Impact of Guided Implant Dentistry on Patient Quality of Life, Satisfaction, and Psychological Well-Being: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection and Data Extraction
2.2. Data Analysis
2.3. Quality of Included Studies
3. Results
3.1. Search Results
3.2. Characteristics of the Included Studies
3.3. Sample Size and Sociodemographic Characteristics
3.4. Quality Assessment
3.5. Results of the Clinical Variables from the Included Studies
3.6. Results of the Instruments Used to Assess Patient Perception
3.7. Results of the Validation of Patient Perception
3.8. Results of the Study Conclusions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marshall, S.; Haywood, K.; Fitzpatrick, R. Impact of patient-reported outcome measures on routine practice: A structured review. J. Eval. Clin. Pract. 2006, 12, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Allen, P.F. Assessment of oral health related quality of life. Health Qual. Life Outcomes 2003, 1, 40. [Google Scholar] [CrossRef]
- McGrath, C.; Bedi, R. A national study of the importance of oral health to life quality to inform scales of oral health related quality of life. Qual. Life Res. 2004, 13, 813–818. [Google Scholar] [CrossRef]
- Stucki, G. International Classification of Functioning, Disability, and Health (ICF): A Promising Framework and Classification for Rehabilitation Medicine. Am. J. Phys. Med. Rehabil. 2005, 84, 733–740. [Google Scholar] [CrossRef]
- Knezović Zlatarić, D.; Čelebić, A.; Valentić-Peruzović, M. The effect of removable partial dentures on periodontal health of abutment and non-abutment teeth. J. Periodontol. 2002, 73, 137–144. [Google Scholar] [CrossRef]
- Cunha, L.D.A.P.; Pellizzer, E.P.; Verri, F.R.; Pereira, J.A. Evaluation of the influence of location of osseointegrated implants associated with mandibular removable partial dentures. Implant Dent. 2008, 17, 278–287. [Google Scholar] [CrossRef]
- Velasco-Ortega, E.; Jiménez-Martin, I.R.; Moreno-Muñoz, J.; Nuñez-Márquez, E.; Rondón-Romero, J.L.; Cabanillas-Balsera, D.; Jiménez-Guerra, A.; Ortiz-Garcia, I.; López-López, J.; Monsalve-Guil, L. Long-term treatment outcomes of implant prostheses in partially and totally edentulous patients. Materials 2022, 15, 4910. [Google Scholar] [CrossRef] [PubMed]
- AL-Omiri, M.; Hantash, R.A.; AL-Wahadni, A. Satisfaction with dental implants: A literature review. Implant Dent. 2005, 14, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Monsalve-Guil, L.; Velasco-Ortega, E.; Ortiz-Garcia, I.; Matos-Garrido, N.; Moreno-Muñoz, J.; Núñez-Márquez, E.; Rondón-Romero, J.L.; López-López, J.; Jiménez-Guerra, Á. Retrospective clinical follow-up of implants placed in edentulous jaws after computer-guided surgery and immediate loading, in geriatric patients. Med. Oral Patol. Oral Cir. Bucal 2025, 30, e76–e85. [Google Scholar] [CrossRef]
- Rouzé l’Alzit, F.; Cade, R.; Naveau, A.; Babilotte, J.; Meglioli, M.; Catros, S. Accuracy of commercial 3D printers for the fabrication of surgical guides in dental implantology. J. Dent. 2022, 117, 103909. [Google Scholar] [CrossRef]
- Tahmaseb, A.; Wismeijer, D.; Coucke, W.; Derksen, W. Computer technology applications in surgical implant dentistry: A systematic review. Int. J. Oral Maxillofac. Implant. 2014, 29, 25–42. [Google Scholar] [CrossRef]
- Zhao, W.; Teng, W.; Su, Y.; Zhou, L. Accuracy of dental implant surgery with freehand, static computer-aided, dynamic computer-aided, and robotic computer-aided implant systems: An in vitro study. J. Prosthet. Dent. 2024. [Google Scholar] [CrossRef]
