Impact of Punctate Hyperfluorescence Status on Treatment Outcomes of Faricimab Versus Aflibercept in Neovascular Age-Related Macular Degeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Treatment Method and Data Collection
2.3. Outcome Measures
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics in the Study Population
3.2. Treatment Outcomes in the Loading Dose Regimen
3.3. Retreatment Rate After the Loading Dose Regimen over 1 Year
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bressler, N.M.; Bressler, S.B.; Fine, S.L. Age-related macular degeneration. Surv. Ophthalmol. 1988, 32, 375–413. [Google Scholar] [CrossRef]
- Ferrara, N.; Damico, L.; Shams, N.; Lowman, H.; Kim, R. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina 2006, 26, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, N.; Martin, J.; Ruan, Q.; Rafique, A.; Rosconi, M.P.; Shi, E.; Pyles, E.A.; Yancopoulos, G.D.; Stahl, N.; Wiegand, S.J. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 2012, 15, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.D.; Das, A.; Do, D.V.; Dugel, P.U.; Gomes, A.; Holz, F.G.; Koh, A.; Pan, C.K.; Sepah, Y.J.; Patel, N.; et al. Brolucizumab: Evolution through preclinical and clinical studies and the implications for the management of neovascular age-related macular degeneration. Ophthalmology 2020, 127, 963–976. [Google Scholar] [CrossRef] [PubMed]
- Regula, J.T.; von Lundh, P.; Foxton, R.; Barathi, V.A.; Cheung, C.M.; Bo Tun, S.B.; Wey, Y.S.; Iwata, D.; Dostalek, M.; Moelleken, J.; et al. Targeting key angiogenic pathways with a bispecific CrossMAb optimized for neovascular eye diseases. EMBO Mol. Med. 2016, 8, 1265–1288. [Google Scholar] [CrossRef]
- Maisonpierre, P.C.; Suri, C.; Jones, P.F.; Bartunkova, S.; Wiegand, S.J.; Radziejewski, C.; Compton, D.; McClain, J.; Aldrich, T.H.; Papadopoulos, N.; et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 1997, 277, 55–60. [Google Scholar] [CrossRef]
- Heier, J.S.; Khanani, A.M.; Ruiz, C.Q.; Basu, K.; Ferrone, P.J.; Brittain, C.; Figueroa, M.S.; Lin, H.; Holz, F.G.; Patel, V.; et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): Two randomised, double-masked, phase 3, non-inferiority trials. Lancet 2022, 399, 729–740. [Google Scholar] [CrossRef]
- Pang, C.E.; Freund, K.B. Pachychoroid neovasculopathy. Retina 2015, 35, 1–9. [Google Scholar] [CrossRef]
