Fibrosis in Immune-Mediated and Autoimmune Disorders
Abstract
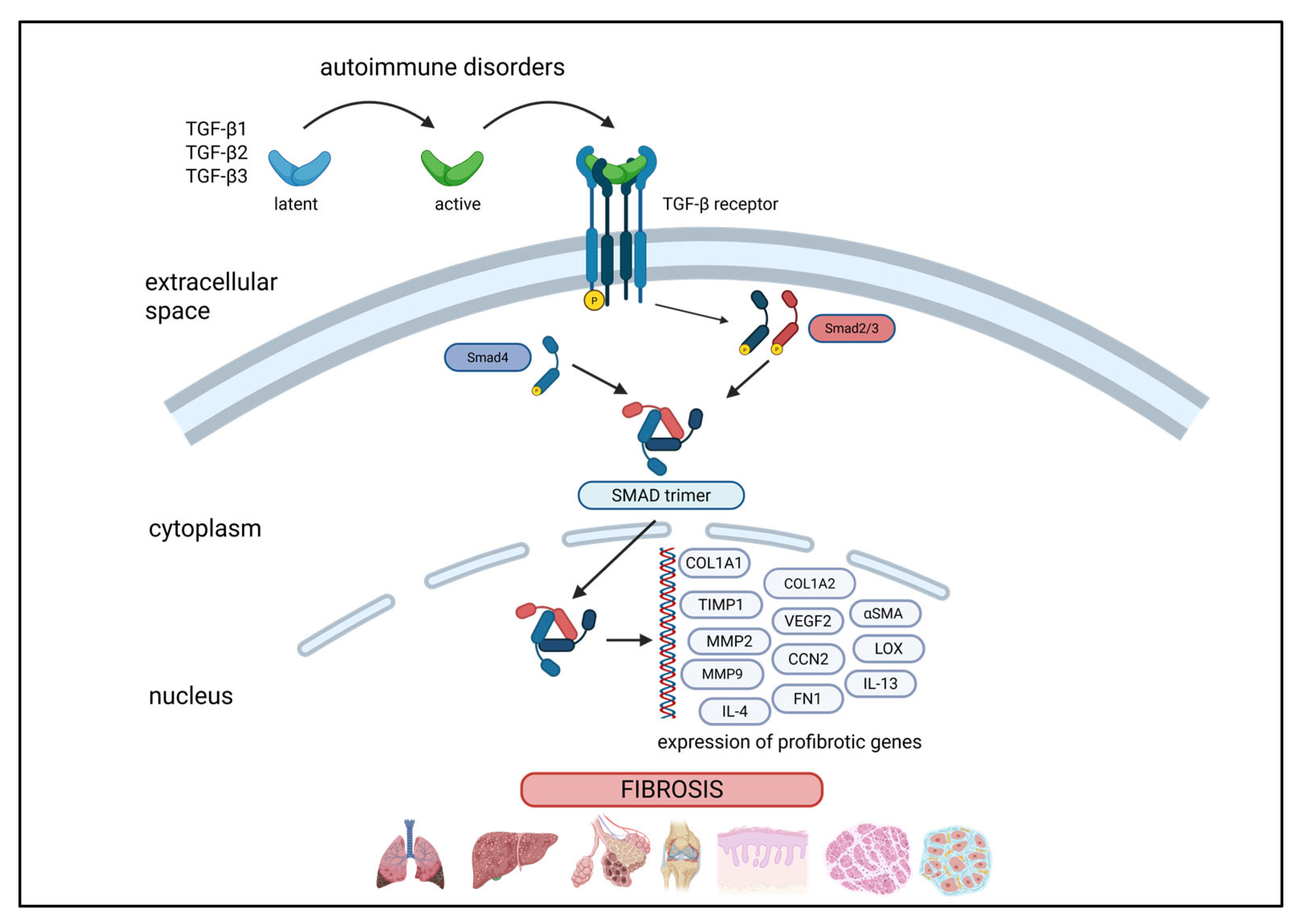
1. Introduction
1.1. “Good” Fibrosis—A Physiological Process in Tissue Repair
1.2. “Bad” Fibrosis—A Pathological Process in Immune-Mediated and Autoimmune Disorders
1.3. Fibroblasts as Central Players in Fibrosis
2. Systemic Sclerosis (SSc)
2.1. Clinical Presentation and Epidemiology of SSc
2.2. Pathogenesis of Fibrosis in SSc
2.3. Current and Emerging Therapies in SSc
3. Morphea
3.1. Clinical Presentation and Epidemiology of Morphea
3.2. Pathogenesis of Fibrosis in Morphea
3.3. Current and Emerging Therapies in Morphea
4. Autoimmune Hepatitis (AIH)
4.1. Clinical Presentation and Epidemiology of AIH
4.2. Pathogenesis of Fibrosis in AIH
4.3. Current and Emerging Therapies in AIH
5. Systemic Lupus Erythematosus (SLE)
5.1. Clinical Presentation and Epidemiology of SLE
5.2. Pathogenesis of Fibrosis in SLE
5.3. Current and Emerging Therapies in SLE
6. Sjögren’s Syndrome (SS)
6.1. Clinical Presentation and Epidemiology of SS
6.2. Pathogenesis of Fibrosis in SS
6.3. Current and Emerging Therapies in SS
7. Inflammatory Bowel Disease (IBD)
7.1. Clinical Presentation and Epidemiology of IBD
7.2. Pathogenesis of Fibrosis in IBD
7.3. Current and Emerging Therapies in IBD
8. Rheumatoid Arthritis (RA)
8.1. Clinical Presentation and Epidemiology of RA
8.2. Pathogenesis of Fibrosis in RA
8.3. Current and Emerging Therapies in RA
9. Fibrosis Regression
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACPA | anti-citrullinated protein antibody |
| AIH | Autoimmune hepatitis |
| ANA | anti-nuclear antibodies |
| APC | antigen-presenting cells |
| ASCs | adult stem cells |
| BAAF | B cell activating factor |
| CCP | cyclic citrullinated peptides |
| CD | Crohn’s disease |
| CI | confidence interval |
| CKD | chronic kidney disease |
| Cmri | cardiac magnetic resonance imaging |
| CRP | C-reactive protein |
| CXCL | C-X-C motif chemokine ligand |
| CYC | cyclophosphamide |
| DAMPs | damage-associated molecular patterns |
| DAS28-ESR | Disease Activity Score in 28 joints-Erythrocyte Sedimentation Rate |
| DEG | differentially expressed gene |
| EC | endothelial cell |
| ECM | extracellular matrix |
| DMARDs | Disease-Modifying Anti-Rheumatic Drugs |
| EMT | epithelial-mesenchymal transition |
| EndoMT | endothelial-mesenchymal transition |
| ESR | erythrocyte sedimentation rate |
| ESRD | end-stage renal disease |
| FLS | fibroblast-like synoviocytes |
| GBD | Global Burden of Disease |
| GERD | Gastroesophageal reflux disease) |
| GWAS | Genome-wide association studies |
| HSCT | hematopoietic stem cell transplantation |
| HSCs | Hepatic stellate cells |
| IBD | Inflammatory Bowel Disease |
| ILD | Interstitial lung disease |
| INF | interferon |
| HLA | human leukocyte antigen |
| HP | fibrosing hypersensitivity pneumonitis |
| LAMP3 | lysosome-associated membrane protein 3 |
| LN | lupus nephritis |
| LPS | lipopolysaccharides |
| LSECs | liver sinusoidal endothelial cells |
| MHC | major histocompatibility complex |
| MMF | mycophenolate mofetil |
| MMPs | matrix metalloproteinases |
| MSCs | mesenchymal stem cells |
| MUC5B | mucin 5B, oligomeric mucus/gel-forming gene |
| NET | neutrophil extracellular trap |
| NSIP | nonspecific interstitial pneumonia |
| PAMPS | pathogen-associated molecular patterns |
| PAH | Pulmonary arterial hypertension |
| pDCs | plasmacytoid dendritic cells |
| PFD | pirfenidone |
| PDGF | platelet-derived growth factor |
| RA | Rheumatoid arthritis |
| RA-ILD | rheumatoid arthritis interstitial lung disease |
| RF | rheumatoid factor |
| RTX | Rituximab |
| SARDs | Systemic Autoimmune Rheumatic Diseases |
| scRNA-seq | single-cell RNA sequencing |
| SLE | Systemic lupus erythematosus |
| SSc | Systemic sclerosis |
| SRC | Scleroderma renal crisis |
| TCZ | Tocilizumab |
| Tfh | T follicular helper cells |
| TGF | transforming growth factor |
| TIMPs | tissue inhibitors of metalloproteinases |
| TLRs | Toll-like receptors |
| TNF | tumor necrosis factor |
| UC | ulcerative colitis |
| UIP | usual interstitial pneumonia |
References
- Weiskirchen, R.; Weiskirchen, S.; Tacke, F. Organ and tissue fibrosis: Molecular signals, cellular mechanisms and translational implications. Mol. Asp. Med. 2019, 65, 2–15. [Google Scholar] [CrossRef]
- Pakshir, P.; Hinz, B. The big five in fibrosis: Macrophages, myofibroblasts, matrix, mechanics, and miscommunication. Matrix Biol. 2018, 68–69, 81–93. [Google Scholar] [CrossRef]
- Hinz, B.; Lagares, D. Evasion of apoptosis by myofibroblasts: A hallmark of fibrotic diseases. Nat. Rev. Rheumatol. 2020, 16, 11–31. [Google Scholar] [CrossRef]
- Volkmann, E.R.; Varga, J. Emerging targets of disease-modifying therapy for systemic sclerosis. Nat. Rev. Rheumatol. 2019, 15, 208–224. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Sepulveda, A.; Esquinca-González, A.; Benavides-Suárez, S.A.; Sordo-Lima, D.E.; Caballero-Islas, A.E.; Cabral-Castañeda, A.R.; Rodríguez-Reyna, T.S. Systemic Sclerosis Pathogenesis and Emerging Therapies, beyond the Fibroblast. BioMed Res. Int. 2019, 2019, 4569826. [Google Scholar]
- Truchetet, M.E.; Brembilla, N.C.; Chizzolini, C. Current Concepts on the Pathogenesis of Systemic Sclerosis. Clin. Rev. Allergy Immunol. 2023, 64, 262–283. [Google Scholar] [CrossRef]
- Truchetet, M.E.; Brembilla, N.C.; Chizzolini, C. Systemic sclerosis. Lancet 2023, 401, 304–318. [Google Scholar]
- Jerjen, R.; Nikpour, M.; Krieg, T.; Denton, C.P.; Saracino, A.M. Systemic sclerosis in adults. Part I: Clinical features and pathogenesis. J. Am. Acad. Dermatol. 2022, 87, 937–954. [Google Scholar] [CrossRef] [PubMed]
- Adigun, R.; Goyal, A.; Hariz, A. Systemic Sclerosis (Scleroderma); StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Rosendahl, A.; Schönborn, K.; Krieg, T. Pathophysiology of systemic sclerosis (scleroderma). Kaohsiung J. Med. Sci. 2022, 38, 187–195. [Google Scholar] [CrossRef]
- Asano, Y. The Pathogenesis of Systemic Sclerosis: An Understanding Based on a Common Pathologic Cascade across Multiple Organs and Additional Organ-Specific Pathologies. J. Clin. Med. 2020, 9, 2687. [Google Scholar] [CrossRef] [PubMed]
- Son, H.H.; Moon, S.J. Pathogenesis of systemic sclerosis: An integrative review of recent advances. J. Rheum. Dis. 2025, 32, 89–104. [Google Scholar] [CrossRef]
- Benfaremo, D.; Svegliati, S.; Paolini, C.; Agarbati, S.; Moroncini, G. Systemic Sclerosis: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines 2022, 10, 163. [Google Scholar] [CrossRef]
- Korman, B. Evolving insights into the cellular and molecular pathogenesis of fibrosis in systemic sclerosis. Transl. Res. 2019, 209, 77–89. [Google Scholar] [CrossRef]
- Rockey, D.C.; Bell, P.D.; Hill, J.A. Fibrosis—A common pathway to organ injury and failure. N. Engl. J. Med. 2015, 372, 1138–1149. [Google Scholar] [CrossRef]
- Muruganandam, M.; Ariza-Hutchinson, A.; Patel, R.A.; Sibbitt, W.L., Jr. Biomarkers in the Pathogenesis, Diagnosis, and Treatment of Systemic Sclerosis. J. Inflamm. Res. 2023, 16, 4633–4660. [Google Scholar] [CrossRef]
- Cavazzana, I.; Vojinovic, T.; Airo’, P.; Fredi, M.; Ceribelli, A.; Pedretti, E.; Lazzaroni, M.G.; Garrafa, E.; Franceschini, F. Systemic Sclerosis-Specific Antibodies: Novel and Classical Biomarkers. Clin. Rev. Allergy Immunol. 2023, 64, 412–430. [Google Scholar] [CrossRef] [PubMed]
- Bagnato, G.; Versace, A.G.; La Rosa, D.; De Gaetano, A.; Imbalzano, E.; Chiappalone, M.; Ioppolo, C.; Roberts, W.N.; Bitto, A.; Irrera, N.; et al. Autologous Haematopoietic Stem Cell Transplantation and Systemic Sclerosis: Focus on Interstitial Lung Disease. Cells 2022, 11, 843. [Google Scholar] [CrossRef]
- Pope, J.E.; Denton, C.P.; Johnson, S.R.; Fernandez-Codina, A.; Hudson, M.; Nevskaya, T. State-of-the-art evidence in the treatment of systemic sclerosis. Nat. Rev. Rheumatol. 2023, 19, 212–226. [Google Scholar] [CrossRef]