- Fonteyne, E.; De Bruyn, H.; De Fruyt, F. Quality of life and social participation in dental rehabilitation: A personality and multi-informant perspective. J. Dent. 2020, 103S, 100021. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The STROBE Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Clark, H.D.; Wells, G.A.; Huët, C.; McAlister, F.A.; Salmi, L.R.; Fergusson, D.; Laupacis, A. Assessing the quality of randomized trials: Reliability of the Jadad scale. Control. Clin. Trials 1999, 20, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Evid. Synth. 2020, 18, 2127–2133. [Google Scholar] [CrossRef]
- Montero, J.; Dolz, J.; Silvestre, F.J.; Flores, J.; Dib, A.; Gómez-Polo, C. Changes in oral health-related quality of life after three different strategies of implant therapy: A clinical trial. Odontology 2019, 107, 383–392. [Google Scholar] [CrossRef]
- Merli, M.; Bernardelli, F.; Esposito, M. Computer-guided flapless placement of immediately loaded dental implants in the edentulous maxilla: A pilot prospective case series. Eur. J. Oral Implantol. 2008, 1, 61–69. [Google Scholar] [PubMed]
- Fortin, T.; Bosson, J.L.; Isidori, M.; Blanchet, E. Effect of flapless surgery on pain experienced in implant placement using an image-guided system. Int. J. Oral Maxillofac. Implant. 2006, 21, 298–304. [Google Scholar] [PubMed]
- Lindeboom, J.A.; Van Wijk, A.J. A comparison of two implant techniques on patient-based outcome measures: A report of flapless vs. conventional flapped implant placement. Clin. Oral Implant. Res. 2010, 21, 366–370. [Google Scholar] [CrossRef]
- Vercruyssen, M.; De Laat, A.; Coucke, W.; Quirynen, M. An RCT comparing patient-centred outcome variables of guided surgery (bone- or mucosa-supported) with conventional implant placement. J. Clin. Periodontol. 2014, 41, 724–732. [Google Scholar] [CrossRef]
- Vercruyssen, M.; Van De Wiele, G.; Teughels, W.; Naert, I.; Jacobs, R.; Quirynen, M. Implant- and patient-centred outcomes of guided surgery, a 1-year follow-up: An RCT comparing guided surgery with conventional implant placement. J. Clin. Periodontol. 2014, 41, 1154–1160. [Google Scholar] [CrossRef]
- Jorba-García, A.; Bara-Casaus, J.J.; Camps-Font, O.; Sánchez-Garcés, M.Á.; Figueiredo, R.; Valmaseda-Castellón, E. Accuracy of dental implant placement with or without the use of a dynamic navigation-assisted system: A randomized clinical trial. Clin. Oral Implant. Res. 2023, 34, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, A.; Tallarico, M.; Moy, P.K. Four-implant overdenture fully supported by a CAD-CAM titanium bar: A single-cohort prospective 1-year preliminary study. J. Prosthet. Dent. 2016, 116, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Sancho-Puchades, M.; Alfaro, F.; Naenni, N.; Jung, R.; Hämmerle, C.; Schneider, D. A randomized controlled clinical trial comparing conventional and computer-assisted implant planning and placement in partially edentulous patients. Part 2: Patient-related outcome measures. Int. J. Periodontics Restor. Dent. 2019, 39, e99–e110. [Google Scholar] [CrossRef] [PubMed]
- Frizzera, F.; Calazans, N.N.N.; Pascoal, C.H.; Martins, M.E.; Mendonça, G. Flapless guided implant surgeries compared with conventional surgeries performed by nonexperienced individuals: Randomized and controlled split-mouth clinical trial. Int. J. Oral Maxillofac. Implant. 2021, 36, 755–761. [Google Scholar] [CrossRef]
