Redo-Transcatheter Aortic Valve Replacement: Current Evidence and Procedural Considerations
Abstract
1. Introduction
2. Pre-Procedural Aspects of Redo-TAVR
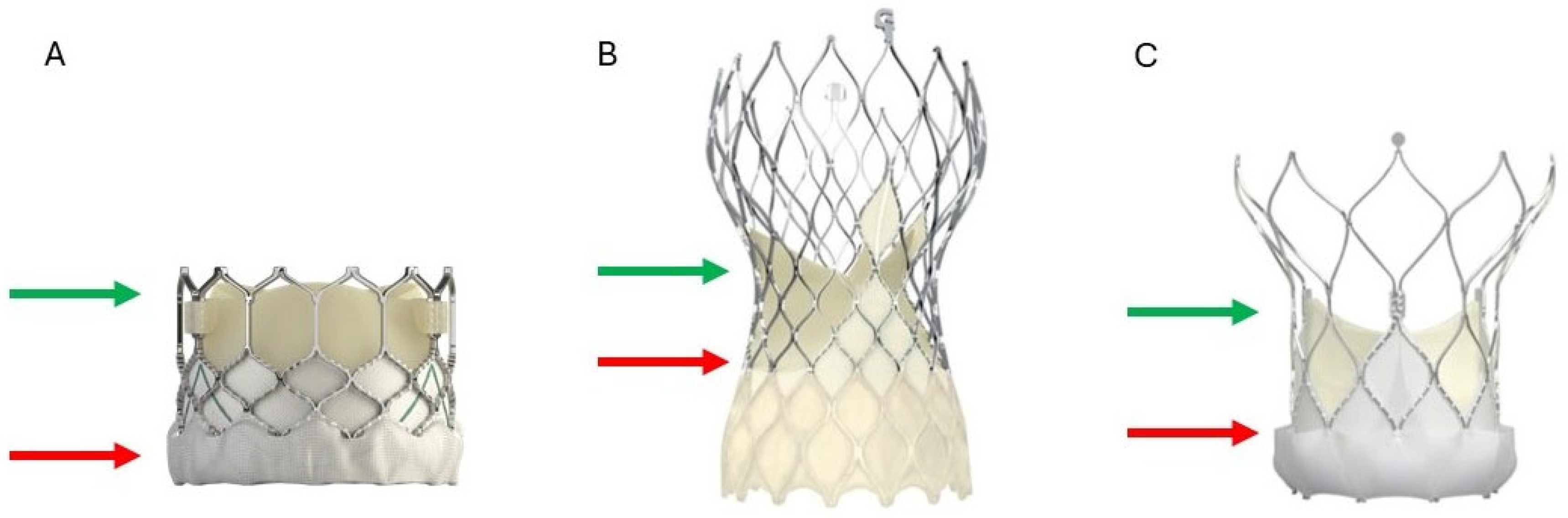
2.1. THV Durability
2.2. Bioprosthetic Valve Dysfunction (BVD)
