Outcomes of Iso-Elastic Acetabular Cup in Primary Total Hip Arthroplasty with 5-Year Minimum Follow-Up: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Survivorship and Complications
3.2. Clinical Outcomes
3.3. Implant-Related Outcomes
3.4. Quality Assessment
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
- No language restrictions
- From inception to 22 April 2025
References
- Morscher, E.W. Current status of acetabular fixation in primary total hip arthroplasty. Clin. Orthop. 1992, 274, 172–193. [Google Scholar] [CrossRef]
- Gaffey, J.L.; Callaghan, J.J.; Pedersen, D.R.; Goetz, D.D.; Sullivan, P.M.; Johnston, R.C. Cementless acetabular fixation at 15 years: A comparison with the same surgeonís results following acetabular fixation with cement. J. Bone Jt. Surg. 2004, 86-A, 257–261. [Google Scholar] [CrossRef]
- Brodt, S.; Jacob, B.; Nowack, D.; Zippelius, T.; Strube, P.; Matziolis, G. An Isoelastic Monoblock Cup Retains More Acetabular and Femoral Bone Than a Modular Press-Fit Cup: A Prospective Randomized Controlled Trial. J. Bone Jt. Surg. 2021, 103, 992–999. [Google Scholar] [CrossRef] [PubMed]
- Levenston, M.E.; Beaupré, G.; Schurman, D.; Carter, D. Computer simulations of stress-related bone remodeling around noncemented acetabular components. J. Arthroplast. 1993, 8, 595. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.M.; Pellicci, P.M.; Salvati, E.A.; Ghelman, B.; Roberts, M.M.; Koh, J.L. Bone density adjacent to press-fit acetabular components. A prospective analysis with quantitative computed tomography. J. Bone Jt. Surg. 2001, 83, 529–536. [Google Scholar] [CrossRef]
- Ridzwan, M.I.Z.; Shuib, S.; Hassan, A.Y.; Shokri, A.A.; Mohamad Ibrahim, M.N. Problem of Stress Shielding and Improvement to the Hip Implant Designs: A Review. J. Med. Sci. 2007, 7, 460–467. [Google Scholar] [CrossRef]
- Pitto, R.P.; Bhargava, A.; Pandit, S.; Munro, J.T. Retroacetabular stress-shielding in THA. Clin. Orthop. Relat. Res. 2008, 466, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Gasser, B. Biomechanical principles and studies. In Hip Joint Surgery—The RM Cup; Horne, G., Ed.; Einhorn-Presse Verlag GmbH: Hamburg, Germany, 2008; p. 16. [Google Scholar]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann. Intern. Med. 2009, 151, W65–W94. [Google Scholar] [CrossRef] [PubMed]
- Afghanyar, Y.; Afghanyar, B.; Loweg, L.; Drees, P.; Gercek, E.; Dargel, J.; Rehbein, P.; Kutzner, K.P. Ten-year clinical and radiological outcomes with a vitamin E-infused highly cross-linked polyethylene acetabular cup. Bone Jt. Open 2024, 5, 825–831. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Afghanyar, Y.; Möller, J.H.; Wunderlich, F.; Dargel, J.; Rehbein, P.; Gercek, E.; Drees, P.; Kutzner, K.P. An isoelastic monoblock cup versus a modular metal-back cup: A matched-pair analysis of clinical and radiological results using Einzel-Bild-Röntgen-Analyse software. Arch. Orthop. Trauma Surg. 2024, 144, 493–500. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anderl, C.; Steinmair, M.; Hochreiter, J. Bone Preservation in Total Hip Arthroplasty. J. Arthroplast. 2022, 37, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Haefeli, P.C.; Zwahlen, Z.M.; Baumgärtner, R.; Link, B.-C.; Beck, M. RM Pressfit vitamys: The 10-year follow-up. HIP Int. 2025, 35, 142–149. [Google Scholar] [CrossRef]
- Snijders, T.E.; Halma, J.J.; Massier, J.R.A.; van Gaalen, S.M.; de Gast, A. The Survivorship of the Uncemented Iso-Elastic Monoblock Acetabular Component at a Mean of 6-Year Follow-up. HSS J. 2020, 16, 15–22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mahmood, F.F.; Beck, M.; de Gast, A.; Rehbein, P.; French, G.J.; Becker, R.; Dominkus, M.; Helmy, N.; Hollmann, L.; Baines, J. Survivorship and Patient-Reported Outcomes of an Uncemented Vitamin E-Infused Monoblock Acetabular Cup: A Multicenter Prospective Cohort Study. J. Arthroplast. 2021, 36, 1700–1706. [Google Scholar] [CrossRef] [PubMed]
- Erivan, R.; Eymond, G.; Villatte, G.; Mulliez, A.; Myriam, G.; Descamps, S.; Boisgard, S. RM Pressfit® cup: Good preliminary results at 5 to 8 years follow-up for 189 patients. Hip Int. 2016, 26, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Ihle, M.; Mai, S.; Pfluger, D.; Siebert, W. The results of the titanium-coated RM acetabular component at 20 years: A long-term follow-up of an uncemented primary total hip replacement. J. Bone Jt. Surg. 2008, 90, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Wyss, T.; Kägi, P.; Mayrhofer, P.; Nötzli, H.; Pfluger, D.; Knahr, K. Five-year results of the uncemented RM pressfit cup clinical evaluation and migration measurements by EBRA. J. Arthroplast. 2013, 28, 1291–1296. [Google Scholar] [CrossRef] [PubMed]
- Portet, A.; Besnard, M.; Ratsimbazafy, C.; Berhouet, J.; Samargandi, R.; Le Nail, L.R. The RM Press fit cup™: An investigation in 182 hips at ten-year follow-up. Orthop. Traumatol. Surg. Res. 2024, 103988. [Google Scholar] [CrossRef] [PubMed]
- Massier, J.R.A.; Van Erp, J.H.J.; Snijders, T.E.; Gast, A. A vitamin E blended highly cross-linked polyethylene acetabular cup results in less wear: 6-year results of a randomized controlled trial in 199 patients. Acta Orthop. 2020, 91, 705–710. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rochcongar, G.; Remazeilles, M.; Bourroux, E.; Dunet, J.; Chapus, V.; Feron, M.; Praz, C.; Buia, G.; Hulet, C. Reduced wear in vitamin E-infused highly cross-linked polyethylene cups: 5-year results of a randomized controlled trial. Acta Orthop. 2021, 92, 151–155. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Afghanyar, Y.; Joser, S.; Tecle, J.; Drees, P.; Dargel, J.; Rehbein, P.; Kutzner, K.P. The concept of a cementless isoelastic monoblock cup made of highly cross-linked polyethylene infused with vitamin E: Radiological analyses of migration and wear using EBRA and clinical outcomes at mid-term follow-up. BMC Musculoskelet. Disord. 2021, 22, 107. [Google Scholar] [CrossRef]
- Mathys Ltd. RM Classic Cup: Surgical Technique/Product Information. 2016. Available online: https://www.mathysmedical.com/Storages/User/Dokumente/Operationstechnik/Huefte/OP-Technik_RM_Classic_Pfanne_EN.pdf (accessed on 10 July 2025).