- Distler, O.; Highland, K.B.; Gahlemann, M.; Azuma, A.; Fischer, A.; Mayes, M.D.; Raghu, G.; Sauter, W.; Girard, M.; Alves, M.; et al. Nintedanib for Systemic Sclerosis-Associated Interstitial Lung Disease. N. Engl. J. Med. 2019, 380, 2518–2528. [Google Scholar] [CrossRef] [PubMed]
- Auth, J.; Müller, F.; Völkl, S.; Bayerl, N.; Distler, J.H.W.; Tur, C.; Raimondo, M.G.; Chenguiti Fakhouri, S.; Atzinger, A.; Coppers, B.; et al. CD19-targeting CAR T-cell therapy in patients with diffuse systemic sclerosis: A case series. Lancet Rheumatol. 2025, 7, e83–e93. [Google Scholar] [CrossRef] [PubMed]
- Moriana, C.; Moulinet, T.; Jaussaud, R.; Decker, P. JAK inhibitors and systemic sclerosis: A systematic review of the literature. Autoimmun. Rev. 2022, 21, 103168. [Google Scholar] [CrossRef]
- Campochiaro, C.; Allanore, Y. An update on targeted therapies in systemic sclerosis based on a systematic review from the last 3 years. Arthritis Res. Ther. 2021, 23, 155. [Google Scholar] [CrossRef]
- Papara, C.; De Luca, D.A.; Bieber, K.; Vorobyev, A.; Ludwig, R.J. Morphea: The 2023 update. Front. Med. 2023, 10, 1108623. [Google Scholar] [CrossRef]
- Wenzel, D.; Haddadi, N.S.; Afshari, K.; Richmond, J.M.; Rashighi, M. Upcoming treatments for morphea. Immun. Inflamm. Dis. 2021, 9, 1101–1145. [Google Scholar] [CrossRef]
- McGaugh, S.; Kallis, P.; De Benedetto, A.; Thomas, R.M. Janus kinase inhibitors for treatment of morphea and systemic sclerosis: A literature review. Dermatol. Ther. 2022, 35, e15437. [Google Scholar] [CrossRef] [PubMed]
- Vichaikul, S.; Gurrea-Rubio, M.; Amin, M.A.; Campbell, P.L.; Wu, Q.; Mattichak, M.N.; Brodie, W.D.; Palisoc, P.J.; Ali, M.; Muraoka, S.; et al. Inhibition of bromodomain extraterminal histone readers alleviates skin fibrosis in experimental models of scleroderma. JCI Insight 2022, 7, e150871. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Kang, G.; Eom, G.H. HDAC Inhibitors: Therapeutic Potential in Fibrosis-Associated Human Diseases. Int. J. Mol. Sci. 2019, 20, 1329. [Google Scholar] [CrossRef]
- Stein, T.; Cieplewicz-Guźla, P.; Iżykowska, K.; Pieniawska, M.; Żaba, R.; Dańczak-Pazdrowska, A.; Polańska, A. What Is New in Morphea-Narrative Review on Molecular Aspects and New Targeted Therapies. J. Clin. Med. 2024, 13, 7134. [Google Scholar] [CrossRef] [PubMed]
- Pellicano, R.; Ferro, A.; Cicerchia, F.; Mattivi, S.; Fagoonee, S.; Durazzo, M. Autoimmune Hepatitis and Fibrosis. J. Clin. Med. 2023, 12, 1979. [Google Scholar] [CrossRef]
- Sirbe, C.; Badii, M.; Crişan, T.O.; Bența, G.; Gram, A.; Joosten, L.A.B.; Rednic, S.; Pop, T.L. Detection of Novel Biomarkers in Pediatric Autoimmune Hepatitis by Proteomic Profiling. Int. J. Mol. Sci. 2023, 24, 7479. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, R.; Li, H.; Shuai, Z. Clinical management of autoimmune liver diseases: Juncture, opportunities, and challenges ahead. Immunol. Res. 2025, 73, 67. [Google Scholar] [CrossRef]
- Tatour, M.; Baker, F.A.; Saadi, T.; Yahia, A.; Hazzan, R. Advancements in autoimmune hepatitis epidemiology, treatment and complication—A 15-year retrospective study. Clin. Res. Hepatol. Gastroenterol. 2025, 49, 102570. [Google Scholar] [CrossRef] [PubMed]
- Hahn, J.W.; Yang, H.R.; Moon, J.S.; Chang, J.Y.; Lee, K.; Kim, G.A.; Rahmati, M.; Koyanagi, A.; Smith, L.; Kim, M.; et al. Global incidence and prevalence of autoimmune hepatitis, 1970–2022: A systematic review and meta-analysis. EClinicalMedicine 2023, 65, 102280. [Google Scholar] [CrossRef] [PubMed]
- Halliday, N.; Dyson, J.K.; Thorburn, D.; Lohse, A.W.; Heneghan, M.A. Review article: Experimental therapies in autoimmune hepatitis. Aliment. Pharmacol. Ther. 2020, 52, 1134–1149. [Google Scholar] [CrossRef]
- Roehlen, N.; Crouchet, E.; Baumert, T.F. Liver Fibrosis: Mechanistic Concepts and Therapeutic Perspectives. Cells 2020, 9, 875. [Google Scholar] [CrossRef] [PubMed]
- Acharya, P.; Crouchet, E.; Baumert, T.F. Cellular Mechanisms of Liver Fibrosis. Front. Pharmacol. 2021, 12, 671640. [Google Scholar] [CrossRef]
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef]
- Schuppan, D.; Afdhal, N.H. Liver cirrhosis. Lancet 2008, 371, 838–851. [Google Scholar] [CrossRef]
- Reau, N.S.; Lammert, C.S.; Weinberg, E.M. Autoimmune hepatitis: Current and future therapies. Hepatol. Commun. 2024, 8, e0458. [Google Scholar] [CrossRef]
- Dong, B.; Chen, Y.; Lyu, G.; Yang, X. Aspartate Aminotransferase to Platelet Ratio Index and Fibrosis-4 Index for Detecting Liver Fibrosis in Patients With Autoimmune Hepatitis: A Meta-Analysis. Front. Immunol. 2022, 13, 892454. [Google Scholar] [CrossRef]
- Moriya, K.; Sato, S.; Nishimura, N.; Kawaratani, H.; Takaya, H.; Kaji, K.; Namisaki, T.; Uejima, M.; Nagamatsu, S.; Matsuo, H.; et al. Efficacy of Serum Ferritin-Zinc Ratio for Predicting Advanced Liver Fibrosis in Patients with Autoimmune Hepatitis. J. Clin. Med. 2023, 12, 4463. [Google Scholar] [CrossRef]
- Tadokoro, T.; Morishita, A.; Masaki, T. Diagnosis and Therapeutic Management of Liver Fibrosis by MicroRNA. Int. J. Mol. Sci. 2021, 22, 8139. [Google Scholar] [CrossRef]
- Czaja, A.J. Epigenetic Aspects and Prospects in Autoimmune Hepatitis. Front. Immunol. 2022, 13, 921765. [Google Scholar] [CrossRef]
- Arinaga-Hino, T.; Ide, T.; Akiba, J.; Suzuki, H.; Kuwahara, R.; Amano, K.; Kawaguchi, T.; Sano, T.; Inoue, E.; Koga, H.; et al. Growth differentiation factor 15 as a novel diagnostic and therapeutic marker for autoimmune hepatitis. Sci. Rep. 2022, 12, 8759. [Google Scholar] [CrossRef]
- Lotfy, A.; Elgamal, A.; Burdzinska, A.; Swelum, A.A.; Soliman, R.; Hassan, A.A.; Shiha, G. Stem cell therapies for autoimmune hepatitis. Stem Cell Res. Ther. 2021, 12, 386. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, L.; Huang, M.; Wu, Y.; Jin, S.; Zhang, Y.; Gan, X.; Yu, T.; Yu, G.; Zhang, J.; et al. Mesenchymal Stem Cell-Derived Exosomes: Emerging as a Promising Cell-Free Therapeutic Strategy for Autoimmune Hepatitis. Biomolecules 2024, 14, 1353. [Google Scholar] [CrossRef]
- Heneghan, M.A.; Lohse, A.W. Update in clinical science: Autoimmune hepatitis. J. Hepatol. 2025, 82, 926–937. [Google Scholar] [CrossRef]
- Rahman, A.; Isenberg, D.A. Systemic lupus erythematosus. N. Engl. J. Med. 2008, 358, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.D.; Cyr, M. The history of lupus erythematosus: From Hippocrates to Osler. Rheum. Dis. Clin. N. Am. 1988, 14, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.Y.; Costenbader, K.H. Understanding the Concept of Pre-Clinical Autoimmunity: Prediction and Prevention of Systemic Lupus Erythematosus: Identifying Risk Factors and Developing Strategies Against Disease Development. Front. Immunol. 2022, 13, 890522. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Fan, Y.; Zhao, X. Systemic lupus erythematosus: Updated insights on the pathogenesis, diagnosis, prevention and therapeutics. Signal Transduct. Target. Ther. 2025, 10, 102. [Google Scholar] [CrossRef]
- Kaul, A.; Gordon, C.; Crow, M.K.; Touma, Z.; Urowitz, M.B.; van Vollenhoven, R.; Ruiz-Irastorza, G.; Hughes, G. Systemic lupus erythematosus. Nat. Rev. Dis. Primers 2016, 2, 16039. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.S.; Jung, S.M.; Yoo, J.; Lee, S.W.; Song, J.J.; Park, Y.B. Anti-Smith antibody is associated with disease activity in patients with new-onset systemic lupus erythematosus. Rheumatol. Int. 2019, 39, 1937–1944. [Google Scholar] [CrossRef] [PubMed]
- Weckerle, C.E.; Franek, B.S.; Kelly, J.A.; Kumabe, M.; Mikolaitis, R.A.; Green, S.L.; Utset, T.O.; Jolly, M.; James, J.A.; Harley, J.B.; et al. Network analysis of associations between serum interferon-alpha activity, autoantibodies, and clinical features in systemic lupus erythematosus. Arthritis Rheum. 2011, 63, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.C.; Chun, S.; Kim, K.; Mak, A. Update on the Genetics of Systemic Lupus Erythematosus: Genome-Wide Association Studies and Beyond. Cells 2019, 8, 1180. [Google Scholar] [CrossRef]
