Sarcopenia and Myosteatosis as a Predictor of Post-Operative Outcomes in Patients Undergoing Laparotomy for Abdominal Emergencies
Abstract
1. Introduction
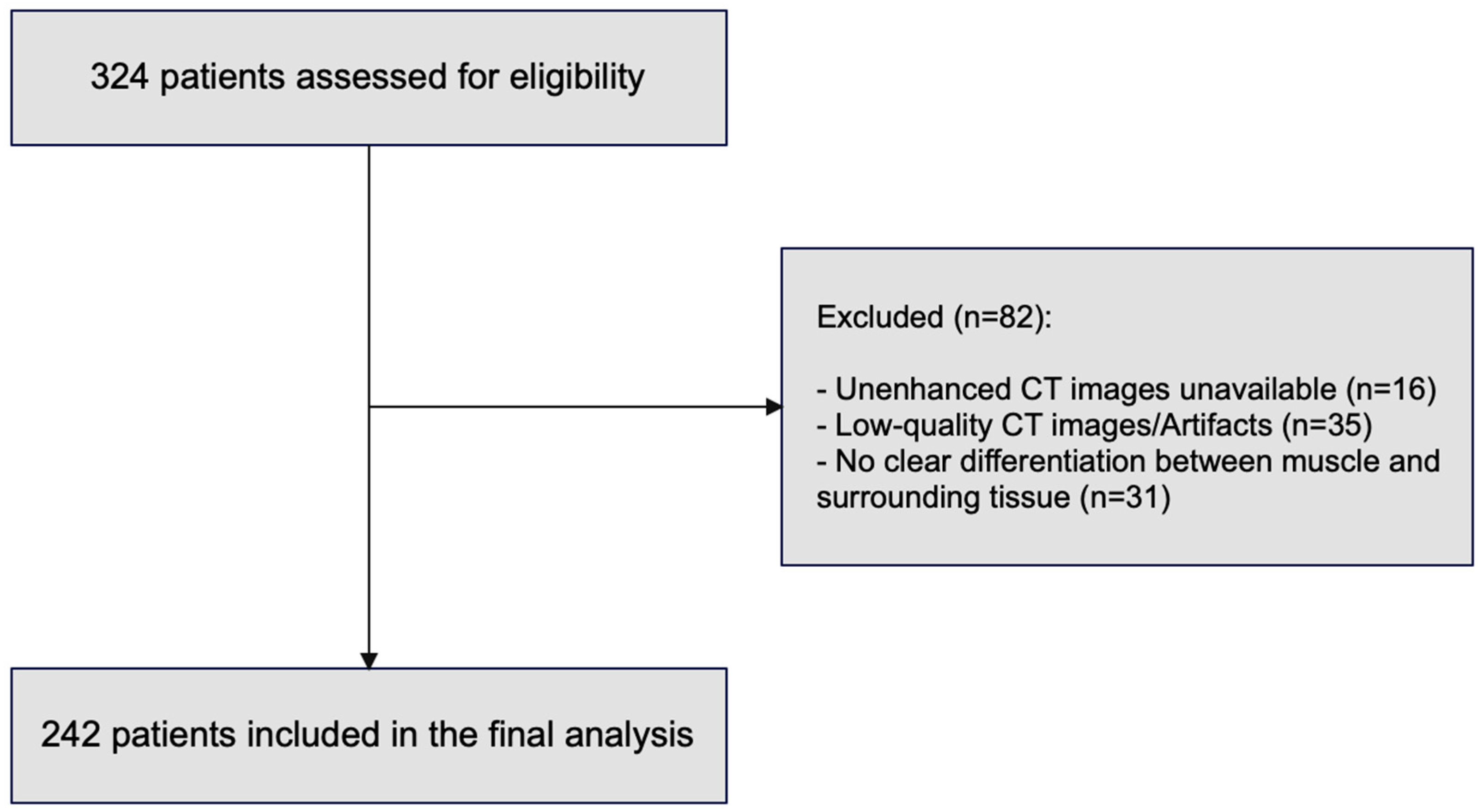
2. Materials and Methods
2.1. Study Design
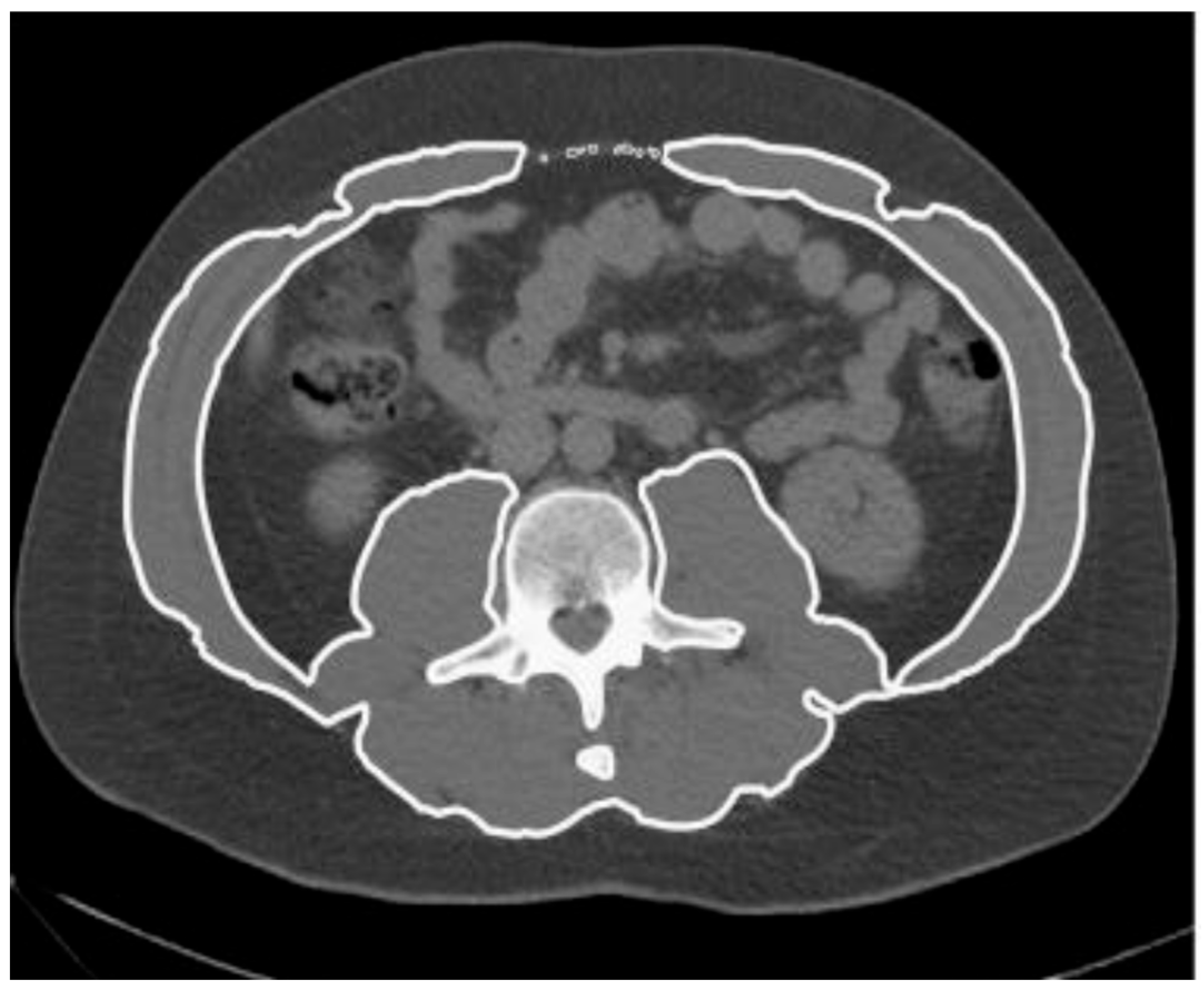
2.2. Image Analysis
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Sarcopenic and Myosteatosic Patient Subgroups
3.3. Mortality
3.4. Severe Complications
3.5. ICU Admission
3.6. Length of Hospitalization
4. Discussions
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EL | Emergency Laparotomy |
| SMA | Skeletal Muscle Area |
| SMI | Skeletal Muscle Index |
| MRA | Muscle Radiation Attenuation |
| CT | Computed Tomography |
| BMI | Body Mass Index |
| ASA | American Society of Anesthesiologists |
| CFS | Clinical Frailty Scale |
| CCI | Charlson Comorbidity Index |
| ICU | Intensive Care Unit |
| h-LOS | hospital Length Of Stay |
| CD | Clavien-Dindo |
| HU | Hounsfield Units |
References
- Kelly, N.; Murray, D. Assessing risk in emergency laparotomy. Anaesthesia 2023, 78, 949–952. [Google Scholar] [CrossRef]
- Havens, J.M.; Columbus, A.B.; Seshadri, A.J.; Brown, C.V.R.; Tominaga, G.T.; Mowery, N.T.; Crandall, M. Risk stratification tools in emergency general surgery. Trauma Surg. Acute Care Open 2018, 3, e000160. [Google Scholar] [CrossRef]
- Eugene, N.; Oliver, C.M.; Bassett, M.G.; Poulton, T.E.; Kuryba, A.; Johnston, C.; Anderson, I.D.; Moonesinghe, S.R.; Grocott, M.P.; Murray, D.M.; et al. Development and internal validation of a novel risk adjustment model for adult patients undergoing emergency laparotomy surgery: The National Emergency Laparotomy Audit risk model. Br. J. Anaesth. 2018, 121, 739–748. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Wiedmer, P.; Jung, T.; Castro, J.P.; Pomatto, L.C.D.; Sun, P.Y.; Davies, K.J.A.; Grune, T. Sarcopenia-Molecular mechanisms and open questions. Ageing Res. Rev. 2021, 65, 101200. [Google Scholar] [CrossRef] [PubMed]
- Kuk, J.L.; Saunders, T.J.; Davidson, L.E.; Ross, R. Age-related changes in total and regional fat distribution. Ageing Res. Rev. 2009, 8, 339–348. [Google Scholar] [CrossRef]