- Mathys Ltd. RM Pressfit Cup: Product Information. 2013. Available online: https://www.mathysmedical.com/Storages/User/Dokumente/Produktinfo/Huefte/Produktinfo_RMPressfit_E_V01.pdf (accessed on 26 September 2019).
- Harris, W.H. Traumatic Arthritis of the Hip after Dislocation and Acetabular Fractures: Treatment by Mold Arthroplasty: An End-Result Study Using a New Method of Result Evaluation. J. Bone Jt. Surg. 1969, 51, 737–755. [Google Scholar] [CrossRef]
- D’Aubigne, R.M.; Postel, M. Functional results of hip arthroplasty with acrylic prosthesis. J. Bone Jt. Surg. 1954, 36-A, 451–475. [Google Scholar] [CrossRef]
- D’Augibné, R.M. Numerical evaluation of hip function. Rev. Chir. Orthopédique Réparatrice Appar. Mot. 1970, 56, 481–486. (In French) [Google Scholar]
- Delaunay, C.; Epinette, J.-A.; Dawson, J.; Murray, D.; Jolles, B.-M. Cross-cultural adaptations of the Oxford-12 HIP score to the French speaking population. Orthop. Traumatol. Surg. Res. 2009, 95, 89–99. [Google Scholar] [CrossRef]
- Krismer, M.; Bauer, R.; Tschupik, J.; Mayrhofer, P. EBRA: A method to measure migration of acetabular components. J. Biomech. 1995, 28, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Bottner, F.; Su, E.; Nestor, B.; Azzis, B.; Sculco, T.P.; Bostrom, M. Radiostereometric analysis: The hip. HSS J. 2005, 1, 94–99. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Geerdink, C.H.; Grimm, B.; Vencken, W.; Heyligers, I.C.; Tonino, A.J. The determination of linear and angular penetration of the femoral head into the acetabular component as an assessment of wear in total hip replacement: A comparison of four computer-assisted methods. J. Bone Jt. Surg. 2008, 90, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Dowd, J.E.; Sychterz, C.J.; Young, A.M.; Engh, C.A. Characterization of long-term femoral-head-penetration rates. Association with and prediction of osteolysis. J. Bone Jt. Surg. 2000, 82, 1102–1107. [Google Scholar] [CrossRef] [PubMed]
- Sarry, L.; Tilmant, C.; Boisgard, S.; Boire, J.Y.; Levai, J.P. Monitoring of polyethylene wear in nonmetal-backed acetubular cups by digitized anteroposterior pelvic radiography. IEEE Trans. Med. Imaging 2003, 22, 1172–1182. [Google Scholar] [CrossRef]
- Sarry, L.; Descamps, S.; Boisgard, S.; Levai, J.-P.; Boire, J.-Y. Radiographic stereometry for non-metal-backed acetubular cups: 3D wear estimation and related uncertainty. Med. Image Anal. 2005, 9, 267–279. [Google Scholar] [CrossRef]
- Livermore, J.; Ilstrup, D.; Morrey, B. Effect of femoral head size on wear of the polyethylene acetabular component. J. Bone Jt. Surg. 1990, 72, 518–528. [Google Scholar] [CrossRef]
- Rho, J.Y.; Ashman, R.B.; Turner, C.H. Young’s modulus of trabecular and cortical bone material: Ultrasonic and microtensile measurements. J. Biomech. 1993, 26, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.F.; Bayraktar, H.H.; Keaveny, T.M. Trabecular bone modulus-density relationships depend on anatomic site. J. Biomech. 2003, 36, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Engh, C.A.; Bobyn, J.D.; Glassman, A.H. Porous-coated hip replacement. The factors governing bone ingrowth, stress shielding, and clinical results. J. Bone Jt. Surg. 1987, 69, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Huiskes, R. Stress shielding and bone resorption in THA: Clinical versus computer-simulation studies. Acta Orthop. Belg. 1993, 59 (Suppl. S1), 118–129. [Google Scholar] [PubMed]