- Nirula, P.; Selvaganesh, S.; Thiyaneswaran, N. Feedback on dental implants with dynamic navigation versus freehand. Bioinformation 2023, 19, 290–294. [Google Scholar] [CrossRef]
- Engkawong, S.; Mattheos, N.; Pisarnturakit, P.P.; Pimkhaokham, A.; Subbalekha, K. Comparing patient-reported outcomes and experiences among static, dynamic computer-aided, and conventional freehand dental implant placement: A randomized clinical trial. Clin. Implant. Dent. Relat. Res. 2021, 23, 660–670. [Google Scholar] [CrossRef]
- Campos, L.A.; Peltomäki, T.; Marôco, J.; Campos, J.A.D.B. Use of Oral Health Impact Profile-14 (OHIP-14) in different contexts: What is being measured? Int. J. Environ. Res. Public Health 2021, 18, 13412. [Google Scholar] [CrossRef]
- Sischo, L.; Broder, H.L. Oral health-related quality of life: What, why, how, and future implications. J. Dent. Res. 2011, 90, 1264–1270. [Google Scholar] [CrossRef]
- Delgado, D.A.; Lambert, B.S.; Boutris, N.; McCulloch, P.C.; Robbins, A.B.; Moreno, M.R.; Harris, J.D. Validation of digital visual analog scale pain scoring with a traditional paper-based visual analog scale in adults. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2018, 2, e088. [Google Scholar] [CrossRef] [PubMed]
- Black, N.; Varaganum, M.; Hutchings, A. Relationship between patient-reported experience (PREMs) and patient-reported outcomes (PROMs) in elective surgery. BMJ Qual. Saf. 2014, 23, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Seymour, R.A.; Charlton, J.E.; Phillips, M.E. An evaluation of dental pain using visual analogue scales and the Mcgill Pain Questionnaire. J. Oral Maxillofac. Surg. 1983, 41, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, M.; Wilner, N.; Alvarez, W. Impact of Event Scale: A measure of subjective stress. Psychosom. Med. 1979, 41, 209–218. [Google Scholar] [CrossRef]
- Kvale, G.; Berg, E.; Raadal, M. The ability of Corah’s Dental Anxiety Scale and Spielberger’s State Anxiety Inventory to distinguish between fearful and regular Norwegian dental patients. Acta Odontol. Scand. 1998, 56, 105–109. [Google Scholar] [CrossRef]
- Yao, C.J.; Cao, C.; Bornstein, M.M.; Mattheos, N. Patient-reported outcome measures of edentulous patients restored with implant-supported removable and fixed prostheses: A systematic review. Clin. Oral Implant. Res. 2018, 29 (Suppl. S16), 241–254. [Google Scholar] [CrossRef]
- Yeo, X.H.; Uei, L.J.; Yi, M.; Kungsadalpipob, K.; Subbalehka, K.; Al-Nawas, B.; Mattheos, N. Computer-Assisted Implant Surgery: Patients’ Experience and Perspectives. Clin. Exp. Dent. Res. 2025, 11, e70143. [Google Scholar] [CrossRef]
- Lanis, A.; Peña-Cardelles, J.F.; Negreiros, W.M.; Hamilton, A.; Gallucci, G.O. Impact of digital technologies on implant surgery in fully edentulous patients: A scoping review. Clin. Oral Implant. Res. 2024, 35, 1000–1010. [Google Scholar] [CrossRef]
- Ashy, L.M. Clinicians’ Attitude Toward Computer-Guided Implant Surgery Approach: Survey in Saudi Arabia. Pragmat. Obs. Res. 2021, 12, 1–8. [Google Scholar] [CrossRef]
- Alnafaiy, S.M.; Alyousef, H.; Aljabr, R.; Tounsi, A.; Almutairi, R.; Albaijan, R.S. Digital technology implementation in prosthodontics postgraduate programs in Saudi Arabia: A multi-institutional survey of program directors. BMC Oral Health 2024, 24, 1136. [Google Scholar] [CrossRef]
- Pjetursson, B.E.; Tan, W.C.; Zwahlen, M.; Lang, N.P. A systematic review of the success of sinus floor elevation and survival of implants inserted in combination with sinus floor elevation: Part I: Lateral approach. J. Clin. Periodontol. 2008, 35 (Suppl. S8), 216–240. [Google Scholar] [CrossRef]