- Brown, J.C.; Caan, B.J.; Meyerhardt, J.A.; Weltzien, E.; Xiao, J.; Cespedes Feliciano, E.M.; Kroenke, C.H.; Castillo, A.; Kwan, M.L.; Prado, C.M. The deterioration of muscle mass and radiodensity is prognostic of poor survival in stage I-III colorectal cancer: A population-based cohort study (C-SCANS). J. Cachexia Sarcopenia Muscle 2018, 9, 664–672. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.P.; Wang, X.L.; Tian, H.; Gao, T.T.; Tang, L.M.; Tian, F.; Wang, J.W.; Zheng, H.J.; Zhang, L.; et al. Computed tomography-quantified body composition predicts short-term outcomes after gastrectomy in gastric cancer. Curr. Oncol. 2018, 25, e411–e422. [Google Scholar] [CrossRef]
- Daabiss, M. American Society of Anaesthesiologists physical status classification. Indian J. Anaesth. 2011, 55, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- van der Werf, A.; Langius, J.A.E.; de van der Schueren, M.A.E.; Nurmohamed, S.A.; van der Pant, K.A.M.I.; Blauwhoff-Buskermolen, S.; Wierdsma, N.J. Percentiles for skeletal muscle index, area and radiation attenuation based on computed tomography imaging in a healthy Caucasian population. Eur. J. Clin. Nutr. 2018, 72, 288–296. [Google Scholar] [CrossRef]
- Pearse, R.M.; Moreno, R.P.; Bauer, P.; Pelosi, P.; Metnitz, P.; Spies, C.; Vallet, B.; Vincent, J.-L.; Hoeft, A.; Rhodes, A.; et al. Mortality after surgery in Europe: A 7 day cohort study. Lancet 2012, 380, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Jansson Timan, T.; Hagberg, G.; Sernert, N.; Karlsson, O.; Prytz, M. Mortality following emergency laparotomy: A Swedish cohort study. BMC Surg. 2021, 21, 322. [Google Scholar] [CrossRef] [PubMed]
- Barazanchi, A.W.H.; Xia, W.; MacFater, W.; Bhat, S.; MacFater, H.; Taneja, A.; Hill, A.G. Risk factors for mortality after emergency laparotomy: Scoping systematic review. ANZ J. Surg. 2020, 90, 1895–1902. [Google Scholar] [CrossRef]
- Lai, C.P.T.; Goo, T.T.; Ong, M.W.; Prakash, P.S.; Lim, W.W.; Drakeford, P.A. A Comparison of the P-POSSUM and NELA Risk Score for Patients Undergoing Emergency Laparotomy in Singapore. World J. Surg. 2021, 45, 2439–2446. [Google Scholar] [CrossRef] [PubMed]
- Peponis, T.; Bohnen, J.D.; Sangji, N.F.; Nandan, A.R.; Han, K.; Lee, J.; Yeh, D.D.; de Moya, M.A.; Velmahos, G.C.; Chang, D.C.; et al. Does the emergency surgery score accurately predict outcomes in emergent laparotomies? Surgery 2017, 162, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejska, K.; Witowski, J.; Tylec, P.; Grochowska, A.; Przytuła, N.; Lis, M.; Pędziwiatr, M.; Rubinkiewicz, M. Radiological Features for Frailty Assessment in Patients Requiring Emergency Laparotomy. J. Clin. Med. 2022, 11, 5365. [Google Scholar] [CrossRef]