- Moudi, B.; Salimi, S.; Farajian Mashhadi, F.; Sandoughi, M.; Zakeri, Z. Association of FAS and FAS ligand genes polymorphism and risk of systemic lupus erythematosus. Sci. World J. 2013, 2013, 176741. [Google Scholar] [CrossRef]
- Xiong, W.; Lahita, R.G. Pragmatic approaches to therapy for systemic lupus erythematosus. Nat. Rev. Rheumatol. 2014, 10, 97–107. [Google Scholar] [CrossRef]
- Mahieu, M.A.; Strand, V.; Simon, L.S.; Lipsky, P.E.; Ramsey-Goldman, R. A critical review of clinical trials in systemic lupus erythematosus. Lupus 2016, 25, 1122–1140. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, D.; Yao, X.; Huang, Y.; Lu, Q. Global epidemiology of systemic lupus erythematosus: A comprehensive systematic analysis and modelling study. Ann. Rheum. Dis. 2023, 82, 351–356. [Google Scholar] [CrossRef]
- Pons-Estel, G.J.; Alarcón, G.S.; Scofield, L.; Reinlib, L.; Cooper, G.S. Understanding the epidemiology and progression of systemic lupus erythematosus. Semin. Arthritis Rheum. 2010, 39, 257–268. [Google Scholar] [CrossRef]
- Yee, C.S.; Su, L.; Toescu, V.; Hickman, R.; Situnayake, D.; Bowman, S.; Farewell, V.; Gordon, C. Birmingham SLE cohort: Outcomes of a large inception cohort followed for up to 21 years. Rheumatology 2015, 54, 836–843. [Google Scholar] [CrossRef]
- Sexton, D.J.; Reule, S.; Solid, C.; Chen, S.C.; Collins, A.J.; Foley, R.N. ESRD from lupus nephritis in the United States, 1995–2010. Clin. J. Am. Soc. Nephrol. 2015, 10, 251–259. [Google Scholar] [CrossRef]
- Mosca, M.; Tani, C.; Aringer, M.; Bombardieri, S.; Boumpas, D.; Brey, R.; Cervera, R.; Doria, A.; Jayne, D.; Khamashta, M.; et al. European League Against Rheumatism recommendations for monitoring patients with systemic lupus erythematosus in clinical practice and in observational studies. Ann. Rheum. Dis. 2010, 69, 1269–1274. [Google Scholar] [CrossRef]
- Maria, N.I.; Davidson, A. Protecting the kidney in systemic lupus erythematosus: From diagnosis to therapy. Nat. Rev. Rheumatol. 2020, 16, 255–267. [Google Scholar] [CrossRef]
- Sun, Y.S.; Huang, D.F.; Chang, F.P.; Chen, W.S.; Liao, H.T.; Chen, M.H.; Tsai, H.C.; Tsai, M.T.; Tsai, C.Y.; Lai, C.C.; et al. Interstitial fibrosis increases the risk of end-stage kidney disease in patients with lupus nephritis. Rheumatology 2024, 63, 2467–2472. [Google Scholar] [CrossRef] [PubMed]
- Anders, H.J.; Saxena, R.; Zhao, M.H.; Parodis, I.; Salmon, J.E.; Mohan, C. Lupus nephritis. Nat. Rev. Dis. Primers 2020, 6, 7. [Google Scholar] [CrossRef]
- Mittoo, S.; Fell, C.D. Pulmonary manifestations of systemic lupus erythematosus. Semin. Respir. Crit. Care Med. 2014, 35, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef]
- Falke, L.L.; Gholizadeh, S.; Goldschmeding, R.; Kok, R.J.; Nguyen, T.Q. Diverse origins of the myofibroblast-implications for kidney fibrosis. Nat. Rev. Nephrol. 2015, 11, 233–244. [Google Scholar] [CrossRef]
- Sarrand, J.; Soyfoo, M.S. Involvement of Epithelial-Mesenchymal Transition (EMT) in Autoimmune Diseases. Int. J. Mol. Sci. 2023, 24, 14481. [Google Scholar] [CrossRef] [PubMed]
- Schunk, S.J.; Floege, J.; Fliser, D.; Speer, T. WNT-beta-catenin signalling—A versatile player in kidney injury and repair. Nat. Rev. Nephrol. 2021, 17, 172–184. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Zhao, L.; Tang, S.; Chen, F.; Ren, X.; Han, X.; Zhou, X. Regulation of macrophage polarization by targeted metabolic reprogramming for the treatment of lupus nephritis. Mol. Med. 2024, 30, 96. [Google Scholar] [CrossRef]
- Hu, H.H.; Chen, D.Q.; Wang, Y.N.; Feng, Y.L.; Cao, G.; Vaziri, N.D.; Zhao, Y.Y. New insights into TGF-beta/Smad signaling in tissue fibrosis. Chem. Biol. Interact. 2018, 292, 76–83. [Google Scholar] [CrossRef]
- Meng, X.M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-beta: The master regulator of fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Overstreet, J.M.; Li, Y.; Wang, Y.; Niu, A.; Wang, S.; Fan, X.; Sasaki, K.; Jin, G.N.; Khodo, S.N.; et al. TGF-beta promotes fibrosis after severe acute kidney injury by enhancing renal macrophage infiltration. JCI Insight 2018, 3, e123563. [Google Scholar] [CrossRef] [PubMed]
- Remijsen, Q.; Kuijpers, T.W.; Wirawan, E.; Lippens, S.; Vandenabeele, P.; Vanden Berghe, T. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ. 2011, 18, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Chrysanthopoulou, A.; Mitroulis, I.; Apostolidou, E.; Arelaki, S.; Mikroulis, D.; Konstantinidis, T.; Sivridis, E.; Koffa, M.; Giatromanolaki, A.; Boumpas, D.T.; et al. Neutrophil extracellular traps promote differentiation and function of fibroblasts. J. Pathol. 2014, 233, 294–307. [Google Scholar] [CrossRef]
- Lin, H.; Liu, J.; Li, N.; Zhang, B.; Nguyen, V.D.; Yao, P.; Feng, J.; Liu, Q.; Chen, Y.; Li, G.; et al. NETosis promotes chronic inflammation and fibrosis in systemic lupus erythematosus and COVID-19. Clin. Immunol. 2023, 254, 109687. [Google Scholar] [CrossRef]
- Richeldi, L.; du Bois, R.M.; Raghu, G.; Azuma, A.; Brown, K.K.; Costabel, U.; Cottin, V.; Flaherty, K.R.; Hansell, D.M.; Inoue, Y.; et al. Efficacy and Safety of Nintedanib in Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2015, 373, 782. [Google Scholar] [CrossRef]
- Wollin, L.; Maillet, I.; Quesniaux, V.; Holweg, A.; Ryffel, B. Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis. J. Pharmacol. Exp. Ther. 2014, 349, 209–220. [Google Scholar] [CrossRef]
- Bedinger, D.; Lao, L.; Khan, S.; Lee, S.; Takeuchi, T.; Mirza, A.M. Development and characterization of human monoclonal antibodies that neutralize multiple TGFbeta isoforms. MAbs 2016, 8, 389–404. [Google Scholar] [CrossRef]
- Bonafoux, D.; Lee, W.C. Strategies for TGF-beta modulation: A review of recent patents. Expert. Opin. Ther. Pat. 2009, 19, 1759–1769. [Google Scholar] [CrossRef]
- Huang, X.R.; Chung, A.C.; Wang, X.J.; Lai, K.N.; Lan, H.Y. Mice overexpressing latent TGF-beta1 are protected against renal fibrosis in obstructive kidney disease. Am. J. Physiol. Ren. Physiol. 2008, 295, F118–F127. [Google Scholar] [CrossRef]
- March, J.T.; Golshirazi, G.; Cernisova, V.; Carr, H.; Leong, Y.; Lu-Nguyen, N.; Popplewell, L.J. Targeting TGFbeta Signaling to Address Fibrosis Using Antisense Oligonucleotides. Biomedicines 2018, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.Y.; Liu, X.S.; Huang, X.R.; Yu, X.Q.; Lan, H.Y. Diverse Role of TGF-beta in Kidney Disease. Front. Cell Dev. Biol. 2020, 8, 123. [Google Scholar] [CrossRef] [PubMed]
- Ning, A.; Xiao, N.; Yu, X.; Wang, H.; Guan, C.; Guo, C.; Dong, Y.; Ma, X.; Xia, H. Dimethyloxallyl Glycine Preconditioning Promotes the Anti-inflammatory and Anti-fibrotic Effects of Human Umbilical Cord Mesenchymal Stem Cells on Kidney Damage in Systemic Lupus Erythematosus Related to TGF-beta/Smad Signaling Pathway. Inflammation 2025, 48, 839–854. [Google Scholar] [CrossRef] [PubMed]
- Negrini, S.; Emmi, G.; Greco, M.; Borro, M.; Sardanelli, F.; Murdaca, G.; Indiveri, F.; Puppo, F. Sjogren’s syndrome: A systemic autoimmune disease. Clin. Exp. Med. 2022, 22, 9–25. [Google Scholar] [CrossRef]
- Maslinska, M. and K. Kostyra-Grabczak, The role of virus infections in Sjogren’s syndrome. Front. Immunol. 2022, 13, 823659. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, Z.; Yu, C.; Yang, C. Expression of Toll-like receptors 7, 8, and 9 in primary Sjogren’s syndrome. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 109, 844–850. [Google Scholar] [CrossRef]
- Cruz-Tapias, P.; Rojas-Villarraga, A.; Maier-Moore, S.; Anaya, J.M. HLA and Sjogren’s syndrome susceptibility. A meta-analysis of worldwide studies. Autoimmun. Rev. 2012, 11, 281–287. [Google Scholar] [CrossRef]
- Lessard, C.J.; Li, H.; Adrianto, I.; Ice, J.A.; Rasmussen, A.; Grundahl, K.M.; Kelly, J.A.; Dozmorov, M.G.; Miceli-Richard, C.; Bowman, S.; et al. Variants at multiple loci implicated in both innate and adaptive immune responses are associated with Sjogren’s syndrome. Nat. Genet. 2013, 45, 1284–1292. [Google Scholar] [CrossRef]