- Pommer, B.; Zechner, W.; Watzak, G.; Ulm, C.; Watzek, G.; Tepper, G. Progress and trends in patients’ mindset on dental implants. II: Implant acceptance, patient-perceived costs and patient satisfaction. Clin. Oral Implant. Res. 2011, 22, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Joda, T.; Brägger, U. Digital vs. conventional implant prosthetic workflows: A cost/time analysis. Clin. Oral Implant. Res. 2015, 26, 1430–1435. [Google Scholar] [CrossRef] [PubMed]
- Aghaloo, T.L.; Moy, P.K. Which hard tissue augmentation techniques are the most successful in furnishing bony support for implant placement? Int. J. Oral Maxillofac. Implant. 2007, 22, 49–70. [Google Scholar] [PubMed]
- Sankar, H.; Shalini, M.; Rajagopalan, A.; Gupta, S.; Kumar, A.; Shouket, R. Dental implant placement accuracy with robotic surgery compared to free-hand, static and dynamic computer assisted techniques: Systematic review and meta-analysis. J. Oral Biol. Craniofac. Res. 2025, 15, 69–76. [Google Scholar] [CrossRef]
- Schiavon, L.; Mancini, L.; Settecase, E.; Jung, R.E.; Joda, T. Does Computer-Assisted Surgery Improve the Accuracy of Immediate Implant Placement? A Systematic Review and Network Meta-Analysis. J. Periodontal Res. 2025. [Google Scholar] [CrossRef]
- Mittal, H.; John, M.T.; Sekulić, S.; Theis-Mahon, N.; Rener-Sitar, K. Patient-Reported Outcome Measures for Adult Dental Patients: A Systematic Review. J. Evid. Based Dent. Pract. 2019, 19, 53–70. [Google Scholar] [CrossRef]
- Hua, F. Dental patient-reported outcomes update 2022. J. Evid. Based Dent. Pract. 2023, 23, 101802. [Google Scholar] [CrossRef]
- Schierz, O.; Reissmann, D.R. Dental patient-reported outcomes—The promise of dental implants. J. Evid. Based Dent. Pract. 2021, 21, 101541. [Google Scholar] [CrossRef]
- Omara, M.; Stamm, T.; Bekes, K. Lessons learned from the first steps of implementing value-based oral health care: A case study from the Medical University of Vienna. J. Evid. Based Dent. Pract. 2023, 23, 101791. [Google Scholar] [CrossRef]
- Reissmann, D.R. Methodological considerations when measuring oral health-related quality of life. J. Oral Rehabil. 2021, 48, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Chanthavisouk, P.; John, M.T.; Paulson, D.; Pattanaik, S. Commonalities among dental patient-reported outcomes (dPROs)-A Delphi consensus study. PLoS ONE 2022, 17, e0268750. [Google Scholar] [CrossRef] [PubMed]
- Ala, L.A.B.; Nogueira, T.E.; Leles, C.R. One-year prospective study on single short (7-mm) implant overdentures in patients with severely resorbed mandibles. Clin. Oral Implant. Res. 2022, 33, 291–301. [Google Scholar] [CrossRef]
- Thoma, D.S.; Strauss, F.J.; Mancini, L.; Gasser, T.J.W.; Jung, R.E. Minimal invasiveness in soft tissue augmentation at dental implants: A systematic review and meta-analysis of patient-reported outcome measures. Periodontology 2000 2023, 91, 182–198. [Google Scholar] [CrossRef] [PubMed]
- Kunavisarut, C.; Santivitoonvong, A.; Chaikantha, S.; Pornprasertsuk-Damrongsri, S.; Joda, T. Patient-reported outcome measures comparing static computer-aided implant surgery and conventional implant surgery for single-tooth replacement: A randomized controlled trial. Clin. Oral Implant. Res. 2022, 33, 278–290. [Google Scholar] [CrossRef]