- Delfosse, D.; Lerf, R.; Adlhart, C. What happens to the vitamin E in a vitamin-stabilised HXLPE? In Tribology in Total Hip and Knee Arthroplasty: Potential Drawbacks and Benefits of Commonly Used Materials; Knahr, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 197–205. [Google Scholar]
- Halma, J.J.; Vogely, H.C.; Dhert, W.J.; Van Gaalen, S.M.; de Gast, A. Do monoblock cups improve survivorship, decrease wear, or reduce osteolysis in uncemented total hip arthroplasty? Clin. Orthop. Relat. Res. 2013, 471, 3572–3580. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weiss, R.J.; Hailer, N.P.; Stark, A.; Kärrholm, J. Survival of uncemented acetabular monoblock cups: Evaluation of 210 hips in the Swedish Hip Arthroplasty Register. Acta Orthop. 2012, 83, 214–219. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Messer-Hannemann, P.; Campbell, G.M.; Morlock, M.M. Deformation of acetabular press-fit cups: Influence of design and surgical factors. Clin. Biomech. 2019, 69, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Oral, E.; Wannomae, K.K.; Hawkins, N.; Harris, W.H.; Muratoglu, O.K. Alpha-tocopherol-doped irradiated UHMWPE for high fatigue resistance and low wear. Biomaterials 2004, 25, 5515–5522. [Google Scholar] [CrossRef] [PubMed]
- Oral, E.; Christensen, S.D.; Malhi, A.S.; Wannomae, K.K.; Muratoglu, O.K. Wear resistance and mechanical properties of highly crosslinked UHMWPE doped with vitamin-E. J. Arthroplast. 2006, 21, 580–591. [Google Scholar] [CrossRef]
- Cheng, Q.Y.; Zhang, B.F.; Wen, P.F.; Wang, J.; Hao, L.-J.; Wang, T.; Cheng, H.-G.; Wang, Y.-K.; Guo, J.-B.; Zhang, Y.-M. Vitamin E-enhanced liners in primary total hip arthroplasty: A systematic review and meta-analysis. BioMed Res. Int. 2021, 2021, e3236679. [Google Scholar] [CrossRef]
- Miettinen, S.S.; Mäkinen, T.J.; Laaksonen, I.; Mäkelä, K.; Huhtala, H.; Kettunen, J.; Remes, V. Early aseptic loosening of cementless monoblock acetabular components. Int. Orthop. 2017, 41, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Bitar, D.; Parvizi, J. Biological response to prosthetic debris. World J. Orthop. 2015, 6, 172–189. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dumbleton, J.H.; Manley, M.T.; Edidin, A.A. A literature review of the association between wear rate and osteolysis in total hip arthroplasty. J. Arthroplast. 2002, 17, 649–661. [Google Scholar] [CrossRef] [PubMed]
- García-Rey, E.; García-Cimbrelo, E.; Cruz-Pardos, A. Cup press fit in uncemented THA depends on sex, acetabular shape, and surgical technique. Clin. Orthop. Relat. Res. 2012, 470, 3014–3023. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Hulskes, R. Finite element analysis of acetabular reconstruction: Noncemented threaded cups. Acta Orthop. 1987, 58, 620–625. [Google Scholar] [CrossRef]
- Lewinnek, G.E.; Lewis, J.L.; Tarr, R.; Compere, C.L.; Zimmerman, J.R. Dislocations after total hip-replacement arthroplasties. J. Bone Jt. Surg. Am. 1978, 60, 217–220. [Google Scholar] [CrossRef]
- Feng, X.; Gu, J.; Zhou, Y. Primary total hip arthroplasty failure: Aseptic loosening remains the most common cause of revision. Am. J. Transl. Res. 2022, 14, 7080–7089. [Google Scholar] [PubMed] [PubMed Central]
- Ben-Shlomo, Y.; Blom, A.; Boulton, C.; Brittain, R.; Clark, E.; Dawson-Bowling, S.; Deere, K.; Esler, C.; Espinoza, O.; Evans, J.; et al. National Joint Registry Annual Reports. Available online: https://www.ncbi.nlm.nih.gov/books/NBK587525/ (accessed on 10 July 2025).