- Selmi, C.; Gershwin, M.E. Chronic Autoimmune Epithelitis in Sjogren’s Syndrome and Primary Biliary Cholangitis: A Comprehensive Review. Rheumatol. Ther. 2017, 4, 263–279. [Google Scholar] [CrossRef]
- Ittah, M.; Miceli-Richard, C.; Eric Gottenberg, J.; Lavie, F.; Lazure, T.; Ba, N.; Sellam, J.; Lepajolec, C.; Mariette, X. B cell-activating factor of the tumor necrosis factor family (BAFF) is expressed under stimulation by interferon in salivary gland epithelial cells in primary Sjogren’s syndrome. Arthritis Res. Ther. 2006, 8, R51. [Google Scholar] [CrossRef]
- Amft, N.; Curnow, S.J.; Scheel-Toellner, D.; Devadas, A.; Oates, J.; Crocker, J.; Hamburger, J.; Ainsworth, J.; Mathews, J.; Salmon, M.; et al. Ectopic expression of the B cell-attracting chemokine BCA-1 (CXCL13) on endothelial cells and within lymphoid follicles contributes to the establishment of germinal center-like structures in Sjogren’s syndrome. Arthritis Rheum. 2001, 44, 2633–2641. [Google Scholar] [CrossRef]
- Barrera, M.J.; Bahamondes, V.; Sepúlveda, D.; Quest, A.F.; Castro, I.; Cortés, J.; Aguilera, S.; Urzúa, U.; Molina, C.; Pérez, P.; et al. Sjogren’s syndrome and the epithelial target: A comprehensive review. J. Autoimmun. 2013, 42, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Shahane, A. The epidemiology of Sjogren’s syndrome. Clin. Epidemiol. 2014, 6, 247–255. [Google Scholar] [PubMed]
- Brito-Zeron, P.; Theander, E.; Baldini, C.; Seror, R.; Retamozo, S.; Quartuccio, L.; Bootsma, H.; Bowman, S.J.; Dörner, T.; Gottenberg, J.E.; et al. Early diagnosis of primary Sjogren’s syndrome: EULAR-SS task force clinical recommendations. Expert. Rev. Clin. Immunol. 2016, 12, 137–156. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Wang, J.; Yang, Z.; Yang, M.; Ma, N.; Huang, F.; Zhong, R. Epidemiology of primary Sjogren’s syndrome: A systematic review and meta-analysis. Ann. Rheum. Dis. 2015, 74, 1983–1989. [Google Scholar] [CrossRef]
- Maldini, C.; Seror, R.; Fain, O.; Dhote, R.; Amoura, Z.; De Bandt, M.; Delassus, J.L.; Falgarone, G.; Guillevin, L.; Le Guern, V.; et al. Epidemiology of primary Sjogren’s syndrome in a French multiracial/multiethnic area. Arthritis Care Res. 2014, 66, 454–463. [Google Scholar] [CrossRef]
- Brito-Zeron, P.; Acar-Denizli, N.; Zeher, M.; Rasmussen, A.; Seror, R.; Theander, E.; Li, X.; Baldini, C.; Gottenberg, J.E.; Danda, D.; et al. Influence of geolocation and ethnicity on the phenotypic expression of primary Sjogren’s syndrome at diagnosis in 8310 patients: A cross-sectional study from the Big Data Sjogren Project Consortium. Ann. Rheum. Dis. 2017, 76, 1042–1050. [Google Scholar] [CrossRef]
- Singh, A.G.; Singh, S.; Matteson, E.L. Rate, risk factors and causes of mortality in patients with Sjogren’s syndrome: A systematic review and meta-analysis of cohort studies. Rheumatology 2016, 55, 450–460. [Google Scholar]
- Ramos-Casals, M.; Brito-Zerón, P.; Bombardieri, S.; Bootsma, H.; De Vita, S.; Dörner, T.; Fisher, B.A.; Gottenberg, J.-E.; Hernandez-Molina, G.; Kocher, A.; et al. EULAR recommendations for the management of Sjogren’s syndrome with topical and systemic therapies. Ann. Rheum. Dis. 2020, 79, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Sisto, M.; Ribatti, D.; Lisi, S. Sjogren’s Syndrome-Related Organs Fibrosis: Hypotheses and Realities. J. Clin. Med. 2022, 11, 3551. [Google Scholar] [CrossRef] [PubMed]
- Leehan, K.M.; Pezant, N.P.; Rasmussen, A.; Grundahl, K.; Moore, J.S.; Radfar, L.; Lewis, D.M.; Stone, D.U.; Lessard, C.J.; Rhodus, N.L.; et al. Minor salivary gland fibrosis in Sjogren’s syndrome is elevated, associated with focus score and not solely a consequence of aging. Clin. Exp. Rheumatol. 2018, 36 (Suppl. S112), 80–88. [Google Scholar]
- Sisto, M.; Ribatti, D.; Lisi, S. ADAM 17 and Epithelial-to-Mesenchymal Transition: The Evolving Story and Its Link to Fibrosis and Cancer. J. Clin. Med. 2021, 10, 3373. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, Y.; Fujisawa, T.; Kono, M.; Nakamura, H.; Yokomura, K.; Koshimizu, N.; Toyoshima, M.; Imokawa, S.; Sumikawa, H.; Johkoh, T.; et al. Prognostic factors for primary Sjogren’s syndrome-associated interstitial lung diseases. Respir. Med. 2019, 159, 105811. [Google Scholar] [CrossRef]
- Roca, F.; Dominique, S.; Schmidt, J.; Smail, A.; Duhaut, P.; Lévesque, H.; Marie, I. Interstitial lung disease in primary Sjogren’s syndrome. Autoimmun. Rev. 2017, 16, 48–54. [Google Scholar] [CrossRef]
- He, C.; Chen, Z.; Liu, S.; Chen, H.; Zhang, F. Prevalence and risk factors of interstitial lung disease in patients with primary Sjogren’s syndrome: A systematic review and meta-analysis. Int. J. Rheum. Dis. 2020, 23, 1009–1018. [Google Scholar] [CrossRef]
- Kakugawa, T.; Sakamoto, N.; Ishimoto, H.; Shimizu, T.; Nakamura, H.; Nawata, A.; Ito, C.; Sato, S.; Hanaka, T.; Oda, K.; et al. Lymphocytic focus score is positively related to airway and interstitial lung diseases in primary Sjogren’s syndrome. Respir. Med. 2018, 137, 95–102. [Google Scholar] [CrossRef]
- Aiyegbusi, O.; McGregor, L.; McGeoch, L.; Kipgen, D.; Geddes, C.C.; Stevens, K.I. Renal Disease in Primary Sjogren’s Syndrome. Rheumatol. Ther. 2021, 8, 63–80. [Google Scholar] [CrossRef]
- Maripuri, S.; Grande, J.P.; Osborn, T.G.; Fervenza, F.C.; Matteson, E.L.; Donadio, J.V.; Hogan, M.C. Renal involvement in primary Sjogren’s syndrome: A clinicopathologic study. Clin. J. Am. Soc. Nephrol. 2009, 4, 1423–1431. [Google Scholar] [CrossRef]
- Goules, A.; Geetha, D.; Arend, L.J.; Baer, A.N. Renal involvement in primary Sjogren’s syndrome: Natural history and treatment outcome. Clin. Exp. Rheumatol. 2019, 37 (Suppl. S118), 123–132. [Google Scholar]
- Nishiwaki, A.; Kobayashi, H.; Ikumi, N.; Kobayashi, Y.; Yokoe, I.; Sugiyama, K.; Matsukawa, Y.; Takei, M.; Kitamura, N. Salivary Gland Focus Score Is Associated With Myocardial Fibrosis in Primary Sjogren Syndrome Assessed by a Cardiac Magnetic Resonance Approach. J. Rheumatol. 2021, 48, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Yokoe, I.; Kobayashi, H.; Nishiwaki, A.; Nagasawa, Y.; Kitamura, N.; Haraoka, M.; Kobayashi, Y.; Takei, M.; Nakamura, H. Asymptomatic myocardial dysfunction was revealed by feature tracking cardiac magnetic resonance imaging in patients with primary Sjogren’s syndrome. Int. J. Rheum. Dis. 2021, 24, 1482–1490. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Feng, Y.; Lin, X. Immune and non-immune mediators in the fibrosis pathogenesis of salivary gland in Sjogren’s syndrome. Front. Immunol. 2024, 15, 1421436. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, X.; Shi, X.; Liu, Y.; Cheng, D.; Tian, Q.; Lin, N.; Wei, W.; Wu, H. CXCL9, 10, 11/CXCR3 Axis Contributes to the Progress of Primary Sjogren’s Syndrome by Activating GRK2 to Promote T Lymphocyte Migration. Inflammation 2023, 46, 1047–1060. [Google Scholar] [CrossRef]
- Ruffilli, I. Sjogren’s syndrome and chemokines. Clin. Ter. 2014, 165, e464–e469. [Google Scholar]
- Dong, Y.; Wang, T.; Wu, H. The role of cytokines from salivary gland epithelial cells in the immunopathology of Sjogren’s syndrome. Front. Immunol. 2024, 15, 1443455. [Google Scholar] [CrossRef]
- Tan, Z.; Wang, L.; Li, X. Composition and regulation of the immune microenvironment of salivary gland in Sjogren’s syndrome. Front. Immunol. 2022, 13, 967304. [Google Scholar] [CrossRef]
- Sisto, M.; Lorusso, L.; Tamma, R.; Ingravallo, G.; Ribatti, D.; Lisi, S. Interleukin-17 and -22 synergy linking inflammation and EMT-dependent fibrosis in Sjogren’s syndrome. Clin. Exp. Immunol. 2019, 198, 261–272. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Maeno, T.; Nomura, M.; Aoyagi-Ikeda, K.; Matsui, H.; Hara, K.; Tanaka, T.; Iso, T.; Suga, T.; Kurabayashi, M. Hypoxia-inducible factor-1alpha mediates TGF-beta-induced PAI-1 production in alveolar macrophages in pulmonary fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2011, 300, L740–L752. [Google Scholar] [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef]