- Salem, S.A.; Almogy, G.; Lev-Cohain, N.; Bala, M.; Cohen, N.; Issachar, O.; Pikarsky, A.J.; Yuval, J.B. Psoas Attenuation and Mortality of Elderly Patients Undergoing Nontraumatic Emergency Laparotomy. J. Surg. Res. 2021, 257, 252–259. [Google Scholar] [CrossRef]
- Giani, M.; Rezoagli, E.; Grassi, A.; Porta, M.; Riva, L.; Famularo, S.; Barbaro, A.; Bernasconi, D.; Ippolito, D.; Bellani, G.; et al. Low skeletal muscle index and myosteatosis as predictors of mortality in critically ill surgical patients. Nutrition 2022, 101, 111687. [Google Scholar] [CrossRef]
- Park, B.; Vandal, A.; Welsh, F.; Eglinton, T.; Koea, J.; Taneja, A.; Barazanchi, A.; Hill, A.G.; MacCormick, A.D. Sarcopenia, myosteatosis, and frailty parameters to predict adverse outcomes in patients undergoing emergency laparotomy: Prospective observational multicentre cohort study. BJS Open 2025, 9, zraf016. [Google Scholar] [CrossRef]
- Yang, T.-R.; Luo, K.; Deng, X.; Xu, L.; Wang, R.-R.; Ji, P. Effect of sarcopenia in predicting postoperative mortality in emergency laparotomy: A systematic review and meta-analysis. World J. Emerg. Surg. 2022, 17, 36. [Google Scholar] [CrossRef]
- Simonsen, C.; de Heer, P.; Bjerre, E.D.; Suetta, C.; Hojman, P.; Pedersen, B.K.; Svendsen, L.B.; Christensen, J.F. Sarcopenia and Postoperative Complication Risk in Gastrointestinal Surgical Oncology: A Meta-analysis. Ann. Surg. 2018, 268, 58–69. [Google Scholar] [CrossRef]
- Li, S.; Xie, K.; Xiao, X.; Xu, P.; Tang, M.; Li, D. Correlation between sarcopenia and esophageal cancer: A narrative review. World J. Surg. Oncol. 2024, 22, 27. [Google Scholar] [CrossRef]
- Body, S.; Ligthart, M.A.; Rahman, S.; Ward, J.; May-Miller, P.; Pucher, P.H.; Curtis, N.J.; West, M.A. Sarcopenia and Myosteatosis Predict Adverse Outcomes After Emergency Laparotomy: A Multi-center Observational Cohort Study. Ann. Surg. 2022, 275, 1103–1111. [Google Scholar] [CrossRef]
- Thu, M.M.; Ng, H.J.; Moug, S. The influence between frailty, sarcopenia and physical status on mortality in patients undergoing emergency laparotomy. World J. Emerg. Surg. 2025, 20, 38. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-L.; Shen, J.; Danzeng, C.-D.; Xu, X.-S.; Cao, Z.-X.; Jiang, W. CT psoas calculations on the prognosis prediction of emergency laparotomy: A single-center, retrospective cohort study in eastern Asian population. World J. Emerg. Surg. 2022, 17, 31. [Google Scholar] [CrossRef] [PubMed]