- Miyake, M.; Ooto, S.; Yamashiro, K.; Takahashi, A.; Yoshikawa, M.; Akagi-Kurashige, Y.; Ueda-Arakawa, N.; Oishi, A.; Nakanishi, H.; Tamura, H.; et al. Pachychoroid neovasculopathy and age-related macular degeneration. Sci. Rep. 2015, 5, 16204. [Google Scholar] [CrossRef]
- Matsumoto, H.; Hiroe, T.; Morimoto, M.; Mimura, K.; Ito, A.; Akiyama, H. Efficacy of treat-and-extend regimen with aflibercept for pachychoroid neovasculopathy and Type 1 neovascular age-related macular degeneration. Jpn. J. Ophthalmol. 2018, 62, 144–150. [Google Scholar] [CrossRef]
- Tsujikawa, A.; Ojima, Y.; Yamashiro, K.; Ooto, S.; Tamura, H.; Nakagawa, S.; Yoshimura, N. Punctate hyperfluorescent spots associated with central serous chorioretinopathy as seen on indocyanine green angiography. Retina 2010, 30, 801–809. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, B.H.; Park, K.H.; Woo, S.J. Punctate hyperfluorescence spot as a common choroidopathy of central serous chorioretinopathy and polypoidal choroidal vasculopathy. Am. J. Ophthalmol. 2014, 158, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Kamao, H.; Goto, K.; Date, Y.; Hiraki, R.; Mizukawa, K.; Miki, A. Clinical characteristics of punctate hyperfluorescence spots in the fellow eye of patients with unilateral macular neovascularization with no drusen. J. Clin. Med. 2024, 13, 5394. [Google Scholar] [CrossRef] [PubMed]
- Kamao, H.; Goto, K.; Mizukawa, K.; Hiraki, R.; Miki, A.; Kimura, S. Punctate hyperfluorescence as a favorable predictive factor for treatment response following a switch to brolucizumab for patients with aflibercept-refractory neovascular age-related macular degeneration. J. Clin. Med. 2025, 14, 5141. [Google Scholar] [CrossRef] [PubMed]
- Kamao, H.; Goto, K.; Mito, Y.; Miki, A.; Kiryu, J. Effects of smoking on outcomes of antivascular endothelial growth factor therapy in patients with neovascular age-related macular degeneration smoking and anti-VEGF Therapy in nAMD. J. Ophthalmol. 2018, 2018, 2353428. [Google Scholar] [CrossRef]
- Kamao, H.; Mitsui, E.; Date, Y.; Goto, K.; Mizukawa, K.; Miki, A. Clinical characteristics of unilateral macular neovascularization patients with pachydrusen in the fellow eye. J. Clin. Med. 2024, 13, 3757. [Google Scholar] [CrossRef]