- Brito-Zeron, P.; Baldini, C.; Bootsma, H.; Bowman, S.J.; Jonsson, R.; Mariette, X.; Sivils, K.; Theander, E.; Tzioufas, A.; Ramos-Casals, M. Sjogren syndrome. Nat. Rev. Dis. Primers 2016, 2, 16047. [Google Scholar] [CrossRef]
- Alvarez-Rivas, N.; Sang-Park, H.; Díaz Del Campo, P.; Fernández-Castro, M.; Corominas, H.; Andreu, J.L.; Navarro-Compán, V. Efficacy of belimumab in Primary Sjogren’s syndrome: A systematic review. Reumatol. Clínica 2021, 17, 170–174. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Y.; Shi, G. Anti-CD20 antibody in primary Sjogren’s syndrome management. Curr. Pharm. Biotechnol. 2014, 15, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Steinfeld, S.D.; Tant, L.; Burmester, G.R.; Teoh, N.K.; Wegener, W.A.; Goldenberg, D.M.; Pradier, O. Epratuzumab (humanised anti-CD22 antibody) in primary Sjogren’s syndrome: An open-label phase I/II study. Arthritis Res. Ther. 2006, 8, R129. [Google Scholar] [CrossRef] [PubMed]
- Noll, B.; Mougeot, F.B.; Brennan, M.T.; Mougeot, J.C. Regulation of MMP9 transcription by ETS1 in immortalized salivary gland epithelial cells of patients with salivary hypofunction and primary Sjogren’s syndrome. Sci. Rep. 2022, 12, 14552. [Google Scholar] [CrossRef]
- Marinkovic, M.; Tran, O.N.; Wang, H.; Abdul-Azees, P.; Dean, D.D.; Chen, X.D.; Yeh, C.K. Extracellular matrix turnover in salivary gland disorders and regenerative therapies: Obstacles and opportunities. J. Oral Biol. Craniofac. Res. 2023, 13, 693–703. [Google Scholar] [CrossRef]
- Lozito, T.P.; Jackson, W.M.; Nesti, L.J.; Tuan, R.S. Human mesenchymal stem cells generate a distinct pericellular zone of MMP activities via binding of MMPs and secretion of high levels of TIMPs. Matrix Biol. 2014, 34, 132–143. [Google Scholar] [CrossRef]
- Chihaby, N.; Orliaguet, M.; Le Pottier, L.; Pers, J.O.; Boisramé, S. Treatment of Sjogren’s Syndrome with Mesenchymal Stem Cells: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 10474. [Google Scholar] [CrossRef]
- Xu, J.; Wang, D.; Liu, D.; Fan, Z.; Zhang, H.; Liu, O.; Ding, G.; Gao, R.; Zhang, C.; Ding, Y.; et al. Allogeneic mesenchymal stem cell treatment alleviates experimental and clinical Sjogren syndrome. Blood 2012, 120, 3142–3151. [Google Scholar] [CrossRef]
- Tian, J.; Hong, Y.; Zhu, Q.; Zhou, H.; Zhang, Y.; Shen, Z.; Guo, H.; Zhang, Y.; Ai, X.; Zhao, F.; et al. Mesenchymal Stem Cell Enhances the Function of MDSCs in Experimental Sjogren Syndrome. Front. Immunol. 2020, 11, 604607. [Google Scholar] [CrossRef]
- Shi, B.; Qi, J.; Yao, G.; Feng, R.; Zhang, Z.; Wang, D.; Chen, C.; Tang, X.; Lu, L.; Chen, W.; et al. Mesenchymal stem cell transplantation ameliorates Sjogren’s syndrome via suppressing IL-12 production by dendritic cells. Stem Cell Res. Ther. 2018, 9, 308. [Google Scholar] [CrossRef]
- Yao, G.; Qi, J.; Liang, J.; Shi, B.; Chen, W.; Li, W.; Tang, X.; Wang, D.; Lu, L.; Chen, W.; et al. Mesenchymal stem cell transplantation alleviates experimental Sjogren’s syndrome through IFN-beta/IL-27 signaling axis. Theranostics 2019, 9, 8253–8265. [Google Scholar] [CrossRef]
- Qin, L.; Liu, N.; Bao, C.L.; Yang, D.Z.; Ma, G.X.; Yi, W.H.; Xiao, G.Z.; Cao, H.L. Mesenchymal stem cells in fibrotic diseases-the two sides of the same coin. Acta Pharmacol. Sin. 2023, 44, 268–287. [Google Scholar] [CrossRef] [PubMed]
- Salazar, K.D.; Lankford, S.M.; Brody, A.R. Mesenchymal stem cells produce Wnt isoforms and TGF-beta1 that mediate proliferation and procollagen expression by lung fibroblasts. Am. J. Physiol. Lung Cell Mol. Physiol. 2009, 297, L1002–L1011. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, U.; Mayshar, Y.; Benvenisty, N. Large-scale analysis reveals acquisition of lineage-specific chromosomal aberrations in human adult stem cells. Cell Stem Cell 2011, 9, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhang, H.; Kong, W.; Deng, W.; Wang, D.; Feng, X.; Zhao, C.; Hua, B.; Wang, H.; Sun, L. Safety analysis in patients with autoimmune disease receiving allogeneic mesenchymal stem cells infusion: A long-term retrospective study. Stem Cell Res. Ther. 2018, 9, 312. [Google Scholar] [CrossRef]
- Vatn, M.H. Natural history and complications of IBD. Curr. Gastroenterol. Rep. 2009, 11, 481–487. [Google Scholar] [CrossRef]
- Xin, S.; Liu, X.; He, C.; Gao, H.; Wang, B.; Hua, R.; Gao, L.; Shang, H.; Sun, F.; Xu, J. Inflammation accelerating intestinal fibrosis: From mechanism to clinic. Eur. J. Med. Res. 2024, 29, 335. [Google Scholar] [CrossRef]
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Yassin, N.; Marley, A.; Bellato, V.; Foppa, C.; Pellino, G.; Myrelid, P.; Millan, M.; Gros, B.; Avellaneda, N.; et al. Crossing barriers: The burden of inflammatory bowel disease across Western Europe. Ther. Adv. Gastroenterol. 2023, 16, 17562848231218615. [Google Scholar] [CrossRef] [PubMed]
- Nakase, H.; Uchino, M.; Shinzaki, S.; Matsuura, M.; Matsuoka, K.; Kobayashi, T.; Saruta, M.; Hirai, F.; Hata, K.; Hiraoka, S.; et al. Evidence-based clinical practice guidelines for inflammatory bowel disease 2020. J. Gastroenterol. 2021, 56, 489–526. [Google Scholar] [CrossRef] [PubMed]
- M’Koma, A.E. Inflammatory Bowel Disease: Clinical Diagnosis and Pharmaceutical Management. Med. Res. Arch. 2023, 11, 10–18103. [Google Scholar]
- Yang, B.; Zhang, G.; Elias, M.; Zhu, Y.; Wang, J. The role of cytokine and immune responses in intestinal fibrosis. J. Dig. Dis. 2020, 21, 308–314. [Google Scholar] [CrossRef]
- Macias-Ceja, D.C.; Mendoza-Ballesteros, M.T.; Ortega-Albiach, M.; Barrachina, M.D.; Ortiz-Masià, D. Role of the epithelial barrier in intestinal fibrosis associated with inflammatory bowel disease: Relevance of the epithelial-to mesenchymal transition. Front. Cell Dev. Biol. 2023, 11, 1258843. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lu, Q.; Liu, Y.; Shi, Z.; Hu, L.; Zeng, Z.; Tu, Y.; Xiao, Z.; Xu, Q. Th17 Cells in Inflammatory Bowel Disease: Cytokines, Plasticity, and Therapies. J. Immunol. Res. 2021, 2021, 8816041. [Google Scholar] [CrossRef]
- Meng, X.M.; Huang, X.R.; Xiao, J.; Chung, A.C.; Qin, W.; Chen, H.Y.; Lan, H.Y. Disruption of Smad4 impairs TGF-beta/Smad3 and Smad7 transcriptional regulation during renal inflammation and fibrosis in vivo and in vitro. Kidney Int. 2012, 81, 266–279. [Google Scholar] [CrossRef]
- Di Sabatino, A.; Jackson, C.L.; Pickard, K.M.; Buckley, M.; Rovedatti, L.; Leakey, N.A.; Picariello, L.; Cazzola, P.; Monteleone, G.; Tonelli, F.; et al. Transforming growth factor beta signalling and matrix metalloproteinases in the mucosa overlying Crohn’s disease strictures. Gut 2009, 58, 777–789. [Google Scholar] [CrossRef]
- Fabre, T.; Kared, H.; Friedman, S.L.; Shoukry, N.H. IL-17A enhances the expression of profibrotic genes through upregulation of the TGF-beta receptor on hepatic stellate cells in a JNK-dependent manner. J. Immunol. 2014, 193, 3925–3933. [Google Scholar] [CrossRef]
- Lu, Q.; Yang, M.F.; Liang, Y.J.; Xu, J.; Xu, H.M.; Nie, Y.Q.; Wang, L.S.; Yao, J.; Li, D.F. Immunology of Inflammatory Bowel Disease: Molecular Mechanisms and Therapeutics. J. Inflamm. Res. 2022, 15, 1825–1844. [Google Scholar] [CrossRef]
- Fujii, T.; Fuchs, B.C.; Yamada, S.; Lauwers, G.Y.; Kulu, Y.; Goodwin, J.M.; Lanuti, M.; Tanabe, K.K. Mouse model of carbon tetrachloride induced liver fibrosis: Histopathological changes and expression of CD133 and epidermal growth factor. BMC Gastroenterol. 2010, 10, 79. [Google Scholar] [CrossRef]
- D’Alessio, S.; Ungaro, F.; Noviello, D.; Lovisa, S.; Peyrin-Biroulet, L.; Danese, S. Revisiting fibrosis in inflammatory bowel disease: The gut thickens. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 169–184. [Google Scholar] [CrossRef]
- Arpino, V.; Brock, M.; Gill, S.E. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol. 2015, 44–46, 247–254. [Google Scholar] [CrossRef]