- Yeroushalmi, D.; Singh, V.; Maher, N.; Gabor, J.A.; Zuckerman, J.D.; Schwarzkopf, R. Excellent mid-term outcomes with a hemispheric titanium porous-coated acetabular component for total hip arthroplasty: 7–10 year follow-up. Hip Int. 2023, 33, 404–410. [Google Scholar] [CrossRef] [PubMed]
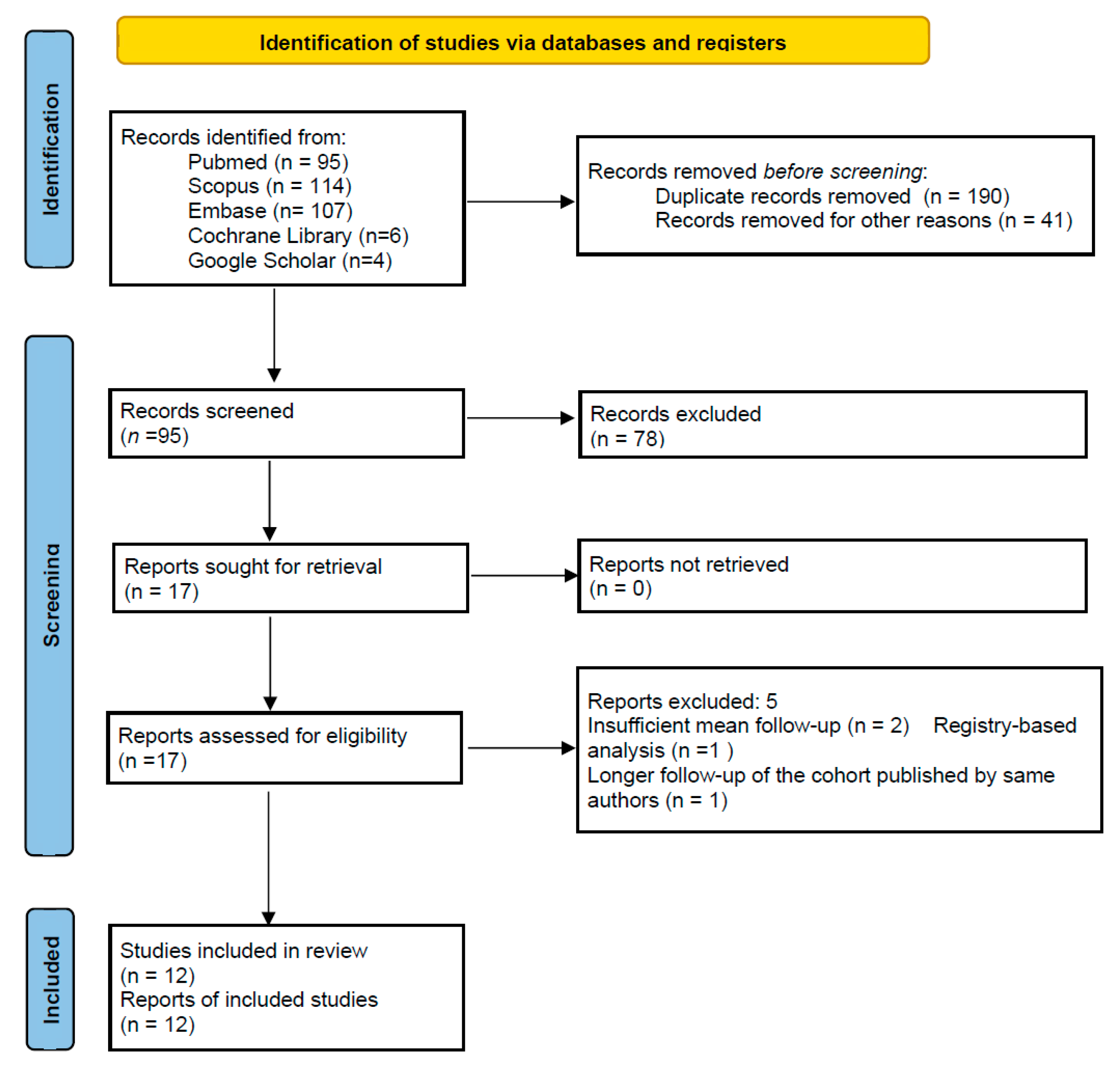
| Study | Selection: Representativeness of Exposed Cohort (★) | Selection: Selection of Non-Exposed Cohort (★) | Selection: Ascertainment of Exposure (★) | Selection: Outcome Not Present at Start (★) | Comparability: Control for Confounding (★★) | Outcome: Assessment of Outcome (★) | Outcome: Follow-Up Long Enough (★) | Outcome: Adequacy of Follow-Up (★) | Total Stars | Notes |
|---|---|---|---|---|---|---|---|---|---|---|
| Afghanyar et al., 2024 [10] | ★ | × | ★ | ★ | × | ★ | ★ | ★ | 6 | Prospective design; no control group; validated and standardized outcome measures; 10-year follow-up; high % of loss to follow-up, 29.7%, but good description provided of those lost |
| Afghanyar et al., 2023 [11] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 | Retrospective matched-pair study; time lapse during which patients underwent surgery was not reported; groups matched by sex, age, body mass index (BMI), and ASA classification; EBRA method on radiographs; mean follow-up: 5 years |
| Anderl et al., 2022 [12] | ★ | × | ★ | ★ | × | ★ | ★ | ★ | 6 | Prospective design; no control group; small cohort: 47 patients; 5-year follow-up with clinical and radiographic outcomes; lack of adjustment for potential confounder; dual-energy X-ray absorptiometry used to assess instrumental outcomes |
| Haefeli et al., 2025 [13] | ★ | × | ★ | ★ | × | ★ | ★ | ★ | 6 | Prospective design; no control group; use of validated tools to assess outcomes; 10-year follow-up; 38% loss of follow-up (though well documented) and cross-checked with national registry (SIRIS) improves ascertainment of revisions |
| Snijders et al., 2020 [14] | ★ | × | ★ | ★ | × | ★ | ★ | ★ | 6 | Prospective design; clear inclusion criteria; no control group; surgical approach not reported; standardized clinical and instrumental outcomes assessment; 6-year mean follow-up; loss of follow-up was 15.2%, but good description provided of those lost |
| Mahmood et al., 2014 [15] | ★ | × | ★ | ★ | × | ★ | ★ | ★ | 6 | Prospective multicenter study; standardized data collection; validated clinical outcomes and radiographic monitoring; no control arm; no adjustment for potential confounders; long mean follow-up (8.9 years) with detailed survival analysis; 66.7% of enrolled patients completed clinical/radiographic follow-up |
| Erivan et al., 2020 [16] | ★ | × | ★ | ★ | × | ★ | ★ | ★ | 6 | Prospective design; no control group; validated clinical score to assess outcome; radiographic evaluation was not blinded; mean follow-up: 6.5 years with low rate of loss of follow-up (2.1%); use of survival analysis with clear endopoints; no statistical adjustment for confounders |
| Ihle et al., 2008 [17] | ★ | × | ★ | ★ | × | ★ | ★ | ★ | 6 | Prospective series with clinical and radiographic evaluation; no comparator group; subgroup analyses by head size and age groups were performed; long mean follow-up: 19.3 years; high percentage of loss of follow-up (52.6%), but good description provided of those lost |
| Wyss et al., 2013 [18] | ★ | × | ★ | ★ | × | ★ | ★ | ★ | 6 | Prospective multicenter study; small cohort; 5-year radiographic and clinical analysis; EBRA method used on radiographs; no comparator and 28% loss of follow-up (even if good descriptions were provided) |
| Portet et al., 2024 [19] | ★ | × | ★ | ★ | × | ★ | ★ | ★ | 6 | Retrospective study; validated tools to assess clinical and radiographic outcomes; 10.5-year data; limited by absence of control group and absence of adjustment for potential confounders; radiological measurements were performed by a single observer. |
| Study | Randomization Process | Deviations from Intended Interventions | Missing Outcome Data | Measurement of the Outcome | Selection of the Reported Result | Overall Risk of Bias | Notes |
|---|---|---|---|---|---|---|---|