- Abou-Ayash, S.; Fonseca, M.; Pieralli, S.; Reissmann, D.R. Treatment effect of implant-supported fixed complete dentures and implant overdentures on patient-reported outcomes: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2023, 34 (Suppl. S26), 177–195. [Google Scholar] [CrossRef]
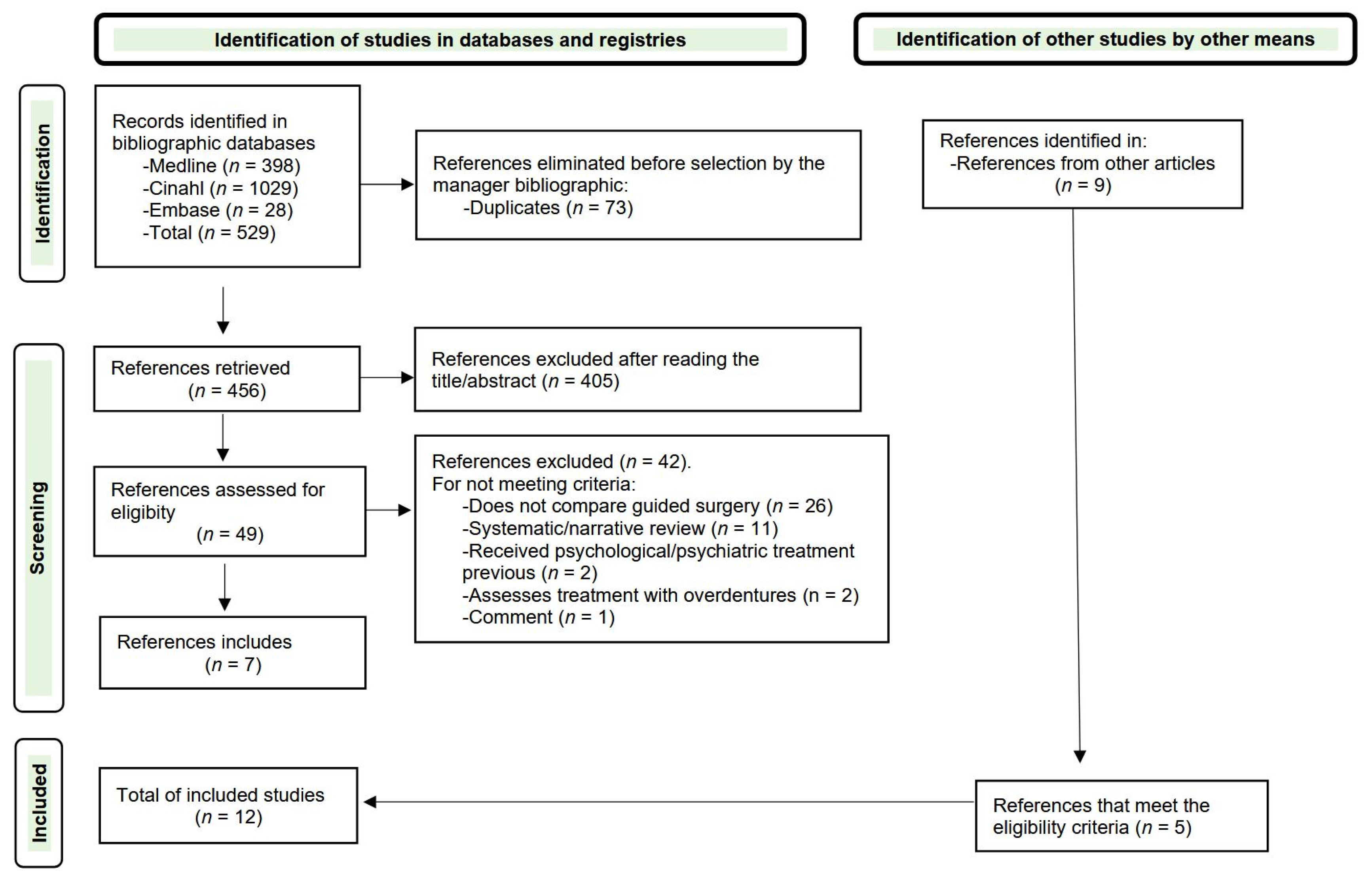
| Database | Search Strategy | Number of Articles |
|---|---|---|
| Medline | ((“Dental Implants” OR “guided implant surgery”) AND (“Psychological Phenomena” OR “psychological impact” OR “mental health” OR “anxiety” OR “Quality of Life” OR “Stress”) AND (“Patient Satisfaction” OR “treatment outcomes”)) | 398 |
| Cinhal | (MH “Dental Implants” OR “guided implant surgery”) AND (MH “Psychological Phenomena” OR “psychological impact” OR MH “Mental Health” OR MH “Anxiety” OR MH “Quality of Life” OR MH “Stress”) AND (MH “Patient Satisfaction” OR “treatment outcomes”) | 103 |
| Embase | (“dental implants”/exp OR “guided implant surgery”) AND (“psychological phenomena”/exp OR “psychological phenomena” OR “psychological impact”/exp OR “psychological impact”) AND (“patient satisfaction”/exp OR “patient satisfaction” OR “treatment outcomes”) | 28 |
| Author and Year of Publication (Country of Execution) | SD | Duration of the Study | SS (NI) | AA [Sex] | Funding [Type of Funding] | Conflict of Interest [Quality Rating] |
|---|---|---|---|---|---|---|
| Sancho-Puchades et al., 2019 [26] (Switzerland) | RCT | NR | 73 (NR) | NR [NR] | Yes [Public: University of Geneva and University of Zurich] | No [2 Low] |
| Vercruyssen et al., 2014 [23] (Belgium) | RCT | 1 year | 59 (314) | Avg. 58 years [Both] | Yes [Private: Nobel Biocare] | No [3 Low] |
| Vercruyssen et al., 2014 [22] (Belgium) | RCT | 7 days | 59 (314) | Avg. 58 years [Both] | Yes [Private: Nobel Biocare] | No [2 Low] |
| Nirula et al., 2023 [28] (India) | RCT | 4 months | 60 (210) | Avg. 48 years [Both] | NR [NR] | No [2 Low] |
| Frizzera et al., 2011 [27] (Brazil) | RCT | 3 months | 10 (20) | Over 18 years old | Yes [Private: FAESA and FAPES and Derig-Implants] | No [5 High] |
| Montero et al., 2019 [18] (Spain) | N-RCT | 6 months | 104 (399) | Avg. 55 years [Both] | Yes [Public: University of Salamanca] | No [1 Low] |
| Merli et al., 2008 [19] (Italy) | CS | 8 months | 13 (89) | Avg. 62 years [Both] | Yes [Private: Nobel Biocare] | NR [66% Low] |
| Fortin et al., 2006 [20] (France) | CS | 7 months | 60 (152) | 19–82 years [Both] | NR [NR] | NR [89% High] |
| Pozzi et al., 2014 [25] (Italy] | RCT | 1 year | 51 (202) | 62.7–63.4 years [Both] | NR [NR] | No [3 Low] |
| Engkawong et al., 2021 [29] (Thailand) | RCT | 2 weeks | 88 (179) | 32–74 years [Both] | NR [NR] | No [3 Low] |
| Lindeboom & Van Wijk, 2009 [21] (Netherlands) | CS | 10 months | 16 (96) | 54–58 years [NR] | NR [NR] | NR [77.7% High] |
| Jorba-García et al., 2023 [24] (Spain) | RCT | 8 months | 29 (44) | 59–61 years [Both] | NR [NR] | No. [3 Low] |
| Total | SS = 622 (NI) = (192) |
| Author | Comparison Group | [Intervention] Flap | Features/Software | Load |
|---|---|---|---|---|
| Sancho-Puchades et al. [26] | Conventional and static guided surgery with radiological splint and surgical splint and guided surgery with surgical splint | [Static] Flap: all groups | Simplant Dentsply Sirona software. Bone regeneration and bone elevation, if necessary | NR |
| Vercruyssen et al. [23] | conventional and static guided surgery | [Static] Without flap: mucosal support groups (Mat Mu and Fac Mu). With flap: Materialize Universal/bone (Mat Bo), Facilitate/bone (Fac Bo), freehand navigation (Freehand), and pilot drill template (Templ) | It uses two guided surgery systems: Materialize Universal (Materialize, Leuven, Belgium) and FacilitateTM (DENTSPLY Implants, Molndal, Sweden) with depth control stops | 3–4 months |