- Li, S.; Li, H.; Qi, M. Exploring shared pathogenic mechanisms and biomarkers in hepatic fibrosis and inflammatory bowel disease through bioinformatics and machine learning. Front. Immunol. 2025, 16, 1533246. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, L.; Hua, H.; Liu, L.; Mao, Y.; Wang, R. Interactions between toll-like receptors signaling pathway and gut microbiota in host homeostasis. Immun. Inflamm. Dis. 2024, 12, e1356. [Google Scholar] [CrossRef] [PubMed]
- Henderson, N.C.; Rieder, F.; Wynn, T.A. Fibrosis: From mechanisms to medicines. Nature 2020, 587, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.B.; Xavier, R.J. Pathway paradigms revealed from the genetics of inflammatory bowel disease. Nature 2020, 578, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Kim, J.; Lee, Y.J.; Bae, S.U.; Lee, H.W. Inflammatory bowel disease-associated intestinal fibrosis. J. Pathol. Transl. Med. 2023, 57, 60–66. [Google Scholar] [CrossRef]
- Macias-Ceja, D.C.; Barrachina, M.D.; Ortiz-Masia, D. Autophagy in intestinal fibrosis: Relevance in inflammatory bowel disease. Front. Pharmacol. 2023, 14, 1170436. [Google Scholar] [CrossRef] [PubMed]
- Imbrizi, M.; Magro, F.; Coy, C.S.R. Pharmacological Therapy in Inflammatory Bowel Diseases: A Narrative Review of the Past 90 Years. Pharmaceuticals 2023, 16, 1272. [Google Scholar] [CrossRef] [PubMed]
- Rieder, F.; Mukherjee, P.K.; Massey, W.J.; Wang, Y.; Fiocchi, C. Fibrosis in IBD: From pathogenesis to therapeutic targets. Gut 2024, 73, 854–866. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, G.; Lenti, M.V.; Di Sabatino, A. Therapeutic Targeting of Intestinal Fibrosis in Crohn’s Disease. Cells 2022, 11, 429. [Google Scholar] [CrossRef]
- Wengrower, D.; Zanninelli, G.; Pappo, O.; Latella, G.; Sestieri, M.; Villanova, A.; Faitelson, Y.; Pines, M.; Goldin, E. Prevention of fibrosis in experimental colitis by captopril: The role of tgf-beta1. Inflamm. Bowel Dis. 2004, 10, 536–545. [Google Scholar] [CrossRef]
- Speca, S.; Rousseaux, C.; Dubuquoy, C.; Rieder, F.; Vetuschi, A.; Sferra, R.; Giusti, I.; Bertin, B.; Dubuquoy, L.; Gaudio, E.; et al. Novel PPARgamma Modulator GED-0507-34 Levo Ameliorates Inflammation-driven Intestinal Fibrosis. Inflamm. Bowel Dis. 2016, 22, 279–292. [Google Scholar] [CrossRef]
- Li, G.; Ren, J.; Hu, Q.; Deng, Y.; Chen, G.; Guo, K.; Li, R.; Li, Y.; Wu, L.; Wang, G.; et al. Oral pirfenidone protects against fibrosis by inhibiting fibroblast proliferation and TGF-beta signaling in a murine colitis model. Biochem. Pharmacol. 2016, 117, 57–67. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Vanderkerken, M.; Hammad, H. The emerging role of ADAM metalloproteinases in immunity. Nat. Rev. Immunol. 2018, 18, 745–758. [Google Scholar] [CrossRef]
- Holvoet, T.; Devriese, S.; Castermans, K.; Boland, S.; Leysen, D.; Vandewynckel, Y.P.; Devisscher, L.; Van den Bossche, L.; Van Welden, S.; Dullaers, M.; et al. Treatment of Intestinal Fibrosis in Experimental Inflammatory Bowel Disease by the Pleiotropic Actions of a Local Rho Kinase Inhibitor. Gastroenterology 2017, 153, 1054–1067. [Google Scholar] [CrossRef]
- Li, Y.; Xu, F.; Fang, Y.; Cui, Y.; Zhu, Z.; Wu, Y.; Tong, Y.; Hu, J.; Zhu, L.; Shen, H. Inflammation-fibrosis interplay in inflammatory bowel disease: Mechanisms, progression, and therapeutic strategies. Front. Pharmacol. 2025, 16, 1530797. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, X.; Liu, S.; Wang, Q.; Wang, Y.; Hou, S.; Wang, J.; Zhang, Y. Global, regional, and national epidemiology of rheumatoid arthritis among people aged 20–54 years from 1990 to 2021. Sci. Rep. 2025, 15, 10736. [Google Scholar] [CrossRef]
- Chai, D.; Sun, D.; Wang, Y.; Song, Y.; Wu, N.; Ye, Q. Progression of radiographic fibrosis in rheumatoid arthritis-associated interstitial lung disease. Front. Med. 2023, 10, 1265355. [Google Scholar] [CrossRef]
- Conforti, A.; Di Cola, I.; Pavlych, V.; Ruscitti, P.; Berardicurti, O.; Ursini, F.; Giacomelli, R.; Cipriani, P. Beyond the joints, the extra-articular manifestations in rheumatoid arthritis. Autoimmun. Rev. 2021, 20, 102735. [Google Scholar] [CrossRef] [PubMed]
- Juge, P.A.; Lee, J.S.; Ebstein, E.; Furukawa, H.; Dobrinskikh, E.; Gazal, S.; Kannengiesser, C.; Ottaviani, S.; Oka, S.; Tohma, S.; et al. MUC5B Promoter Variant and Rheumatoid Arthritis with Interstitial Lung Disease. N. Engl. J. Med. 2018, 379, 2209–2219. [Google Scholar] [CrossRef]
- Juge, P.A.; Borie, R.; Kannengiesser, C.; Gazal, S.; Revy, P.; Wemeau-Stervinou, L.; Debray, M.P.; Ottaviani, S.; Marchand-Adam, S.; Nathan, N.; et al. Shared genetic predisposition in rheumatoid arthritis-interstitial lung disease and familial pulmonary fibrosis. Eur. Respir. J. 2017, 49, 1602314. [Google Scholar] [CrossRef]
- Okada, Y.; Suzuki, A.; Ikari, K.; Terao, C.; Kochi, Y.; Ohmura, K.; Higasa, K.; Akiyama, M.; Ashikawa, K.; Kanai, M.; et al. Contribution of a Non-classical HLA Gene, HLA-DOA, to the Risk of Rheumatoid Arthritis. Am. J. Hum. Genet. 2016, 99, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Adegunsoye, A.; Vij, R.; Noth, I. Integrating Genomics Into Management of Fibrotic Interstitial Lung Disease. Chest 2019, 155, 1026–1040. [Google Scholar] [CrossRef]
- Lepri, G.; Markovic, M.; Bellando-Randone, S.; Sebastiani, M.; Guiducci, S. The Burden of Interstitial Lung Involvement in Rheumatoid Arthritis: Could Lung Ultrasound Have a Role in Its Detection? A Literature Review. Diagnostics 2024, 14, 1430. [Google Scholar] [CrossRef] [PubMed]
- Kadura, S.; Raghu, G. Rheumatoid arthritis-interstitial lung disease: Manifestations and current concepts in pathogenesis and management. Eur. Respir. Rev. 2021, 30, 210011. [Google Scholar] [CrossRef] [PubMed]
- Neofotistou-Themeli, E.; Goutakoli, P.; Chanis, T.; Semitekolou, M.; Sevdali, E.; Sidiropoulos, P. Fibroblasts in rheumatoid arthritis: Novel roles in joint inflammation and beyond. Front. Med. 2024, 11, 1376925. [Google Scholar] [CrossRef]
- Damerau, A.; Rosenow, E.; Alkhoury, D.; Buttgereit, F.; Gaber, T. Fibrotic pathways and fibroblast-like synoviocyte phenotypes in osteoarthritis. Front. Immunol. 2024, 15, 1385006. [Google Scholar] [CrossRef]
- Bhamidipati, K.; McIntyre, A.B.R.; Kazerounian, S.; Ce, G.; Tran, M.; Prell, S.A.; Lau, R.; Khedgikar, V.; Altmann, C.; Small, A.; et al. Spatial patterning of fibroblast TGFbeta signaling underlies treatment resistance in rheumatoid arthritis. bioRxiv 2025. [Google Scholar] [CrossRef]
- Wilson, T.M.; Bolt, M.; Stahly, A.; Lee, J.S.; Bang, T.J.; Sachs, P.B.; Deane, K.D.; Humphries, S.M.; Solomon, J.J.; Demoruelle, M.K. Transforming growth factor-beta is increased in sputum from individuals with rheumatoid arthritis-associated pulmonary fibrosis. Rheumatology 2025, 64, 3989–3995. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, B.; Sun, X.; Li, H.; Ouyang, X.; Wei, J.; Dai, B.; Zhang, Y.; Li, X. Rheumatoid arthritis fibroblast-like synoviocytes co-cultured with PBMC increased peripheral CD4+CXCR5+ICOS+ T cell numbers. Clin. Exp. Immunol. 2017, 190, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Chen, X.; Sun, Y.; Zhang, Y.; Dong, R.; Wang, X.; Chen, S. Exploring the molecular mechanisms and shared potential drugs between rheumatoid arthritis and arthrofibrosis based on large language model and synovial microenvironment analysis. Sci. Rep. 2024, 14, 18939. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, Y.; Wu, R.; Ding, T.; Xue, H.; Gao, C.; Li, X.; Wang, C. New Insights From Single-Cell Sequencing Data: Synovial Fibroblasts and Synovial Macrophages in Rheumatoid Arthritis. Front. Immunol. 2021, 12, 709178. [Google Scholar] [CrossRef]
- Steenvoorden, M.M.; Tolboom, T.C.; van der Pluijm, G.; Löwik, C.; Visser, C.P.; DeGroot, J.; Gittenberger-DeGroot, A.C.; DeRuiter, M.C.; Wisse, B.J.; Huizinga, T.W.; et al. Transition of healthy to diseased synovial tissue in rheumatoid arthritis is associated with gain of mesenchymal/fibrotic characteristics. Arthritis Res. Ther. 2006, 8, R165. [Google Scholar] [CrossRef]