- Cheung, C.M.G.; Lim, J.I.; Priglinger, S.; Querques, G.; Margaron, P.; Patel, S.; Souverain, A.; Willis, J.R.; Yang, M.; Guymer, R. Anatomic outcomes with faricimab vs aflibercept in head-to-head dosing phase of the TENAYA/LUCERNE trials in neovascular age-related macular degeneration. Ophthalmology 2025, 132, 519–526. [Google Scholar] [CrossRef]
- Fukuda, Y.; Notomi, S.; Shiose, S.; Maehara, Y.; Kiyohara, K.; Fujiwara, K.; Hashimoto, S.; Kano, K.; Ishikawa, K.; Hisatomi, T.; et al. Three-month outcomes of treatment with faricimab or aflibercept for neovascular age-related macular degeneration: A propensity score matching study in a Japanese population. Graefe’s Arch. Clin. Exp. Ophthalmol. 2024, 262, 3971–3978. [Google Scholar] [CrossRef]
- Hara, C.; Suzue, M.; Fujimoto, S.; Fukushima, Y.; Sayanagi, K.; Nishida, K.; Maruyama, K.; Sato, S.; Nishida, K. Comparison of loading dose between aflibercept and faricimab for neovascular age-related macular degeneration. J. Clin. Med. 2024, 13, 385. [Google Scholar] [CrossRef]
- Iida, T.; Gomi, F.; Yasukawa, T.; Yamashiro, K.; Honda, S.; Maruko, I.; Kataoka, K. Japanese clinical guidelines for neovascular age-related macular degeneration. Jpn. J. Ophthalmol. 2025, 69, 639–660. [Google Scholar] [CrossRef]
- Curcio, C.A.; Presley, J.B.; Malek, G.; Medeiros, N.E.; Avery, D.V.; Kruth, H.S. Esterified and unesterified cholesterol in drusen and basal deposits of eyes with age-related maculopathy. Exp. Eye Res. 2005, 81, 731–741. [Google Scholar] [CrossRef]
- Anderson, D.H.; Ozaki, S.; Nealon, M.; Neitz, J.; Mullins, R.F.; Hageman, G.S.; Johnson, L.V. Local cellular sources of apolipoprotein E in the human retina and retinal pigmented epithelium: Implications for the process of drusen formation. Am. J. Ophthalmol. 2001, 131, 767–781. [Google Scholar] [CrossRef]
- Schutt, F.; Bergmann, M.; Holz, F.G.; Kopitz, J. Proteins modified by malondialdehyde, 4-hydroxynonenal, or advanced glycation end products in lipofuscin of human retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2662–2668. [Google Scholar] [CrossRef] [PubMed]
- Terao, N.; Koizumi, H.; Kojima, K.; Yamagishi, T.; Yamamoto, Y.; Yoshii, K.; Kitazawa, K.; Hiraga, A.; Toda, M.; Kinoshita, S.; et al. Distinct aqueous humour cytokine profiles of patients with pachychoroid neovasculopathy and neovascular age-related macular degeneration. Sci. Rep. 2018, 6, 10520. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Hoshino, J.; Mukai, R.; Nakamura, K.; Kishi, S.; Akiyama, H. Chronic choriocapillaris ischemia in dilated vortex vein region in pachychoroid neovasculopathy. Sci. Rep. 2021, 11, 16274. [Google Scholar] [CrossRef] [PubMed]