| Vercruyssen et al. [22] | Conventional and static guided surgery | [Static] Without flap: mucosal support groups (Mat Mu and Fac Mu). With flap: Materialize Universal/bone (Mat Bo), Facilitate/bone (Fac Bo), freehand navigation (Freehand), and pilot drill template (Templ) | It uses two guided surgery systems: Materialize Universal (Materialize, Leuven, Belgium) and FacilitateTM (DENTSPLY Implants, Molndal, Sweden) with depth control stops | 3–4 months |
| Nirula, P. et al. [28] | Conventional and dynamic guided surgery | [Dynamic] Flap: conventional surgery | Navident Software (Toronto, ON, Canada) | NR |
| Frizzera et al. [27] | Conventional and static guided surgery. | [Static] Flap: conventional surgery | Blue sky plan Software (Libertyville, IL, USA) | NR |
| Montro et al. [18]. | Conventional, static guided surgery with conventional loading and immediate loading surgery | [Static] Flap: conventional surgery | MozoGrau Guided Surgery Software (MG_Fidelis, Mozograu, Valladolid, Spain) | Immediate and conventional |
| Merli et al. [19] | Static guided surgery. | [Static] Flap: 2 patients for bone regeneration | Nobel Biocare, Goteborg, Sweden | Immediate and conventional |
| Fortin et al. [20] | Conventional and static guided surgery. | [Static] Flap: conventional surgery | CADimplant v2.3 Software | NR |
| Pozzi et al. [25] | Conventional and static guided surgery. | [Static] Flapless or mini flap | Software Nobel Biocare, Kloten, Switzerland | Immediate. |
| Engkawong et al. [29] | Conventional, static guided surgery and dynamic guided surgery. | [Static and dynamic] With and without flap depending on the consistency of keratinized mucosa | coDiagnostiX software, Dental Wings, Inc., Montreal, QC, Canada, and Iris-100, EPED, Inc., Taiwan. Bone regeneration and bone elevation, if necessary | NR |
| Lindeboom & Van Wijk [21] | Flap-guided surgery and flapless-guided surgery. | [Static] Flap: guided surgery; Flapless: guided surgery | Nobel Biocare AB Software, Goteborg, Sweden | NR |
| Jorba-García et al. [24] | Conventional and dynamic guided surgery. | [Dynamic] Flap-free whenever possible for both groups | Navident Software (Navident®, ClaroNav Technology Inc.®; Los Ángeles, CA, USA) | NR |
| Study | OHIP | VAS-p | VAS-ql | HRQoL | MPQ-DLV | Likert-a | PREM’s | IES-R | s-DAI | O-3 | Self-d |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sancho-Puchades et al. [26] | X | X | |||||||||
| Vercruyssen et al. [23] | X | ||||||||||
| Vercruyssen et al. [22] | X | X | X | ||||||||
| Nirula et al. [28] | X | X | |||||||||
| Frizzera et al. [27] | X | X | |||||||||
| Montero et al. [18] | X | X | |||||||||
| Merli et al. [19] | X | ||||||||||
| Fortin et al. [20] | X | ||||||||||
| Pozzi et al. [25] | X | X | X | ||||||||
| Engkawong et al. [29] | X | X | |||||||||
| Lindeboom & Van Wijk [21] | X | X | X | ||||||||
| Jorba-García et al. [24] | X | X |
| Author | Instrument | Aspects Assessed | Questionnaire Variables | Time of Questionnaire Administration |
|---|---|---|---|---|
| Sancho-Puchades et al. [26] |
| Intraoperative comfort, perceived duration of the surgical procedure, and intra- and postoperative symptoms | Swelling, bruising, bleeding, nausea, opening, chewing, social interaction, sleep, etc. | Immediately after surgery |