| Massier et al., 2020 [20] | Low risk | Some concerns | Some risk | Low risk | Low risk | Low risk | Well-randomized; allocations performed using an internet randomization system; standardized procedures; blinded assessors; surgeons were not blinded; high rate of loss of follow-up: 11%; the HXLPE group received significantly larger head sizes due to thinner polyethylene liners |
| Rochcongar et al., 2021 [21] | Low risk | Some concerns | Low risk | Low risk | Low risk | Some risk | Validated RSA method to assess wear rate; small sample size with further loss of follow-up (10 patients): only 40 patients followed up at 5 years; high-quality design despite unblinded surgeons; surgical approach was not reported |
| Author | Year | Patients | Total Hips | Cup | FU Hips | Lost to FU (%) | Females | Males | Age (Years) | BMI |
|---|---|---|---|---|---|---|---|---|---|---|
| Afghanyar et al. [10] | 2024 | 96 | 101 | RM Pressfit vitamys cup (HXLPE) | 71 (57 both clinical and radiological FU + 14 only clinical FU) | 29.7% (7 lost, 23 deaths not related to primary THA) | 63 | 38 | 69.4 (50.7–84.3) | 27.5 (19.3–41.5) |
| Afghanyar et al. [11] | 2023 | 98 | 98 | RM Pressfit vitamys cup (HXLPE) | 98 | 0 | 52 | 46 | 67.1 (50.7–78.7) | 26.8 (4.03) |
| Anderl et al. [12] | 2022 | 47 | 47 | RM Pressfit vitamys cup (HXLPE) | 41 | 12.7% (3 lost, 3 deaths not related to primary THA) | 23 | 22 | 66.8 (46.0–80.0) | / |
| Hefaeli et al. [13] | 2025 | 150 | 162 | RM Pressfit vitamys cup (HXLPE) | 100 (99 both clinical and radiological evaluation, 1 only radiological) | 38.3% (20 deaths not related to primary THA, 42 lost) | 74 | 76 | 67.2 (38–88) | 27.3 (16.7–46.9) |
| Snijders et al. [14] | 2019 | 112 | 117 | RM Pressfit Vitamys cup (HXLPE) | 100 | 15.2% (4 deaths not related to primary THA, 13 lost) | 74 | 38 | 63.8 (40.0–86.0) | 26.15 (19.0–35.0) |
| Massier et al. [20] | 2020 | 199 (102 vit E blended HXLPE cup, 99 UHMWPE cup) | 199 | RM PressFit Vitamys cup (HXLPE) and RM Pressfit cup (UHMWPE) | 177 | 11% | 141 | 36 | 65 | / |
| Mahmood F. F. et al. [15] | 2021 | 675 | 675 | RM Pressfit vitamys cup (HXLPE) | 450 | 33.3% (53 deaths not related to primary THA, 172 lost) | 395 | 280 | 68.3 (34.7–93.1) | 27.5 (14.6–46.9) |
| Erivan et al. [16] | 2016 | 189 | 189 | RM Pressfit cup (UHMWPE) | 185 of which only 101 with X-rays | 2.1% (4 lost) | 108 | 81 | 75.4 (29.0–90) | 26.1 (15–39) |
| Ihle et al. [17] | 2008 | 93 | 93 | RM Pressfit cup (UHMWPE) | 44 | 52.6% (25 deaths not related to primary THA, 14 revised, 5 lost) | 41 | 39 | 52.2 (28.0–81) | 26.3 (18.7–36.3) |
| Wyss et al. [18] | 2013 | 50 | 50 | RM Pressfit cup (UHMWPE) | 36 | 28% (8 deaths not related to primary THA, 6 lost for other reasons) | 22 | 28 | 72.3 (54.1–90.1) | 27.9 (16.3–39.2) |
| Rochcongar et al. [21] | 2021 | 62 | 33 HXLPE; 29 UHMWPE | RM Pressfit vitamys cup (HXLPE) or RM Pressfit cup (UHMWPE) | 22 HXLPE; 18 UHMWPE | 33% HXLPE group; 37,9% UHMWPE | 16 HXLPE group; 17 UHMWPE group | 16 HXLPE group; 12 UHMWPE group | 61 | 27 |
| Portet et al. [19] | 2024 | 163 | 207 | RM Pressfit cup (UHMWPE) | 182 hips included for survival analysis, 157 hips included for clinical and radiological evaluation | 12% | 60 | 103 | 63 | 27 (24.5–30) |
| Authors | Followed-Up Hips | Infections | Hematoma | Nerve Injury/Palsy | Periprosthetic Fractures | Heterotopic Ossifications | Aseptic loosening | Osteolysis | Dislocation |
|---|---|---|---|---|---|---|---|---|---|
| Afghanyar et al. (2024) [10] | 71 | 1 superficial | 4 | 1 | 2 | 6 | 0 | 0 | / |
| Afghanyar et al. (2023) [11] | 49 | / | / | / | / | 1 (VEPE) | 0 | 0 | / |
| Anderl et al. [12] | 41 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Hefaeli et al. [13] | 100 | 0 | 0 | 0 | 0 | 35 | 0 | 0 | / |
| Snijders et al. [14] | 100 | 3 superficial, 2 deep | 0 | 0 | 1 femoral | 0 | 0 | 0 | / |
| Massier et al. [20] | 199 | 2 superficial, 1 deep | 0 | 2 | 2 femoral, 1 acetabular | / | 0 | 0 | 6 |
| Mahmood et al. [15] | 450 | 3 deep | 0 | 10 | 12 | / | 0 | 0 | 8 |
| Erivan et al. [16] | 189 | 4 | 1 | 1 | 0 | 1 | 0 | 0 | 2 |
| Ihle et al. [17] | 93 | 1 superficial, 1 deep | 0 | 0 | 18 femoral | 25 | 5 | 8 | 2 |
| Wyss et al. [18] | 36 | / | / | / | 1 | 10 | 0 | 1 | / |
| Rochcongar et al. [21] | 40 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Portet et al. [19] | 182 | 1 deep | 0 | 0 | 0 | 0 | 1 post-traumatic | 0 | 5 |