- Ng, D.S.; Yip, Y.W.; Bakthavatsalam, M.; Chen, L.J.; Ng, T.K.; Lai, T.Y.; Pang, C.P.; Brelén, M.E. Elevated angiopoietin 2 in aqueous of patients with neovascular age related macular degeneration correlates with disease severity at presentation. Sci. Rep. 2017, 27, 45081. [Google Scholar] [CrossRef]
- Oh, H.; Takagi, H.; Suzuma, K.; Otani, A.; Matsumura, M.; Honda, Y. Hypoxia and vascular endothelial growth factor selectively up-regulate angiopoietin-2 in bovine microvascular endothelial cells. J. Biol. Chem. 1999, 274, 15732–15739. [Google Scholar] [CrossRef]
- Lobov, I.B.; Brooks, P.C.; Lang, R.A. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 11205–11210. [Google Scholar] [CrossRef]
- Wei, L.-H.; Kuo, M.-L.; Chen, C.-A.; Chou, C.-H.; Lai, K.-B.; Lee, C.-N.; Hsieh, C.-Y. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene 2003, 22, 1517–1527. [Google Scholar] [CrossRef]
- Izumi-Nagai, K.; Nagai, N.; Ozawa, Y.; Mihara, M.; Ohsugi, Y.; Kurihara, T.; Koto, T.; Satofuka, S.; Inoue, M.; Tsubota, K.; et al. Interleukin-6 receptor-mediated activation of signal transducer and activator of transcription-3 (STAT3) promotes choroidal neovascularization. Am. J. Pathol. 2007, 170, 2149–2158. [Google Scholar] [CrossRef]
- Kayakabe, K.; Kuroiwa, T.; Sakurai, N.; Ikeuchi, H.; Kadiombo, A.T.; Sakairi, T.; Matsumoto, T.; Maeshima, A.; Hiromura, K.; Nojima, Y. Interleukin-6 promotes destabilized angiogenesis by modulating angiopoietin expression in rheumatoid arthritis. Rheumatology 2012, 51, 1571–1579. [Google Scholar] [CrossRef]
- Kuroda, Y.; Yamashiro, K.; Miyake, M.; Yoshikawa, M.; Nakanishi, H.; Oishi, A.; Tamura, H.; Ooto, S.; Tsujikawa, A.; Yoshimura, N. Factors associated with recurrence of age-related macular degeneration after anti-vascular endothelial growth factor treatment: A retrospective cohort study. Ophthalmology 2015, 122, 2303–2310. [Google Scholar] [CrossRef]
- Ho, A.C.; Kleinman, D.M.; Lum, F.C.; Heier, J.S.; Lindstrom, R.L.; Orr, S.C.; Chang, G.C.; Smith, E.L.; Pollack, J.S. Baseline visual acuity at wet AMD diagnosis predicts long-term vision outcomes: An analysis of the IRIS Registry. Ophthalmic Surg. Lasers Imaging Retin. 2020, 51, 633–639. [Google Scholar] [CrossRef]



