L.; Fu, P. Early use of endotoxin absorption by oXiris in abdominal septic shock: A case report. Medicine 2020, 99, e19632. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sun, M.; Mao, X.; Liu, X.; Sun, W. Effectiveness of continuous veno-venous hemofiltration in the treatment of severe acute pancreatitis. Exp. Ther. Med. 2019, 17, 2720–2724. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhou, Q.; Chen, M. High-volume hemofiltration reduces short-term mortality with no influence on the incidence of MODS, hospital stay, and hospitalization cost in patients with severe acute pancreatitis: A meta-analysis. Artif. Organs 2021, 45, 1411–1424. [Google Scholar] [CrossRef]
- Guo, Y.; Cao, F.; Li, C.; Yang, H.; Xia, S.; Li, F. Continuous hemofiltration reduces mortality in severe acute pancreatitis: A meta-analysis. Emerg. Med. Int. 2020, 2020, 6474308. [Google Scholar] [CrossRef]
- Jiang, H.L.; Xue, W.J.; Li, D.Q.; Yin, A.-P.; Xin, X.; Li, C.; Gao, J. Influence of continuous veno-venous hemofiltration on the course of acute pancreatitis. World J. Gastroenterol. 2005, 11, 4815–4821. [Google Scholar] [CrossRef]
- Lin, Y.; He, S.; Gong, J.; Liu, Z.; Ding, X.; Gong, J.; Zeng, Z.; Cheng, Y. Continuous veno-venous hemofiltration for severe acute pancreatitis. Cochrane Database Syst. Rev. 2019, 10, CD012959. [Google Scholar] [CrossRef]
- Xie, Y.; Yuan, Y.; Su, W.; Qing, N.; Xin, H.; Wang, X.; Tian, J.; Li, Y.; Zhu, J. Effect of continuous hemofiltration on severe acute pancreatitis with different intra-abdominal pressure: A cohort study. Medicine 2021, 100, e27641. [Google Scholar] [CrossRef]
- Caronna, R.; Benedetti, M.; Morelli, A.; Rocco, M.; Diana, L.; Prezioso, G.; Cardi, M.; Schiratti, M.; Fanello, G.; Farelli, F.; et al. Clinical effects of laparotomy with perioperative continuous peritoneal lavage and postoperative hemofiltration in patients with severe acute pancreatitis. World J. Emerg. Surg. 2009, 4, 45. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Huang, W.; Yang, X.N.; Jin, Y.; Altaf, K.; Javed, M.; Lin, Z.-Q.; Huang, Z.-W.; Xue, P.; Johnstone, M.; et al. Short-term continuous high-volume hemofiltration on clinical outcomes of severe acute pancreatitis. Pancreas 2014, 43, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wei, S.R.; Ding, T.; Zhang, L.-P.; Weng, Z.H.; Cheng, M.; Zhou, Y.; Zhang, M.; Liu, F.-J.; Yan, B.-B.; et al. Continuous renal replacement therapy with oXiris® in patients with hematologically malignant septic shock: A retrospective study. World J. Clin. Cases 2023, 11, 6073–6082. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dai, G.F.; Xiao, W.B.; Shi, J.-S.; Lin, B.; Lin, J.; Xiao, X. Effects of continuous venous-venous hemofiltration with or without hemoperfusion on patients with hypertriglyceride acute pancreatitis. Clin. Res. Hepatol. Gastroenterol. 2025, 49, 102572. [Google Scholar] [CrossRef]
- Sun, S.; He, L.; Bai, M.; Liu, H.; LI, Y.; Li, L.; Yu, Y.; Shou, M.; Jing, R.; Zhao, L.; et al. High-volume hemofiltration plus hemoperfusion for hyperlipidemic severe acute pancreatitis: A controlled pilot study. Ann. Saudi Med. 2015, 35, 352–358. [Google Scholar] [CrossRef]