| Authors | Cup | Surgical Approach | Score | Mean Follow-Up | Clinical Results | Cup Positioning | Implant-Related Outcomes | FHP Rate | Mean Cup Migration | Mean Wear | Survivorship |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Afghanyar et al. (2024) [10] | RM Pressfit vitamys cup (HXLPE) | Antero-lateral approach | HHS, VAS | 129.3 months (120.0 to 148.9) | Mean HHS improved from 49.9 to 96.4 (p = 0.052). Mean rest pain VAS decreased from 5.0 to 0.0. Mean load pain VAS decreased from 7.7 to 0.2. Mean satisfaction increased from 1.7 to 9.9. | / | Mean cup migration at 5 years was 1.34 mm; it increased to 1.67 mm at 10 years. During the critical first year, we observed a mean cup migration of 0.80 mm. | / | The annual cup migration rate decreased from 0.30 mm at 5 years to 0.16 mm at 10 years. | Mean total wear at last follow-up was 0.35 mm; the measured values decreased slightly from the five-year results at 0.40 mm. Mean annual wear rate was 0.03 mm per year. | 100% (Lost to FU patients not excluded) |
| Afghanyar et al. (2023) [11] | RM Pressfit vitamys cup (HXLPE) | Antero-lateral approach | HHS | 73.2 ± 19.2 months | Mean HHS improved significantly. | Within the Lewinnek safe zone: cup inclination of 40 ± 10° and anteversion of 15 ± 10° | Mean cup migration at the mid-term follow-up was 67 ± 0.92 mm while the annual migration rate was 0.27 ± 0.16 mm/year at 5 years. | / | Mean cup migration at the mid-term follow-up was 1.67 ± 0.92 mm while the annual migration rate was 0.27 ± 0.16 mm/year at 5 years. | Mean total wear at the mid-term follow-up was 0.37 ± 0.28 mm and the mean annual wear rate was 0.06 ± 0.04 mm/year. | 100% (Lost to FU patients not excluded) |
| Anderl et al. [12] | RM Pressfit vitamys cup (HXLPE) | Antero-lateral approach | HHS, VAS | 63.7 months (12.2–68.1) | Mean HHS improved significantly from 56.5 to 95.7 (p < 0.0001). Mean rest pain VAS decreased from 4.5 to 0.7. Mean load pain VAS decreased from 7.2 to 1.3 and satisfaction increased from 1.9 to 9.6. | / | On the acetabular side, DEXA evaluation revealed BMD stabilized in all DeLee and Charnley zones after an initial postoperative decrease. There were non-significant differences in the BMD in DeLee and Charnley zones II and III between 24 and 60 months after surgery (p > 0.05). In zone I, the BMD significantly differed between 24 and 60 months after surgery, with an estimated decrease of 4%. | / | / | / | 100% (Lost to FU patients excluded) |
| Hefaeli et al. [13] | RM Pressfit vitamys cup (HXLPE) | 85.8% anterior, 9.9% anterolateral, 3.7% transgluteal, 0.6% posterior | mHHS, VAS | 120.5 months (118.0–126.0) | Mean rest pain VAS decreased from 3.3 to 0.1. Mean load pain VAS decreased from 6.6 to 0.5 and satisfaction increased from 3.8 to 9.5. Mean mHHS was 94.8, with a mean improvement compared to preoperative mHHS of 33.7. | / | At the ten-year radiographic follow-up, no signs of loosening, acetabular radiolucent lines or osteolysis were observed. | / | / | / | Survival rate was 98%. Survival rate for aseptic loosening was 100%. (Lost to FU patients not excluded) |
| Snijders et al. [14] | RM Pressfit Vitamys cup (HXLPE) | / | HHS, VAS | 71.4 months (57.8–82.4) | Mean rest pain VAS decreased from 4.53 a to 0.45. Mean load pain VAS decreased from 7.41 to 1.26. Mean satisfaction increased from 3.09 to 8.75. Mean HHS increased from 61.1 to 91.8. | / | Mean total FHP on radiographs was 0.249 mm. Mean FHP rate was 0.036 mm/year. | Mean total FHP was 0.249 mm. Mean FHP rate was 0.036 mm/year. | / | / | Survival rate was 97.4%. Survival rate for aseptic loosening was 100%. (Lost to FU patients not excluded) |
| Massier et al. [20] | 102 RM PressFit Vitamys cup (HXLPE) and 97 RM Pressfit cup (UHMWPE) | 49% lateral, 43% posterolateral, 8% anterolateral | VAS, HHS | 70.0 months | Mean preoperative NRS scores for rest pain, load pain, patient satisfaction were around 4, 6, and 4 (only graphically reported). Mean postoperative NRS scores for rest pain, load pain, and patient satisfaction were 0.3, 0.6, and 8.6, respectively. Mean HHS increased from 60 to 93. | Inclination: <35°: 8%; 35–40°: 24%; 41–45°: 27%; 46–50°: 26%; >50°: 16% | Mean FHP rate on radiographs of the HXLPE/VitE cup was lower compared with the FHP rate of the UHMWPE cup (p = 0.002). Multivariate analysis showed no statistically significant effect of head size on the wear rates in either cup. | Total mean FHP was 0.38 mm in the HXLPE/VitE cup and 0.44 mm in the UHMWPE cup (p = 0.01). Mean FHP rate was 0.028 and 0.035 mm/year for the HXLPE/VitE cup and UHMWPE cup, respectively. | / | / | Survival to revision was 98% for both groups. Survival rate for aseptic loosening was 100% in both groups. (Lost to FU patients not excluded) |