| Characteristic | PH Group | Non-PH Group | ||||
|---|---|---|---|---|---|---|
| IVF | IVA | p | IVF | IVA | p | |
| Number of eyes | 28 | 28 | 28 | 28 | ||
| Age (years), mean (SD) | 69.6 (10.0) | 69.2 (6.8) | 0.21 | 75.0 (8.4) | 74.7 (8.3) | 0.33 |
| Sex (female), no. (%) | 11 (39.3) | 7 (25.0) | 0.09 | 11 (39.3) | 9 (32.1) | 0.58 |
| Hypertension, no. (%) | 15 (53.6) | 17 (60.7) | 0.59 | 17 (60.7) | 13 (46.4) | 0.28 |
| Diabetes, no. (%) | 2 (7.1) | 4 (14.3) | 0.38 | 7 (25.0) | 9 (32.1) | 0.55 |
| Smoking history (ever-smoker), no. (%) | 17 (60.7) | 21 (75.0) | 0.59 | 18 (64.3) | 18 (64.3) | 1.00 |
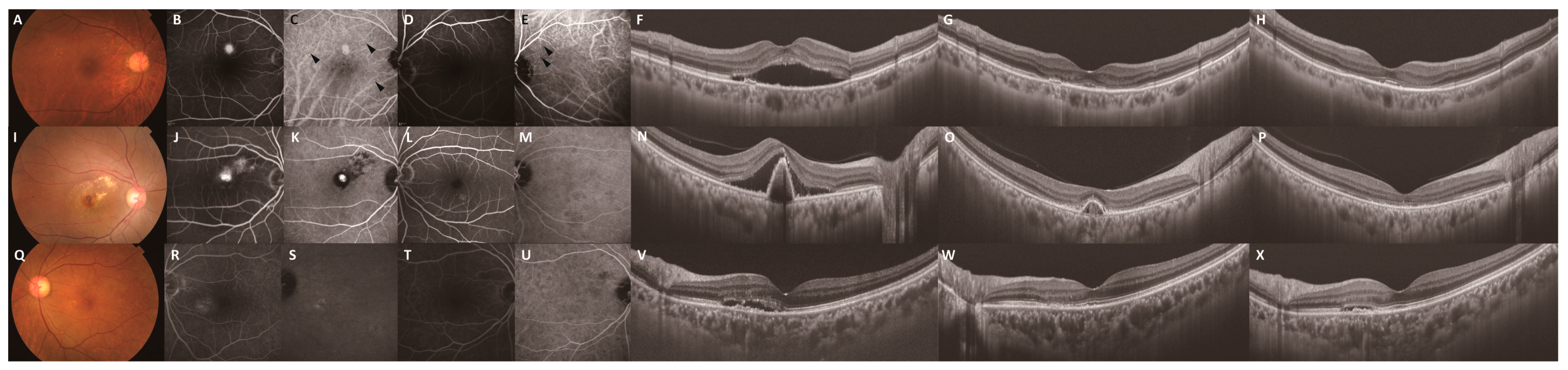
| Presence of SHRM, no. (%) | 0.06 | 0.92 | ||||
| Exudation | 4 (14.3) | 0 (0.0) | 4 (14.3) | 0 (0.0) | ||
| Hemorrhage | 2 (7.1) | 4 (14.3) | 2 (7.1) | 4 (14.3) | ||
| Neovascular tissue | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| No SHRM | 22 (78.6) | 24 (85.7) | 22 (78.6) | 24 (85.7) | ||
| Presence of IRF, no. (%) | 4 (14.3) | 4 (14.3) | 1.00 | 6 (21.4) | 4 (14.3) | 0.48 |
| Presence of SRF, no. (%) | 26 (92.9) | 28 (100) | 0.09 | 26 (92.9) | 28 (100) | 0.09 |
| Presence of polypoidal lesion, no. (%) | 14 (50.0) | 10 (35.7) | 0.28 | 5 (17.9) | 7 (25.0) | 0.51 |
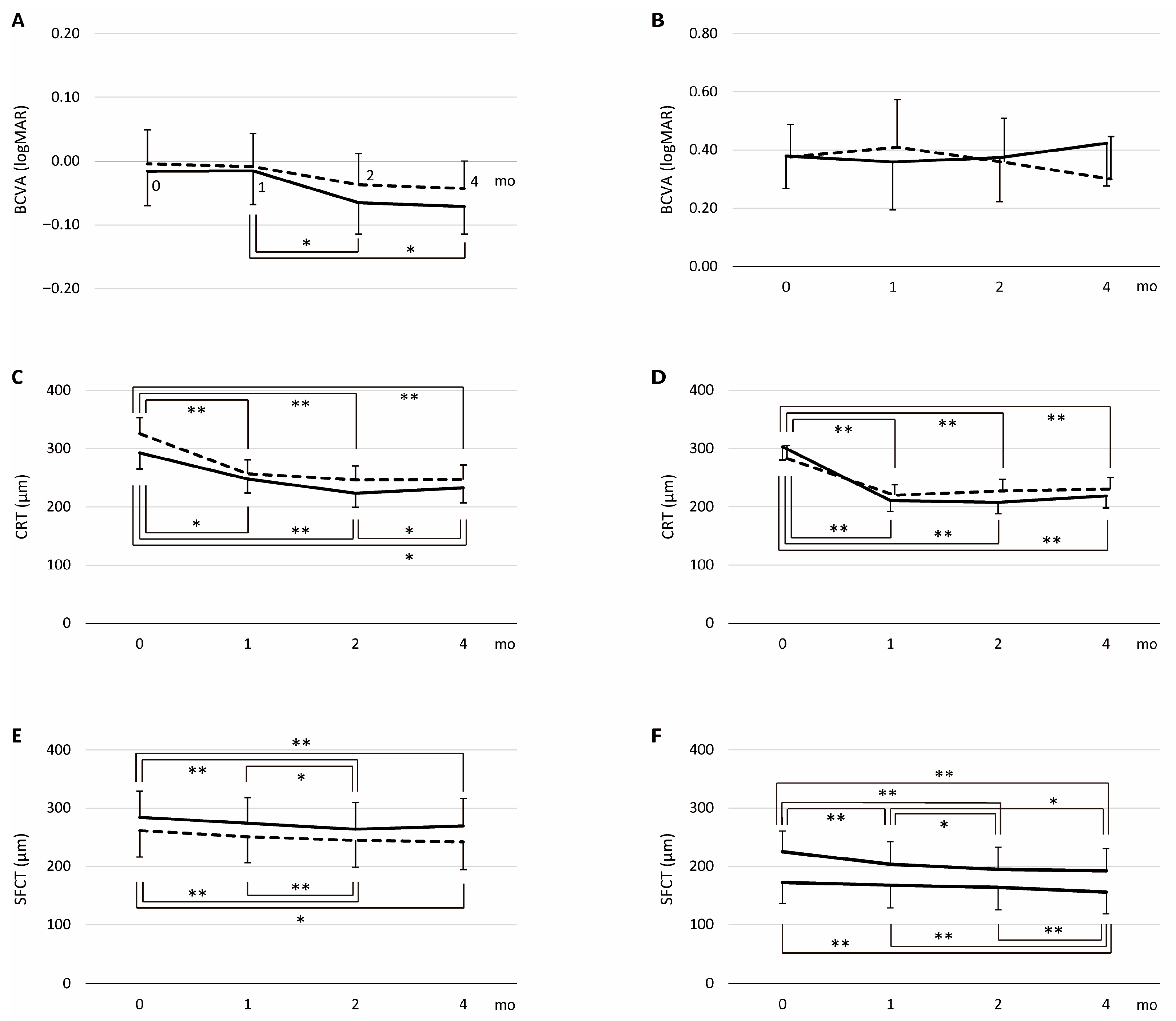
| Group | Outcome | IVF | IVA | Drug, p | Month, p | Drug × Month, p |
|---|---|---|---|---|---|---|
| PH group | logMAR | 0.55 | 0.003 | 0.75 | ||
| baseline, LS mean (95% CI) | −0.02 (−0.07–0.04) | 0.00 (−0.06–0.05) | ||||
| 1M, LS mean (95% CI) | −0.02 (−0.07–0.04) | −0.01 (−0.06–0.04) | ||||
| 2M, LS mean (95% CI) | −0.07 (−0.11–0.02) | −0.04 (−0.09–0.01) | ||||
| 4M, LS mean (95% CI) | −0.07 (−0.11–0.03) | −0.04 (−0.09–0.00) | ||||
| CRT (μm) | 0.13 | <0.001 | 0.09 | |||
| baseline, LS mean (95% CI) | 292.6 (264.8–320.5) | 325.5 (297.7–353.3) | ||||
| 1M, LS mean (95% CI) | 247.8 (223.9–271.7) | 256.7 (232.8–280.6) | ||||
| 2M, LS mean (95% CI) | 223.5 (199.3–247.7) | 246.2 (222.0–270.4) | ||||
| 4M, LS mean (95% CI) | 232.5 (207.2–257.7) | 246.9 (221.6–272.1) | ||||
| SFCT (μm) | 0.47 | <0.001 | 0.06 | |||
| baseline, LS mean (95% CI) | 284.2 (239.1–329.3) | 261.3 (216.2–306.4) | ||||
| 1M, LS mean (95% CI) | 274.0 (229.7–318.3) | 250.9 (206.6–295.1) | ||||
| 2M, LS mean (95% CI) | 263.9 (218.2–309.7) | 244.4 (198.7–290.2) | ||||