| Vercruyssen et al. [23] |
| Quality of life and patient satisfaction | Domains: Functional limitation, physical pain, psychological discomfort, physical disability, social disability and other reasons for discomfort | Preoperative and one year after receiving it |
| Vercruyssen et al. [22] |
| Postoperative pain and quality of life | Pain description and scale. Quality of life variables (masticatory function) | During the following 7 days postoperatively |
| Nirula et al. [28] |
| Expectations, treatment satisfaction and postoperative pain | Experience with robotics, pain perception, and comfort during surgery | Preoperative and during the postoperative period |
| Frizzera F et al. [27] |
| Treatment satisfaction and pain | Intraoperative pain, postoperative pain, treatment satisfaction and surgical time | Preoperative and during the following 7 days postoperatively |
| Montero et al. [18] |
| Quality of life and patient satisfaction | Impact on basic daily activities: eating, speaking, oral hygiene, sleeping, smiling, job functions, emotional stability, and social contact | Preoperative and at 6 months after surgery |
| Merli et al. [19] |
| Patient satisfaction | Quality of life and cost | 7 days after the provisional treatment and 30 days after definitive rehabilitation |
| Fortin et al. [20] |
| Postoperative pain | Postoperative pain and medication | During the following 7 days postoperatively |
| Pozzi et al. [25] |
| Postoperative pain and patient satisfaction | Function, aesthetics, and comfort | Pain: 3 days after treatment |
| Engkawong et al. [29] |
| Postoperative pain and patient satisfaction | Pain, bleeding, difficulty chewing and speaking, oral hygiene, surgical time | 1–2 weeks after treatment |
| Lindeboom & Van Wijk [21] |
| Patient satisfaction, quality of life and dental anxiety | Pain, anxiety, duration of treatment | During the following 7 days postoperatively |
| Jorba-García et al. [24] |
| Quality of life, patient satisfaction and pain | Patient perception, treatment recommendation, intra- and postoperative pain, surgical time | Preoperatively, at the end of surgery, and 7 days postoperatively |
| Study | Conclusions |
|---|---|
| Sancho-Puchades et al. [26] | Overall, patients preferred computer-based technologies. No differences were observed in intra- or postoperative discomfort compared to control protocols. More extensive surgical procedures negatively affected intra- and postoperative quality of life, regardless of treatment group. |
| Vercruyssen et al. [23] | No differences were found at 1-year follow-up between implant and patient outcome variables for guided vs. conventional treatments. Guided surgery appears to be a valid and predictable treatment option. |
| Vercruyssen et al. [22] | Few differences were found in patient outcome variables across treatment groups. However, patients undergoing conventional flap implant placement tended to experience pain for a longer period. |
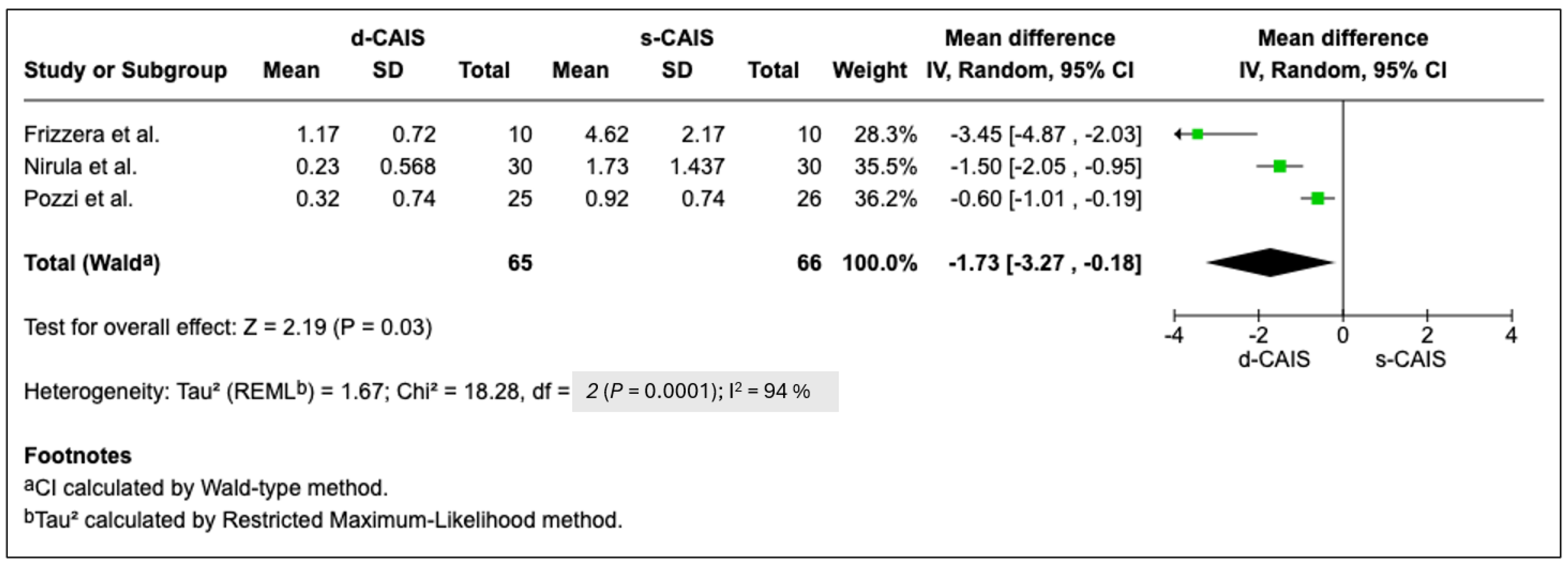
| Nirula et al. [28] | Immediate postoperative pain perception and comfort levels were better with dynamic navigation. Patients showed strong interest in robotic dentistry, appreciating the ability to visualize and understand the procedure on screen. |
| Frizzera et al. [27] | Flapless guided implant surgeries performed by inexperienced clinicians showed reduced surgical time and yielded better patient-reported outcomes both intra- and postoperatively, along with reduced medication usage compared to conventional implant techniques. |