| Mahmood F. F. et al. [15] | RM Pressfit vitamys cup (HXLPE) | / | HHS, VAS | 105.0 months | Mean HHS increased from 54.1 to 93.8. Mean rest pain VAS decreased from 4.5 to 0.3. Mean load pain VAS decreased from 7.2 to 0.7. Mean patient satisfaction improved from 2.7 to 8.9 at 6–12 weeks and was maintained through the 5-year follow-up. | 89% of acetabular cups within the Lewinnek safe zone | A single case with a radiolucent line in zone 2 was identified. There was no evidence of acetabular osteolysis on radiographs in DeLee and Charnley zones 1–3 throughout the follow-up period. | / | / | / | Survival rate was 98.9%. Survival rate for aseptic loosening was 100%. (Lost to FU patients not excluded) |
| Erivan et al. [16] | RM Pressfit cup (UHMWPE) | 96.3% anterolateral, 3.7% anterior | PMA, HHS, Charnley class, Devane’s level of activity, HOOS, WOMAC, SF12 | 6.5 years (5.0–8.0) | Mean PMA score increased 11.4 to 15.8. Mean HHS improved from 56.8 to 84.7 (p < 0.001). Devane classes were preoperatively distributed with 5 patients (4.9%) in class 1, 33 (32%) in class 2, 51 (49.5%) in class 3, 13 (12.6%) in class 4, and 1 (1%) in class 5. Postoperative scores were distributed with 13 (12.6%) in class 1, 24 (23.3%) in class 2, 49 (47.7%) in class 3, 15 (14.6%) in class 4, and 2 (1.9%) in class 5. Postoperative HOOS was measured in 109 patients with the mean at 75.9. WOMAC index was measured at late follow-up with a mean of 23.7. SF-12 quality of life score was found to be 38.3 for the Physical Composite Score (11.6–63.1) and 47.4 for the Mental Composite Score (12.9–72.9). | Mean cup anteversion was 16.3° (0–35.5°). Mean inclination was 42.3° (24–62°). | Mean annual wear rate was calculated on radiographs by MHP software (version not specified) and was found to be 0.065 mm per year. | / | / | Mean annual wear rate was calculated on radiographs by MHP software and was found to be 0.065 mm per year. | Survival rate was 96.8%. Survival rate for aseptic loosening was 100%. (Lost to FU patients not excluded) |
| Ihle et al. [17] | RM Pressfit cup (UHMWPE) | Anterolateral or posterior | HHS, PMA | 19.3 years (17.4–20.9) | Mean HHS was 87.5 at the latest FU. Mean PMA pain score improved from 2.2 to 5.8, mobility from 4.5 to 5.8, ability to walk from 3 to 4.6. | / | A radiolucent line adjacent to the acetabular component was seen in DeLee and Charnley zone 1 on two radiographs, and in zone 3 on one radiograph. None had continuous radiolucencies in all three zones. Osteolysis was seen as a sharply demarcated radiolucent space on eight radiographs: six in zone 3, one in zone 2, and one in zone 1. None of these patients showed any clinical signs of loosening and none are awaiting revision. | / | / | / | Survival rate was 82.7%. Survival rate for aseptic loosening was 94.4%. (Lost to FU patients not excluded) |
| Wyss et al. [18] | RM Pressfit cup (UHMWPE) | Transgluteal | HHS | 60 months | Preoperatively, 90% of the patients had a poor HHS and 10% a moderate HHS. Five years after surgery, 30 patients were examined: 77% reached an excellent, 7% a good, 3% a moderate, and 13% a poor HHS. | / | Mean cup migration rate and annual wear rate analysis revealed a decrease in speed of both phenomenon over time. | / | Mean cup migration was 1.25 mm | The mean annual wear rate was 0.09 mm/year. | 100% (Lost to FU patients not excluded) |