- Zhu, D.; Zhao, J.; Lou, A.; Huang, Q.; OuYang, Q.; Zhu, J.; Fan, M.; He, Y.; Ren, H.; Yang, M. Transforming growth factor beta1 promotes fibroblast-like synoviocytes migration and invasion via TGF-beta1/Smad signaling in rheumatoid arthritis. Mol. Cell. Biochem. 2019, 459, 141–150. [Google Scholar] [CrossRef]
- Zhou, W.; Cheng, H.; Fan, C.; Zhou, X.; Chen, W.; Xie, C.; Hu, Y.; Chen, Y.; Wang, X.; Wu, J. LAMP3-mediated epithelial-mesenchymal transition promotes the invasion and excessive proliferation of fibroblast-like synoviocytes in rheumatoid arthritis. J. Autoimmun. 2025, 151, 103359. [Google Scholar] [CrossRef]
- Elhaj Mahmoud, D.; Kaabachi, W.; Sassi, N.; Mokhtar, A.; Ben Ammar, L.; Rekik, S.; Tarhouni, L.; Kallel-Sellami, M.; Cheour, E.; Laadhar, L. Expression of extracellular matrix components and cytokine receptors in human fibrocytes during rheumatoid arthritis. Connect. Tissue Res. 2021, 62, 720–731. [Google Scholar] [CrossRef]
- Madsen, S.F.; Madsen, S.S.; Madrid, A.S.; Andersen, M.R.; Bay-Jensen, A.C.; Thudium., C.S. Fibrotic remodeling in joint diseases: Induction and inhibition of fibrosis in fibroblast-like synoviocytes. Transl. Med. Commun. 2024, 9, 18. [Google Scholar] [CrossRef]
- Diesler, R.; Cottin, V. Pulmonary fibrosis associated with rheumatoid arthritis: From pathophysiology to treatment strategies. Expert. Rev. Respir. Med. 2022, 16, 541–553. [Google Scholar] [CrossRef]
- Milara, J.; Hernandez, G.; Ballester, B.; Morell, A.; Roger, I.; Montero, P.; Escrivá, J.; Lloris, J.M.; Molina-Molina, M.; Morcillo, E.; et al. The JAK2 pathway is activated in idiopathic pulmonary fibrosis. Respir. Res. 2018, 19, 24. [Google Scholar] [CrossRef]
- Kurushima, S.; Koga, T.; Umeda, M.; Iwamoto, N.; Miyashita, R.; Tokito, T.; Okuno, D.; Yura, H.; Ishimoto, H.; Kido, T.; et al. Impact of Janus kinase inhibitors and methotrexate on interstitial lung disease in rheumatoid arthritis patients. Front. Immunol. 2024, 15, 1501146. [Google Scholar] [CrossRef]
- Gan, D.; Cheng, W.; Ke, L.; Sun, A.R.; Jia, Q.; Chen, J.; Lin, J.; Li, J.; Xu, Z.; Zhang, P. Repurposing of Pirfenidone (Anti-Pulmonary Fibrosis Drug) for Treatment of Rheumatoid Arthritis. Front. Pharmacol. 2021, 12, 631891. [Google Scholar] [CrossRef]
- Yang, M.; Wu, Y.; Liu, X.; Zhao, C.; Li, T.; Li, T.; Zhang, X.; Jiang, H.; Mao, B.; Liu, W. Efficacy and safety of antifibrotic agents in the treatment of CTD-ILD and RA-ILD: A systematic review and meta-analysis. Respir. Med. 2023, 216, 107329. [Google Scholar] [CrossRef]
- Narvaez, J.; Aguilar-Coll, M.; Vicens-Zygmunt, V.; Alegre, J.J.; Bermudo, G.; Molina-Molina, M. Real-World Clinical Effectiveness and Safety of Antifibrotics in Progressive Pulmonary Fibrosis Associated with Rheumatoid Arthritis. J. Clin. Med. 2024, 13, 7074. [Google Scholar] [CrossRef]
- Nemeth, T.; Nagy, G.; Pap, T. Synovial fibroblasts as potential drug targets in rheumatoid arthritis, where do we stand and where shall we go? Ann. Rheum. Dis. 2022, 81, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Glasser, S.W.; Hagood, J.S.; Wong, S.; Taype, C.A.; Madala, S.K.; Hardie, W.D. Mechanisms of Lung Fibrosis Resolution. Am. J. Pathol. 2016, 186, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Drew, L. Tipping the balance. Nature 2018, 564, S74–S75. [Google Scholar] [CrossRef] [PubMed]
- Ellis, E.L.; Mann, D.A. Clinical evidence for the regression of liver fibrosis. J. Hepatol. 2012, 56, 1171–1180. [Google Scholar] [CrossRef]
- van der Meer, A.J.; Berenguer, M. Reversion of disease manifestations after HCV eradication. J. Hepatol. 2016, 65 (Suppl. S1), S95–S108. [Google Scholar] [CrossRef]
- Burnham, E.L.; Janssen, W.J.; Riches, D.W.; Moss, M.; Downey, G.P. The fibroproliferative response in acute respiratory distress syndrome: Mechanisms and clinical significance. Eur. Respir. J. 2014, 43, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Dobrota, R.; Maurer, B.; Graf, N.; Jordan, S.; Mihai, C.; Kowal-Bielecka, O.; Allanore, Y.; Distler, O.; EUSTAR coauthors. Prediction of improvement in skin fibrosis in diffuse cutaneous systemic sclerosis: A EUSTAR analysis. Ann. Rheum. Dis. 2016, 75, 1743–1748. [Google Scholar] [CrossRef]
- Foocharoen, C.; Mahakkanukrauh, A.; Suwannaroj, S.; Nanagara, R. Spontaneous skin regression and predictors of skin regression in Thai scleroderma patients. Clin. Rheumatol. 2011, 30, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, J.C.; Thannickal, V.J. Mechanisms for the Resolution of Organ Fibrosis. Physiology 2019, 34, 43–55. [Google Scholar] [CrossRef]
- Zhao, P.; Sun, T.; Lyu, C.; Liang, K.; Du, Y. Cell mediated ECM-degradation as an emerging tool for anti-fibrotic strategy. Cell Regen. 2023, 12, 29. [Google Scholar] [CrossRef]
- Lopez-Guisa, J.M.; Cai, X.; Collins, S.J.; Yamaguchi, I.; Okamura, D.M.; Bugge, T.H.; Isacke, C.M.; Emson, C.L.; Turner, S.M.; Shankland, S.J.; et al. Mannose receptor 2 attenuates renal fibrosis. J. Am. Soc. Nephrol. 2012, 23, 236–251. [Google Scholar] [CrossRef]
- Kawano, S.; Torisu, T.; Esaki, M.; Torisu, K.; Matsuno, Y.; Kitazono, T. Autophagy promotes degradation of internalized collagen and regulates distribution of focal adhesions to suppress cell adhesion. Biol. Open 2017, 6, 1644–1653. [Google Scholar] [CrossRef]

| Disease | Role of Fibroblasts/Myofibroblasts | Other Key Involved Cells | Main Profibrotic Cytokines |
|---|---|---|---|
| Systemic sclerosis (SSc) | Activated fibroblasts differentiate into myofibroblasts, producing excessive collagen and ECM → progressive skin and organ fibrosis | Th2/Th17 T cells, B cells, M2 macrophages, pDCs, endothelial cells (EndoMT) | TGF-β, IL-6, IL-4, IL-13, PDGF, Endothelin-1, BAFF, IFN-α |
| Morphea | Local skin fibroblasts become activated, producing collagen and ECM → sclerotic plaques | CD4+ T cells (Th1/Th17→Th2), macrophages, dendritic cells, endothelial cells | TGF-β, IL-4, IL-6, IL-13, IL-17, CTGF, PDGF, INF-γ |
| Autoimmune hepatitis (AIH) | Hepatic stellate cells (fibroblast-like) transform into myofibroblasts → collagen I/III production → liver fibrosis/cirrhosis | T cells, NK cells, Kupffer cells, dendritic cells, liver sinusoidal endothelial cells (LSECs) | TGF-β, PDGF, TNF-α, IL-6, IL-1β, IL-17, TIMP-1 |
| Systemic lupus erythematosus (SLE) | Myofibroblasts undergo activation → ECM accumulation → fibrotic remodeling in kidneys, lungs, skin, and heart | NET-forming neutrophils, macrophages | TGF-β, IL-6, IL-1β, TNF-α, IFN-α, PDGF |
| Sjögren’s syndrome (SS) | Epithelial cell reprogramming via EMT, differentiation of fibroblasts into myofibroblasts → ECM accumulation → salivary gland fibrosis | Th1/Th17/Th22, CD8+ T cells, B cells, macrophages | TGF-β1, IFN-γ, TNF-α, IL-6, IL-17, IL-21, CXCL10 |
| Inflammatory bowel disease (IBD) | Intestinal fibroblasts/mesenchymal cells activate into myofibroblasts → collagen deposition → strictures (mainly CD) | Th1/17 T cells, macrophages, epithelial cells (EMT) | TGF-β, TNF-α, IL-6, IL-1β, IL-13/IL-17, TL1A, CTGF, ROCK, microbiota induced TL1A and IL33 |
| Rheumatoid arthritis (RA) | Fibroblast-like synoviocytes (FLS) become aggressive myofibroblast-like cells → synovial and pulmonary fibrosis | Th1/Th17 T cells, macrophages, B cells, neutrophils, endothelial cells, synovial lining cells, circulating fibrocytes | TGF-β1/3, IL-6, TNF-α, IL-17, PDGF, Notch, JAK/STAT, BMP, MMP/TIMP |
| Autoimmune Disease | Key Profibrotic Mediators (Cytokines/Pathways) | Advantages of Inhibition | Potential Drawbacks of Inhibition | Status of Antifibrotic Therapeutic Development |
|---|---|---|---|---|