| Montero et al. [18] | Clear improvement in oral well-being was observed after implant therapy. OHQoL scores and patient satisfaction were notably higher in patients treated with guided surgery and immediate loading protocols, although all groups ultimately achieved similar well-being levels. |
| Merli et al. [19] | Guided implant surgery software can be very helpful in planning and managing complex cases. However, it requires progressive training. The entire flapless and immediate loading process is not simple and should only be used by experienced operators. |
| Fortin et al. [20] | With flapless procedures, patients experienced less intense and shorter-duration pain. |
| Pozzi et al. [25] | When treatment planning was perfomed with CBCT scanning using dedicated 3D planning software, no statistically significant differences were observed between computer-guided and freehand rehabilitations—except that freehand sites, where flaps were more often raised, showed more postoperative pain and inflammation. |
| Engkawong et al. [29] | Conventional freehand, static, and dynamic CAIS techniques for dental implant surgery did not result in any difference in postoperative pain and inflammation levels and appeared to lead to equal levels of patient satisfaction. |
| Lindeboom & Van Wijk [21] | The flap procedure group reported less impact on quality of life and included more patients who reported no pain at all during placement. |
| Jorba-García et al. [24] | d-CAIS systems significantly improve implant placement accuracy in partially edentulous patients compared to the manual approach. However, they significantly increase surgical time and do not appear to enhance patient satisfaction or reduce postoperative pain. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Valdez, D.; Velasco-Ortega, E.; Ortiz-Garcia, I.; Monsalve-Guil, L.; López-López, J.; Núñez-Márquez, E.; Matos-Garrido, N.; Jiménez-Guerra, Á.; Moreno-Muñoz, J.; Rondón-Romero, J.L. Impact of Guided Implant Dentistry on Patient Quality of Life, Satisfaction, and Psychological Well-Being: A Systematic Review. J. Clin. Med. 2025, 14, 6638. https://doi.org/10.3390/jcm14186638
García-Valdez D, Velasco-Ortega E, Ortiz-Garcia I, Monsalve-Guil L, López-López J, Núñez-Márquez E, Matos-Garrido N, Jiménez-Guerra Á, Moreno-Muñoz J, Rondón-Romero JL. Impact of Guided Implant Dentistry on Patient Quality of Life, Satisfaction, and Psychological Well-Being: A Systematic Review. Journal of Clinical Medicine. 2025; 14(18):6638. https://doi.org/10.3390/jcm14186638
Chicago/Turabian StyleGarcía-Valdez, Daniela, Eugenio Velasco-Ortega, Iván Ortiz-Garcia, Loreto Monsalve-Guil, José López-López, Enrique Núñez-Márquez, Nuno Matos-Garrido, Álvaro Jiménez-Guerra, Jesús Moreno-Muñoz, and José Luis Rondón-Romero. 2025. "Impact of Guided Implant Dentistry on Patient Quality of Life, Satisfaction, and Psychological Well-Being: A Systematic Review" Journal of Clinical Medicine 14, no. 18: 6638. https://doi.org/10.3390/jcm14186638
APA StyleGarcía-Valdez, D., Velasco-Ortega, E., Ortiz-Garcia, I., Monsalve-Guil, L., López-López, J., Núñez-Márquez, E., Matos-Garrido, N., Jiménez-Guerra, Á., Moreno-Muñoz, J., & Rondón-Romero, J. L. (2025). Impact of Guided Implant Dentistry on Patient Quality of Life, Satisfaction, and Psychological Well-Being: A Systematic Review. Journal of Clinical Medicine, 14(18), 6638. https://doi.org/10.3390/jcm14186638