- Francomacaro, L.M.; Walker, C.; Jaap, K.; Dove, J.; Hunsinger, M.; Widom, K.; Torres, D.; Shabahang, M.; Blansfield, J.; Wild, J. Sarcopenia predicts poor outcomes in urgent exploratory laparotomy. Am. J. Surg. 2018, 216, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Alani, Z.; Sulistio, E.; Barazanchi, A.W.H.; Koea, J.; Vandal, A.; Hill, A.G.; MacCormick, A.D. Frailty using the Clinical Frailty Scale to predict short- and long-term adverse outcomes following emergency laparotomy: Meta-analysis. BJS Open 2024, 8, zrae078. [Google Scholar] [CrossRef] [PubMed]
- Parmar, K.L.; Law, J.; Carter, B.; Hewitt, J.; Boyle, J.M.; Casey, P.; Maitra, I.; Farrell, I.S.; Pearce, L.; Moug, S.J.; et al. Frailty in Older Patients Undergoing Emergency Laparotomy: Results From the UK Observational Emergency Laparotomy and Frailty (ELF) Study. Ann. Surg. 2021, 273, 709–718. [Google Scholar] [CrossRef]
- Joseph, B.; Zangbar, B.; Pandit, V.; Fain, M.; Mohler, M.J.; Kulvatunyou, N.; Jokar, T.O.; O’Keeffe, T.; Friese, R.S.; Rhee, P. Emergency General Surgery in the Elderly: Too Old or Too Frail? J. Am. Coll. Surg. 2016, 222, 805–813. [Google Scholar] [CrossRef]
- Hewitt, J.; Carter, B.; McCarthy, K.; Pearce, L.; Law, J.; Wilson, F.V.; Tay, H.S.; McCormack, C.; Stechman, M.J.; Moug, S.J.; et al. Frailty predicts mortality in all emergency surgical admissions regardless of age. An observational study. Age Ageing 2019, 48, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.P.T.; Raveshia, D.; Liew, M.S.; Jackowski, A.; Kisiel, A.; Griffiths, E.A.; Tan, B.H.L. Evaluation of sarcopenia and myosteatosis to determine the impact on mortality after emergency laparotomy. BJS Open 2025, 9, zraf092. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S.; Colby, M.; Spencer, J.; Bhimani, N.; Leibman, S.; Laurence, J.M.; Smith, G.; Falk, G.L.; Sandroussi, C. Radiologic myosteatosis predicts major complication risk following esophagectomy for cancer: A multicenter experience. J. Gastrointest. Surg. 2024, 28, 1861–1869. [Google Scholar] [CrossRef] [PubMed]