| 4M, LS mean (95% CI) | 269.5 (222.1–316.9) | 242.0 (194.6–289.4) | ||||
| Non-PH group | logMAR | 0.81 | 0.51 | <0.001 | ||
| baseline, LS mean (95% CI) | 0.38 (0.27–0.49) | 0.38 (0.26–0.49) | ||||
| 1M, LS mean (95% CI) | 0.36 (0.19–0.52) | 0.41 (0.25–0.57) | ||||
| 2M, LS mean (95% CI) | 0.37 (0.22–0.52) | 0.36 (0.21–0.51) | ||||
| 4M, LS mean (95% CI) | 0.42 (0.28–0.57) | 0.30 (0.15–0.45) | ||||
| CRT (μm) | 0.69 | <0.001 | 0.04 | |||
| baseline, LS mean (95% CI) | 302.9 (280.2–325.5) | 282.9 (260.3–305.5) | ||||
| 1M, LS mean (95% CI) | 210.7 (192.0–229.4) | 219.6 (200.9–238.4) | ||||
| 2M, LS mean (95% CI) | 207.7 (188.0–227.4) | 227.2 (207.5–246.9) | ||||
| 4M, LS mean (95% CI) | 218.6 (198.1–239.2) | 230.2 (209.7–250.7) | ||||
| SFCT (μm) | 0.15 | <0.001 | 0.03 | |||
| baseline, LS mean (95% CI) | 172.4 (136.6–208.1) | 224.7 (188.9–260.5) | ||||
| 1M, LS mean (95% CI) | 167.4 (128.7–206.2) | 203.4 (164.6–242.1) | ||||
| 2M, LS mean (95% CI) | 163.6 (125.2–202.0) | 194.6 (156.2–233.0) | ||||
| 4M, LS mean (95% CI) | 155.8 (117.9–193.6) | 192.1 (154.2–229.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamao, H.; Goto, K.; Mizukawa, K.; Hiraki, R.; Miki, A.; Kimura, S. Impact of Punctate Hyperfluorescence Status on Treatment Outcomes of Faricimab Versus Aflibercept in Neovascular Age-Related Macular Degeneration. J. Clin. Med. 2025, 14, 6637. https://doi.org/10.3390/jcm14186637
Kamao H, Goto K, Mizukawa K, Hiraki R, Miki A, Kimura S. Impact of Punctate Hyperfluorescence Status on Treatment Outcomes of Faricimab Versus Aflibercept in Neovascular Age-Related Macular Degeneration. Journal of Clinical Medicine. 2025; 14(18):6637. https://doi.org/10.3390/jcm14186637
Chicago/Turabian StyleKamao, Hiroyuki, Katsutoshi Goto, Kenichi Mizukawa, Ryutaro Hiraki, Atsushi Miki, and Shuhei Kimura. 2025. "Impact of Punctate Hyperfluorescence Status on Treatment Outcomes of Faricimab Versus Aflibercept in Neovascular Age-Related Macular Degeneration" Journal of Clinical Medicine 14, no. 18: 6637. https://doi.org/10.3390/jcm14186637
APA StyleKamao, H., Goto, K., Mizukawa, K., Hiraki, R., Miki, A., & Kimura, S. (2025). Impact of Punctate Hyperfluorescence Status on Treatment Outcomes of Faricimab Versus Aflibercept in Neovascular Age-Related Macular Degeneration. Journal of Clinical Medicine, 14(18), 6637. https://doi.org/10.3390/jcm14186637