| Rochcongar et al. [21] | RM Pressfit vitamys cup (HXLPE) or RM Pressfit cup (UHMWPE) | 51.6% anterolateral, 43.5% posterior, 4.8% transtrochanteric | HHS, PMA | 60 months | Mean HHS and the MAP score improved in both groups (p < 0.001). None of the mean clinical scores differed significantly between the HXLPE/VitE group and the UHMWPE group. | Mean cup inclination angle was similar in both groups (HXLPE/VitE 48° UHMWPE 46°) and remained stable over the entire follow-up period. | Mean femoral head penetration increased 0.08 mm in HXLPE/VitE cups, while it increased 0.2 mm in UHMWPE cups per year. The estimated steady-state rate of wear was approximately 66% lower in the HXLPE/VitE group than in the UHMWPE group (p < 0.001). | Cumulative FHP was 0.24 in the HXLPE/VitE group and 0.45 mm in the UHMWPE group | / | The wear rate averaged 0.02 mm/year in the HXLPE/VitE group compared with 0.06 mm/year in the UHMWPE group. In the HXLPE/VitE group, the wear rate was 66% lower than in the UHMWPE group. Both groups showed no significant correlation of PE wear with cup inclination angles. | 100% (Lost to FU patients excluded) |
| Portet et al. [19] | RM Pressfit cup (UHMWPE) | 69% posterolateral, 30% lateral, 1% transtrochanteric | HHS, Oxford score | 10.5 years | Median postoperative HHS and Oxford scores were 95 (90–98) and 19 (17–23). | Mean inclination was 48° (45−52) | Radiographic analysis showed that 16% of cups exhibited wear greater than 1 mm. In the immediate postoperative period, 27% of cups were not press-fit at the level of the acetabular back rim, 4% of cups were not press-fit at the level of the acetabular roof, and 24% of cups were not press-fit at either location. At 10 years of follow-up, 6% of cups retained a gap at the back rim, and 3% at both locations. At 10 years of follow-up, 3.2% showed periprosthetic osteolysis, and 4.5% had geodes. | / | / | Polyethylene wear on radiographs was 0.058 mm/year. Twenty-five (16%) cups exhibited wear greater than 1 mm at 10 years. | Survival rate was 96.1%. Survival rate for aseptic loosening was 99.5%. (Lost to FU patients excluded) |
| Outcome | No. of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Overall Certainty | Effect/Finding |
|---|---|---|---|---|---|---|---|---|---|
| Implant Survivorship (≥5 years) | 12 | Observational + 2 RCTs | Moderate | Low | No | Low | Not detected | ⬤⬤⬤◯Moderate | Pooled survivorship 97.5% (99.5% excluding septic causes) |
| Revision for Aseptic Loosening | 11 | Observational + RCTs | Low | Low | No | Low | Not detected | ⬤⬤⬤⬤High | <1% revision rate for aseptic loosening |
| Annual Wear Rate (mm/year) | 6 | Observational + RCTs | Moderate | Moderate | No | Moderate | Not detected | ⬤⬤⬤◯Moderate | Mean: 0.05 mm/year (range 0.02–0.09); VEHXLPE < UHMWPE |
| Functional Outcome (HHS) | 11 | Observational + RCTs | Moderate | Moderate | No | Moderate | Not detected | ⬤⬤⬤◯Moderate | Mean postop HHS: 92.6; significant improvement |
| Complication Rates | 12 | Observational + RCTs | Low | Low | No | Low | Not detected | ⬤⬤⬤⬤High | Low rates: dislocation, infection, fracture |
| Periacetabular BMD Preservation | 1 | RCTs | Some concerns | High | No | High | Not detected | ⬤⬤◯◯Low | 1 RCT showed reduced BMD loss in polar region (p = 0.005) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longobardi, V.; Minelli, M.; Pietrogrande, G.; Anzillotti, G.; Della Rocca, F.; Loppini, M. Outcomes of Iso-Elastic Acetabular Cup in Primary Total Hip Arthroplasty with 5-Year Minimum Follow-Up: A Systematic Review. J. Clin. Med. 2025, 14, 6621. https://doi.org/10.3390/jcm14186621
Longobardi V, Minelli M, Pietrogrande G, Anzillotti G, Della Rocca F, Loppini M. Outcomes of Iso-Elastic Acetabular Cup in Primary Total Hip Arthroplasty with 5-Year Minimum Follow-Up: A Systematic Review. Journal of Clinical Medicine. 2025; 14(18):6621. https://doi.org/10.3390/jcm14186621
Chicago/Turabian StyleLongobardi, Vincenzo, Marco Minelli, Giacomo Pietrogrande, Giuseppe Anzillotti, Federico Della Rocca, and Mattia Loppini. 2025. "Outcomes of Iso-Elastic Acetabular Cup in Primary Total Hip Arthroplasty with 5-Year Minimum Follow-Up: A Systematic Review" Journal of Clinical Medicine 14, no. 18: 6621. https://doi.org/10.3390/jcm14186621
APA StyleLongobardi, V., Minelli, M., Pietrogrande, G., Anzillotti, G., Della Rocca, F., & Loppini, M. (2025). Outcomes of Iso-Elastic Acetabular Cup in Primary Total Hip Arthroplasty with 5-Year Minimum Follow-Up: A Systematic Review. Journal of Clinical Medicine, 14(18), 6621. https://doi.org/10.3390/jcm14186621