- Bunino, F.M.; Zulian, D.; Famularo, S.; Persichetti, G.W.L.; Mauri, G.; Del Fabbro, D. Open abdomen versus primary closure in nontrauma patients: A weighted analysis of a single-center experience. J. Trauma Acute Care Surg. 2025, 98, 510–520. [Google Scholar] [CrossRef]
| Patient Characteristics | Whole Cohort (n = 242) |
|---|---|
| n (%)/Median [IQR] | |
| Age, (years) | 70 (58–78) |
| Gender | |
| F | 118 (48.8) |
| M | 124 (51.2) |
| BMI, (kg/m2) | 23.9 [21.1–26.9] |
| Oncologic disease | |
| Yes | 89 (36,8) |
| No | 153 (63,2) |
| White blood cell count, (×103/μL) | 11.1 [7.7–16.2] |
| ASA Physical Status | |
| 1 | 26 (10.7) |
| 2 | 59 (24.4) |
| 3 | 122 (50.4) |
| 4 | 35 (14.5) |
| Charlson Comorbidity Index | |
| <4 | 89 (36.8) |
| ≥4 | 153 (63.2) |
| Clinical Frailty Scale | |
| A (1–3) | 128 (52.9) |
| B (4–5) | 74 (30.6) |
| C (≥6) | 40 (16.5) |
| Indication for surgery | |
| Bowel perforation | 82 (33.9) |
| Mesenteric Ischemia | 27 (11.1) |
| Bowel Occlusion | 106 (43.8) |
| Other | 27 (11.2) |
| Male (124) | Female (118) | Overall (242) | |||
|---|---|---|---|---|---|
| Median (IQR) | Threshold Value | Median (IQR) | Threshold Value | Median (IQR) | |
| Skeletal muscle area–SMA (cm2) | 112.8 [90.2–129.1] | 134 | 102.9 [79.9–127.4] | 89,2 | 107.1 [84.6–128.6] |
| Skeletal muscle index–SMI (cm2/m−2) | 41,1 [36.7–46.3] | 41.6 | 35.5 [6.71–175.5] | 32 | 39,1 [32.5–44.9] |
| Muscle radiation attenuation–MRA (HU) | 19 [6.0–27.5] | 29.3 | 9.5 [5.0–19.0] | 22 | 14.8 [0.0–25.2] |
| Patient Characteristics | Sarcopenia | Myosteatosis | ||||
|---|---|---|---|---|---|---|
| n (%)/Median [IQR] | p Value | n (%)/Median [IQR] | p Value | |||
| Yes | No | Yes | No | |||
| (n = 103, 42.6%) | (n = 139, 57.4%) | (n = 189, 78.1%) | (n = 53, 21.9%) | |||
| Age (years) | 71 [60–80] | 69 [57–77] | 0.067 | 72 [65–80] | 52 [41–63] | <0.001 |
| Gender | 0.001 | 0.793 | ||||
| F | 38 (36.9) | 80 (57.6) | 93 (49.2) | 25 (47.2) | ||
| M | 65 (63.1) | 59 (42.5) | 96 (50.8) | 28 (52.8) | ||
| BMI (kg/m2) | 21.7 | 25.7 | <0.001 | 24.8 | 21.7 | <0.001 |
| [19.4–24.8] | [23.0–28.7] | [21.7–27.3] | [19.5–24.2] | |||
| Oncologic disease | 0.006 | 0.627 | ||||
| Yes | 48 (46.6) | 41 (29.5) | 68 (36.0) | 21 (39.6) | ||
| No | 55 (53.4) | 98 (70.5) | 121 (64.0) | 32 (60.4) | ||
| Lab data | ||||||
| WBC (×103/μL) | 11.14 | 11.1 | 0.334 | 11.4 | 10.8 | 0.751 |
| [7.0–16.1] | [8.0–16.6] | [7.5–16.6] | [8.1–14.8] | |||