| Systemic sclerosis (SSc) | TGF-β, IL-6, IL-4/IL-13, PDGF, CTGF, endothelin-1 (ETaR), AT1R, BAFF, IFN-α/ TGF-β/SMAD pathway, JAK/STAT pathway, EndoMT, TLR-signaling | Inhibition of fibroblast activation and excessive ECM production → slows disease progression, preserves organ function (especially lung), reduces fibrosis-related complications, may improve survival | Immunosuppression leading to infections; treatment-related adverse effects (e.g., diarrhea with nintedanib, hematologic toxicity with HSCT); limited ability to reverse established fibrosis; heterogeneous patient response; off-target effects | Approved: nintedanib (FDA/EMA for SSc-ILD); investigational/repurposed: pirfenidone (not approved in SSc-ILD), biologics: rituximab (anti-CD20), tocilizumab (anti-IL-6), fresolimumab (anti-TGF-β), romilkimab (anti-IL-4/IL-13), abatacept (CTLA-4-Ig), belimumab (BAFF inhibitor); advanced immunotherapies: HSCT; experimental: CD19 CAR-T cells; JAK inhibitors |
| Morphea (localized scleroderma) | TGF-β, IL-4, IL13, IL-6, PDGF, CTGF, IFN-γ, IL-17, vascular adhesion molecules (E-selectin, VCAM-1)/ TGF-β/SMAD pathway, PDGF/c-Abl pathway, JAK/STAT signalling | Blocking profibrotic pathways may reduce fibroblast activation and collagen deposition, control inflammation, halt disease progression, prevent tissue atrophy and deformities, improve skin elasticity and quality of life | Risk of systemic immunosuppression (infection, toxicity), especially in children; limited ability to reverse established fibrosis; heterogeneity of clinical response; relapse after therapy withdrawal; long-term safety concerns | Current: methotrexate ± systemic steroids (first-line), MMF, cyclosporine, hydroxychloroquine, azathioprine, retinoids; topical steroids/tacrolimus; phototherapy, biologics (rituximab; infliximab); emerging: tocilizumab (anti-IL6), abatacept (a CTLA-4-Ig fusion protein), JAK inhibitors (tofacitinib, baricitinib), imatinib (PDGF/c-Abl), anti-TGF-β, BET/HDAC inhibitors—mainly under early clinical investigation |
| Autoimmune hepatitis (AIH) | TGF-β, PDGF, TNF-α, IL-1β, IL-6, IL-17, chemokines (CXCL10), TIMP-1/ TGF-β/SMAD pathway, TLR4/TLR9, NLRP3 inflammasome, Wnt/β-catenin signaling | Suppression of inflammation and HSC activation → inhibits collagen deposition, slows progression to fibrosis/cirrhosis, preserves liver function, lowers portal hypertension risk, may reduce need for transplantation | General immunosuppression → infection risk; steroid/azathioprine toxicity; incomplete response or intolerance in some patients; relapse upon dose reduction; difficulty reversing established fibrosis; off-target effects | Current standard: Prednisolone ± azathioprine; budesonide; MMF; calcineurin (cyclosporine A, tacrolimus), mTOR (everolimus), biologics (rituximab, infliximab) for refractory AIH. Emerging: hematopoietic/mesenchymal stem cell therapy; Experimental immune-targeted: Zetomipzomib (immunoproteasome inhibitor), Ianalumab (anti-BAFF-R), JKB-122 (TLR-4 antagonist), JAK-inhibitors (case reports), adoptive Treg transfer, low-dose IL-2 |
| Systemic lupus erythematosus (SLE) | TGF-β/Smad pathway, IL-6, IL-1β, TNF-α, IFN-α (type I interferon axis), PDGF, Wnt/β-catenin signaling, epithelial-to-mesenchymal transition (EMT), NETosis (NET-induced EMT), M2 macrophages, myofibroblast activation | Reduces progression toward organ fibrosis (kidneys, lungs, skin), preserves organ function, improves long-term outcomes, prevents irreversible damage, may decrease morbidity associated with lupus nephritis and interstitial lung disease | Risk of immunosuppression and infection; potential off-target effects; organ-specific variability and heterogeneity of fibrosis; difficulty reversing already established scarring; possibly poor tolerability or efficacy in some patients | Current: immunosuppressants (steroids, MMF, CYC), biologics (belimumab—anti-BAFF; anifrolumab—anti-IFNAR1). Antifibrotics under investigation: nintedanib (tyrosine-kinase inhibitor), anti-TGF-β agents (fresolimumab), EMT/Wnt inhibitors, stem-cell-based therapies (MSC), NET-targeting strategies, TGF-β antisense oligonucleotides; none yet specifically approved for SLE-related fibrosis |
| Sjögren’s Syndrome (SS) | TGF-β/SMAD/Snail signaling, IFN-γ, TNF-α, IL-6, IL-21, IL-17, CXCL10/CXCR3 axis, EMT of epithelial cells, HIF-1α, MMP/TIMP imbalance, myofibroblast activation, persistent T and B cell–mediated inflammation | Reduces progression of glandular and extraglandular fibrosis (salivary glands, lungs, kidneys, myocardium), preserves secretory and organ function, delays irreversible atrophy and structural remodeling | Immunosuppression risks (infections), difficulty reversing advanced fibrosis, heterogeneous organ involvement, potential off-target effects, possible promotion of additional immune dysregulation | Current: symptomatic (artificial saliva, pilocarpine); immunomodulators (glucocorticoids, hydroxychloroquine, MTX); biologics (rituximab, belimumab, epratuzumab) for systemic disease. Emerging: MSC therapy, siRNAs (e.g., ETS1), anti-TGF-β strategies, FAP-targeted CAR-T cells, anti-fibrotic small molecules—largely experimental or preclinical |
| Inflammatory Bowel Disease (IBD) | TGF-β/Smad, TNF-α, IL-1β, IL-6, IL-17, IL-13, IL-33, TL1A; EMT/EndoMT; myofibroblast activation; MMP/TIMP imbalance; microbial TLR signaling; autophagy-related pathways; ROCK activation; CTGF; angiotensin II; microbiota-derived factors | Prevents fibrostenotic complications and strictures, limits need for surgical resections, preserves intestinal architecture, improves long-term outcomes and quality of life by limiting fibrosis-driven morbidity | Risk of impairing normal mucosal healing; immunosuppression-related infections; heterogeneity of disease sites and fibrosis course; limited ability to reverse established strictures; potential off-target effects | Current: Anti-inflammatory therapy (5-ASA, steroids, thiopurines, MTX, biologics—anti-TNF, anti-integrin, anti-IL, JAK inhibitors, S1P modulators); no approved anti-fibrotic drugs yet. Emerging candidates: ACE inhibitors/sartans; PPAR-γ agonists (GED-0507-34); pirfenidone; ROCK inhibitors (AMA0825); MMP modulators; microbiome therapies; natural compounds (curcumin, resveratrol); combination anti-inflammatory + antifibrotic approaches under investigation |
| Rheumatoid arthritis (RA) | TGF-β (Smad2/3), IL-6, TNF-α, IL-17, PDGF, Notch signaling, EMT/EndoMT, synovial fibroblast activation (FLS → myofibroblasts), MMP/TIMP imbalance, JAK/STAT pathway, BMP signaling, COMP-positive fibrogenic fibroblasts | Limits synovial and pulmonary fibrotic remodeling, preserves joint mobility and lung function, reduces risk of irreversible organ damage (i.e., RA-ILD progression), may complement anti-inflammatory therapy to improve prognosis | Possible interference with physiological repair processes; systemic immunosuppression with infection risk; limited reversal of established fibrosis; heterogeneity in fibrotic phenotypes; could impact cartilage homeostasis/angiogenesis | Current RA therapy: Disease-Modifying Anti-Rheumatic Drugs (DMARDs): MTX, sulfasalazine, biologics (anti-TNF, IL-6 inhibitors, abatacept, rituximab) and JAK inhibitors (tofacitinib). Emerging antifibrotics: pirfenidone (preclinical/clinical for RA and RA-ILD), nintedanib (approved for RA-ILD), tofacitinib and other JAK inhibitors show anti-fibrotic effects, MSC-based therapies in early studies; repositioned drugs (HDAC inhibitors, emodin, silymarin) under investigation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żurawek, M.; Ziółkowska-Suchanek, I.; Iżykowska, K. Fibrosis in Immune-Mediated and Autoimmune Disorders. J. Clin. Med. 2025, 14, 6636. https://doi.org/10.3390/jcm14186636
Żurawek M, Ziółkowska-Suchanek I, Iżykowska K. Fibrosis in Immune-Mediated and Autoimmune Disorders. Journal of Clinical Medicine. 2025; 14(18):6636. https://doi.org/10.3390/jcm14186636
Chicago/Turabian StyleŻurawek, Magdalena, Iwona Ziółkowska-Suchanek, and Katarzyna Iżykowska. 2025. "Fibrosis in Immune-Mediated and Autoimmune Disorders" Journal of Clinical Medicine 14, no. 18: 6636. https://doi.org/10.3390/jcm14186636
APA StyleŻurawek, M., Ziółkowska-Suchanek, I., & Iżykowska, K. (2025). Fibrosis in Immune-Mediated and Autoimmune Disorders. Journal of Clinical Medicine, 14(18), 6636. https://doi.org/10.3390/jcm14186636