- Cui, H.X.; Xu, J.Y.; Li, M.Q. Efficacy of continuous renal replacement therapy in the treatment of severe acute pancreatitis associated acute respiratory distress syndrome. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2523–2526. [Google Scholar]
- Yadav, S.C.; Zhang, B. Effect of early continuous veno-venous haemofiltration in severe acute pancreatitis for the prevention of local pancreatic complications. Gastroenterol. Res. Pract. 2022, 2022, 7575231. [Google Scholar] [CrossRef]
- Mielnicki, W.; Dyla, A.; Zając, M.; Rokicka-Demitraszek, N.; Smeraka, J. Does continuous renal replacement therapy with oXiris in septic shock have any positive impact? Single-centre experience with oXiris therapy in septic shock patients. J. Clin. Med. 2024, 13, 7527. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Y.; Zhao, L.; Kang, K. Continuous renal replacement therapy in combination with oXiris haemofilter in a paediatric patient with sodium valproate-induced acute pancreatitis. BMJ Case Rep. 2025, 18, e258126. [Google Scholar] [CrossRef]
| Sex, n (%) | F: 30 (62.5) M: 18 (37.5) |
| Age, years, mean ± SD | 60.4 ± 18 |
| Aetiology, n (%) | Biliary lithiasis, 34 (70.8) Alcoholic pancreatitis, 8 (16.7) Hypertriglyceridemia, 3 (6.3) Asparaginase-induced pancreatitis (ALL), 3 (6.3) |
| Comorbidities, n (%) | Arterial hypertension, 21 (43.8) COPD, 4 (8.3) CKD, 6 (12.5) DM, 9 (18.8) Obesity (BMI > 30), 17 (35.4) |
| Primary Outcomes | |
| Tolerability, n (%) | 48 (100) |
| Survival, n (%) | 47 (97.9) |
| Secondary Outcomes | |
| Surgical Complications, n (%) | Enteric fistula, 1 (2.1) Major bleeding– ischemic complications– |
| Hospital Stay, days, mean ± SD | 28.5 ± 19 |
| Microorganisms isolated from intraoperative cultures, n (%) | Enterococcus spp., 30 (62.5) Escherichia Coli, 10 (20.8) Pseudomonas Aeruginosa, 5 (10.4) Acinetobacter baumannii, 1 (2.1) Sterile cultures, 2 (4.2) |
| ICU stay, days, mean ± SD | 13.3 ± 11 |
| CVVH adverse events, n (%) | Fever from CVC, 1 (2.1) Hypophosphatemia, 2 (4.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saullo, P.; Caronna, R.; Angelici, A.M.; Rinaldi, V.; Liberatori, G.; Mingoli, A.; Chirletti, P. Value of Continuous Hemofiltration in Patients with Severe Acute Pancreatitis at Onset: Single Centre Experience on 48 Patients. J. Clin. Med. 2025, 14, 6647. https://doi.org/10.3390/jcm14186647
Saullo P, Caronna R, Angelici AM, Rinaldi V, Liberatori G, Mingoli A, Chirletti P. Value of Continuous Hemofiltration in Patients with Severe Acute Pancreatitis at Onset: Single Centre Experience on 48 Patients. Journal of Clinical Medicine. 2025; 14(18):6647. https://doi.org/10.3390/jcm14186647
Chicago/Turabian StyleSaullo, Paolina, Roberto Caronna, Alberto Maria Angelici, Valerio Rinaldi, Giovanni Liberatori, Andrea Mingoli, and Piero Chirletti. 2025. "Value of Continuous Hemofiltration in Patients with Severe Acute Pancreatitis at Onset: Single Centre Experience on 48 Patients" Journal of Clinical Medicine 14, no. 18: 6647. https://doi.org/10.3390/jcm14186647
APA StyleSaullo, P., Caronna, R., Angelici, A. M., Rinaldi, V., Liberatori, G., Mingoli, A., & Chirletti, P. (2025). Value of Continuous Hemofiltration in Patients with Severe Acute Pancreatitis at Onset: Single Centre Experience on 48 Patients. Journal of Clinical Medicine, 14(18), 6647. https://doi.org/10.3390/jcm14186647