| CRP (mg/dL) | 8.7 | 9.5 | 0.543 | 11.1 | 1.76 [0.61–9.5] | 0.001 |
| [1.4–21.0] | [2.1–20.7] | [2.5–21.8] | ||||
| ASA Score | 0.192 | <0.001 | ||||
| 1 | 6 (5.8) | 20 (14.4) | 15 (7.9) | 11 (20.8) | ||
| 2 | 25 (24.3) | 34 (24.5) | 38 (20.1) | 21 (39.6) | ||
| 3 | 56 (54.4) | 66 (47.5) | 105 (55.6) | 17 (32.1) | ||
| 4 | 16 (15.5) | 19 (13.7) | 31 (16.4) | 4 (7.6) | ||
| CCI | 0.001 | <0.001 | ||||
| <4 | 26 (25.2) | 63 (45.3) | 55 (29.1) | 34 (64.2) | ||
| ≥4 | 77 (74.8) | 76 (54.7) | 134 (70.9) | 19 (35.9) | ||
| CFS | 0.301 | 0.001 | ||||
| A (1–3) | 50 (48.5) | 79 (56.8) | 89 (47.1) | 40 (75.5) | ||
| B (4–5) | 33 (32.0) | 42 (30.2) | 65 (34.4) | 10 (18.9) | ||
| C (≥6) | 20 (19.4) | 18 (13.0) | 35 (18.5) | 3 (5.7) | ||
| Outcome | Overall | Sarcopenia | Myosteatosis | ||||
|---|---|---|---|---|---|---|---|
| n (%)/Median [IQR] | n (%)/Median [IQR] | p Value | n (%)/Median [IQR] | p Value | |||
| (n = 242) | Yes | No | Yes | No | |||
| (n = 103, 42.6%) | (n = 139, 57.4%) | (n = 189, 78.1%) | (n = 53, 21.9%) | ||||
| 30-day Mortality | 56 (23.1) | 27 (26.1) | 29 (20.9) | 0.329 | 48 (25.4) | 8 (15.1) | 0.116 |
| Length of hospital stay | 15 [8–30] | 16 [9–30] | 14 [7–31] | 0.554 | 17 [9–34] | 8 [6–16] | <0.001 |
| ICU admission | 106 (43.8) | 46 (44.7) | 60 (43.1) | 0.817 | 91 (48.2) | 15 (28.3) | 0.010 |
| Severe Complications | 82 (33.9) | 41 (39.9) | 41 (29.5) | 0.094 | 70 (37.1) | 12 (22.7) | 0.048 |
| Mortality | Univariate OR | 95% CI | p Value | Multivariate OR | 95% CI | p Value |
| Age (years) | 1.02 | [0.96–1.04] | 0.078 | e | e | e |
| Gender (male vs. female) | 0.57 | [0.32–1.04] | 0.063 | e | e | e |
| BMI (kg/m2) | 1.01 | [0.96–1.06] | 0.723 | e | e | e |
| Oncologic status | 1.04 | [0.56–1.93] | 0.131 | e | e | e |
| CRP | 0.99 | [0.97–1.02] | 1.024 | e | e | e |
| WBC | 1.01 | [0.97–1.04] | 0.677 | e | e | e |
| CCI | 2.28 | [1.15–4.54] | 0.018 | 1.62 | [0.61–4.3] | 0.321 |
| CFS | 3.02 | [1.59–5.72] | <0.001 | 2.46 | [1.15–5.24] | 0.021 |
| ASA score | 1.38 | [0.95–2.00] | 0.087 | 0.95 | [0.6–1.49] | 0.874 |
| Bowel perforation | 0.49 | [0.18–1.34] | 0.438 | e | e | e |
| Mesenteric Ischemia | 3.1 | [1.35–7.11] | 0.007 | 2.77 | [1.11–6.87] | 0.035 |
| Bowel occlusion | 0.62 | [0.24–1.61] | 0.327 | e | e | e |
| Other Diagnosis | 1.65 | [0.56–1.46] | 0.338 | e | e | e |
| Sarcopenia | 1.35 | [0.74–2.46] | 0.336 | e | e | e |
| Myosteatosis | 1.91 | [0.84–4.34] | 0.122 | e | e | e |
| Severe complications | Univariate OR | 95% CI | p value | Multivariate OR | 95% CI | p value |
| Age (years) | 1.01 | [0.99–1.09] | 0.450 | e | e | e |
| Gender (male vs. female) | 0.86 | [0.51–1.47] | 0.509 | e | e | e |
| BMI (kg/m2) | 1.00 | [0.97–1.05] | 0.903 | e | e | e |
| Oncologic status | 1.35 | [0.78–2.34] | 0.283 | e | e | e |
| CRP | 1.03 | [1.01–1.05] | 0.002 | 1.01 | [0.98–1.03] | 0.414 |
| WBC | 1.02 | [0.99–1.06] | 0.102 | e | e | e |
| CCI | 1.04 | [0.97–1.12] | 0.297 | e | e | e |
| CFS | 1.27 | [1.09–1.48] | 0.002 | 1.14 | [0.93–1.41] | 0.205 |
| ASA score | 1.52 | [1.09–2.13] | 0.013 | 1.3 | [0.91–1.85] | 0.143 |
| Bowel Perforation | 0.57 | [0.24–1.36] | 0.204 | e | e | e |
| Mesenteric Ischemia | 2.76 | [1.22–6.22] | 0.013 | 1.5 | [0.62–3.6] | 0.362 |
| Bowel Occlusion | 0.23 | [0.12–0.42] | <0.001 | 0.31 | [0.15–0.64] | 0.011 |
| Other Diagnosis | 1.58 | [0.92–2.70] | 0.081 | e | e | e |
| Sarcopenia | 1.58 | [0.92–2.70] | 0.095 | e | e | e |
| Myosteatosis | 2.0 | [1.12–4.07] | 0.048 | 1.3 | [0.61–2.88] | 0.467 |
| ICU admission | Univariate OR | 95% CI | p value | Multivariate OR | 95% CI | p value |
| Age (years) | 1.02 | [1.01–1.04] | 0.015 | 1.01 | [0.98–1.04] | 0.472 |
| Gender (male vs. female) | 1.24 | [0.75–2.08] | 0.39 | e | e | e |
| BMI (kg/m2) | 1.02 | [0.97–1.06] | 0.48 | e | e | e |
| Oncologic status | 1.24 | [0.73–2.10] | 0.418 | e | e | e |
| CRP | 1.03 | [1.01–1.05] | 0.002 | 1.01 | [0.99–1.04] | 0.312 |
| WBC | 1.02 | [0.99–1.06] | 0.167 | e | e | e |
| CCI | 2.45 | [1.41–4.26] | 0.001 | 1.31 | [0.6–2.89] | 0.517 |
| CFS | 3.66 | [1.97–6.76] | <0.001 | 1.71 | [0.8–3.67] | 0.171 |
| ASA score | 2.33 | [1.63–3.32] | <0.001 | 1.17 | [1.1–2.64] | 0.016 |
| Bowel Perforation | 0.62 | [0.25–1.51] | 0.145 | e | e | e |
| Mesenteric Ischemia | 9.1 | [3.05–27.4] | <0.001 | 4.96 | [1.47–16.75] | 0.013 |
| Bowel Occlusion | 0.19 | [0.1–0.34] | <0.001 | 0.32 | [0.15–0.66] | <0.002 |
| Other Diagnosis | 1.33 | [0.84–1.45] | 0.081 | e | e | e |
| Sarcopenia | 1.06 | [0.64–1.78] | 0.816 | e | e | e |
| Myosteatosis | 2.35 | [1.21–4.56] | 0.010 | 0.89 | [0.36–2.21] | 0.821 |
| Length of hospitalization | Univariate HR | 95% CI | p value | Multivariate HR | 95% CI | p value |
| Age (years) | 1.00 | [0.99–1.01] | 0.982 | e | e | e |
| Gender (male vs. female) | 0.96 | [0.74–1.23] | 0.736 | e | e | e |
| BMI (kg/m2) | 0.99 | [0.97–1.01] | 0.283 | e | e | e |
| Oncologic status | 0.95 | [0.73–1.11] | 0.723 | e | e | e |
| CRP | 0.98 | [0.97–0.99] | 0.001 | 0.99 | [0.99–1.01] | 0.352 |
| WBC | 0.98 | [0.97–1.00] | 0.018 | 0.99 | [0.97–1.00] | 0.106 |
| CCI | 0.98 | [0.96–1.03] | 0.585 | e | e | e |
| CFS | 0.91 | [0.84–0.99] | 0.029 | 1.02 | [0.92–1.14] | 0.683 |
| ASA score | 0.80 | [0.69–0.94] | 0.015 | 0.83 | [0.69–1.00] | 0.052 |
| Bowel Perforation | 1.29 | [0.83–2.00] | 0.261 | e | e | e |
| Mesenteric Ischemia | 1.76 | [1.03–3.05] | 0.038 | 1.83 | [1.05–3.19] | 0.032 |
| Bowel Occlusion | 2.32 | [1.51–3.57] | <0.001 | 1.99 | [1.24–3.17] | 0.004 |
| Other Diagnosis | 1.08 | [0.84–1.4] | 0.548 | e | e | e |
| Sarcopenia | 1.08 | [0.84–1.40] | 0.398 | e | e | e |
| Myosteatosis | 0.65 | [0.48–0.89] | 0.007 | 0.59 | [0.42–0.84] | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giudici, S.; Lanza, E.; Lofino, L.; Barison, A.; Ammirabile, A.; Mauri, G.; Zulian, D.; Ceolin, M.; Brocchi, A.; Del Fabbro, D. Sarcopenia and Myosteatosis as a Predictor of Post-Operative Outcomes in Patients Undergoing Laparotomy for Abdominal Emergencies. J. Clin. Med. 2025, 14, 6639. https://doi.org/10.3390/jcm14186639
Giudici S, Lanza E, Lofino L, Barison A, Ammirabile A, Mauri G, Zulian D, Ceolin M, Brocchi A, Del Fabbro D. Sarcopenia and Myosteatosis as a Predictor of Post-Operative Outcomes in Patients Undergoing Laparotomy for Abdominal Emergencies. Journal of Clinical Medicine. 2025; 14(18):6639. https://doi.org/10.3390/jcm14186639
Chicago/Turabian StyleGiudici, Simone, Ezio Lanza, Ludovica Lofino, Alberto Barison, Angela Ammirabile, Giulia Mauri, Davide Zulian, Martina Ceolin, Andrea Brocchi, and Daniele Del Fabbro. 2025. "Sarcopenia and Myosteatosis as a Predictor of Post-Operative Outcomes in Patients Undergoing Laparotomy for Abdominal Emergencies" Journal of Clinical Medicine 14, no. 18: 6639. https://doi.org/10.3390/jcm14186639
APA StyleGiudici, S., Lanza, E., Lofino, L., Barison, A., Ammirabile, A., Mauri, G., Zulian, D., Ceolin, M., Brocchi, A., & Del Fabbro, D. (2025). Sarcopenia and Myosteatosis as a Predictor of Post-Operative Outcomes in Patients Undergoing Laparotomy for Abdominal Emergencies. Journal of Clinical Medicine, 14(18), 6639. https://doi.org/10.3390/jcm14186639