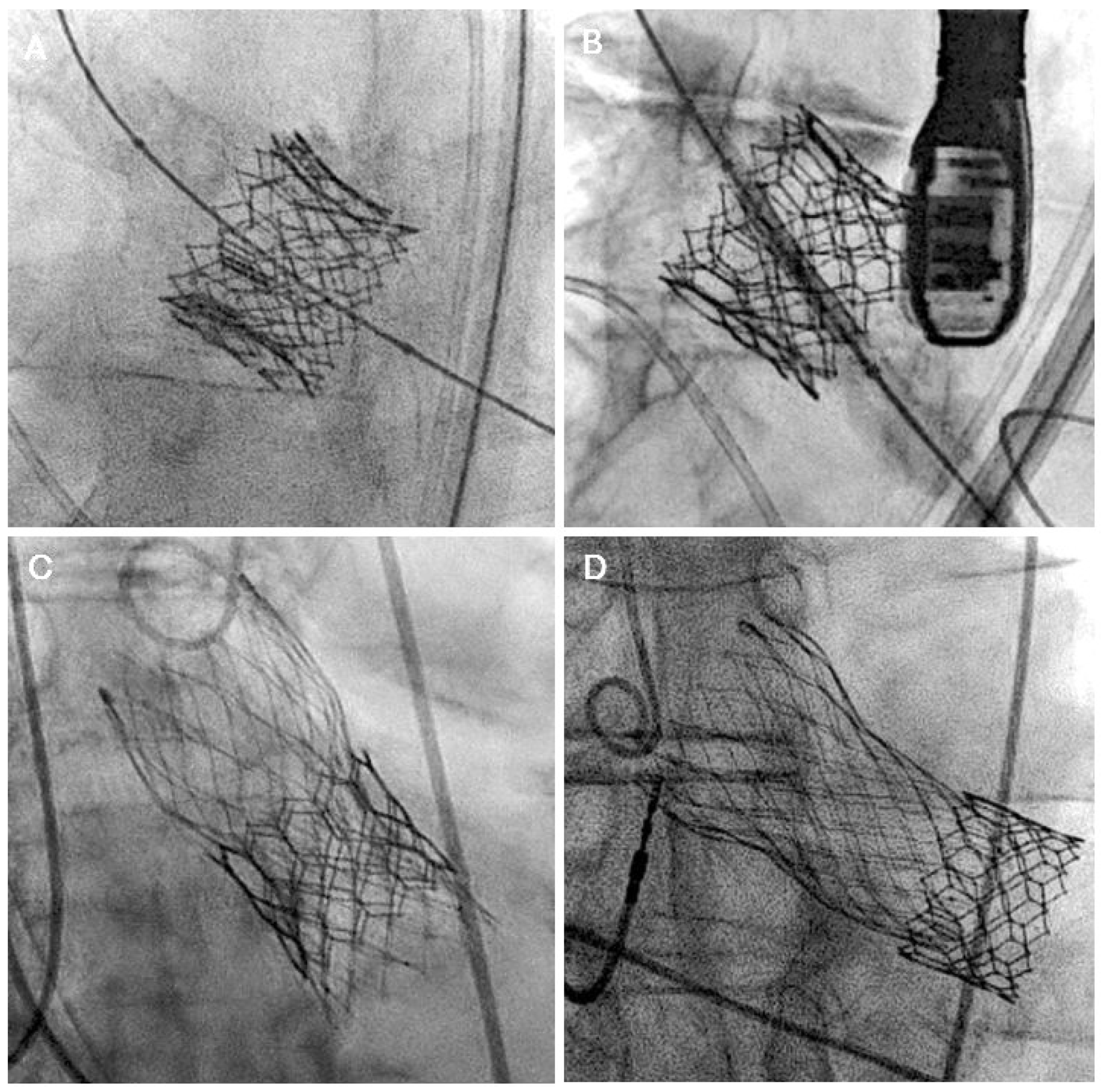
3. TAVR Explantation
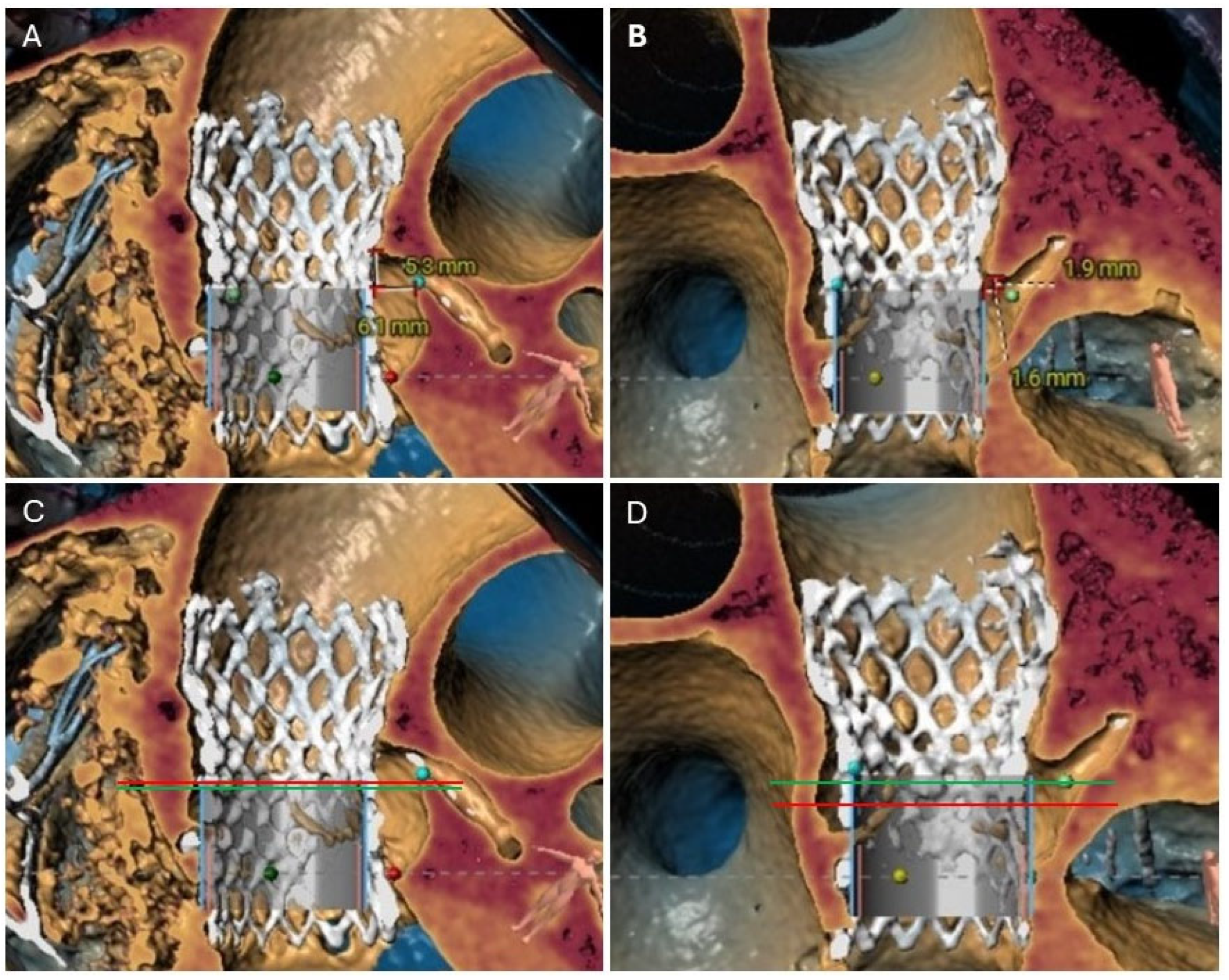
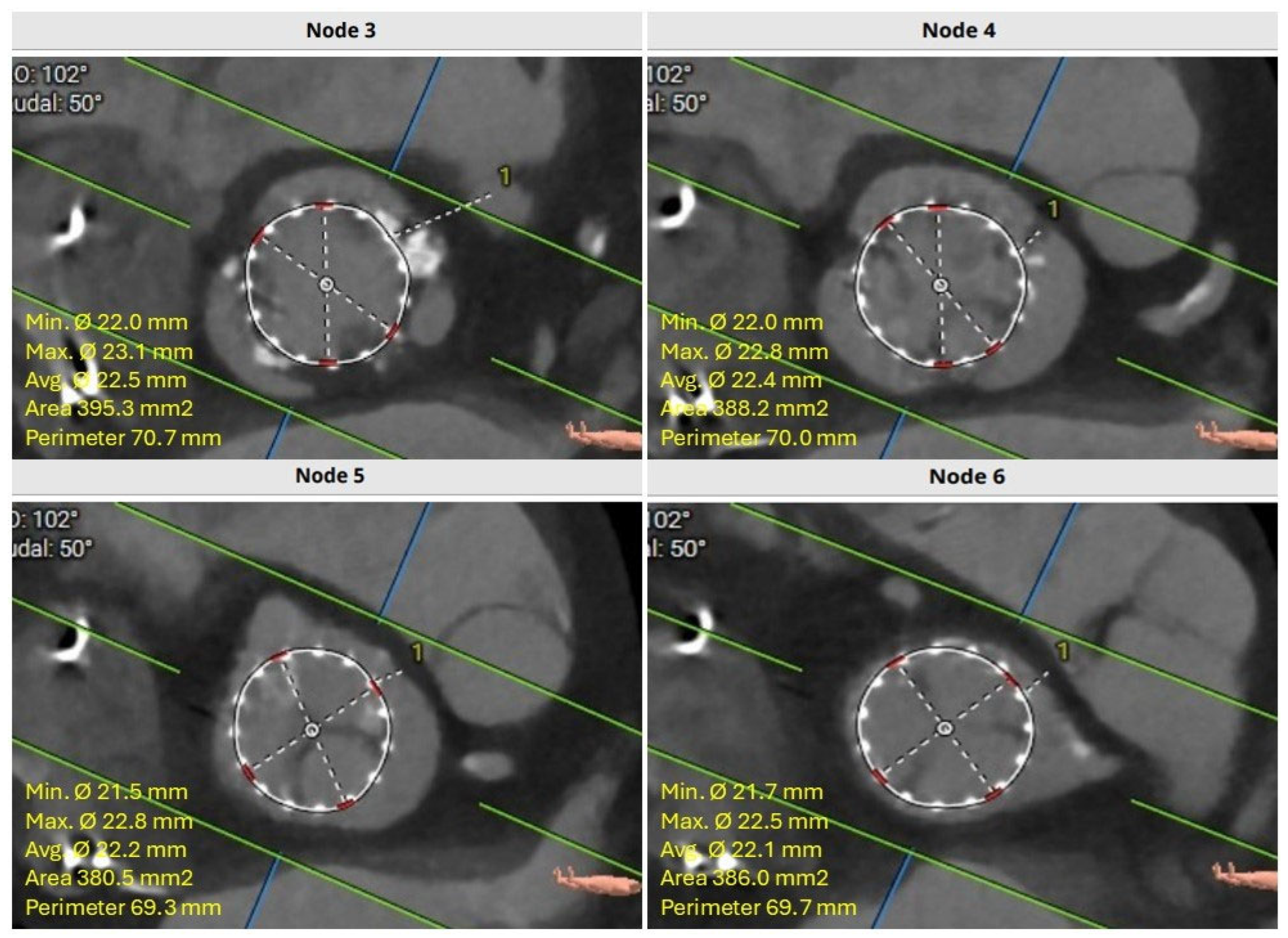
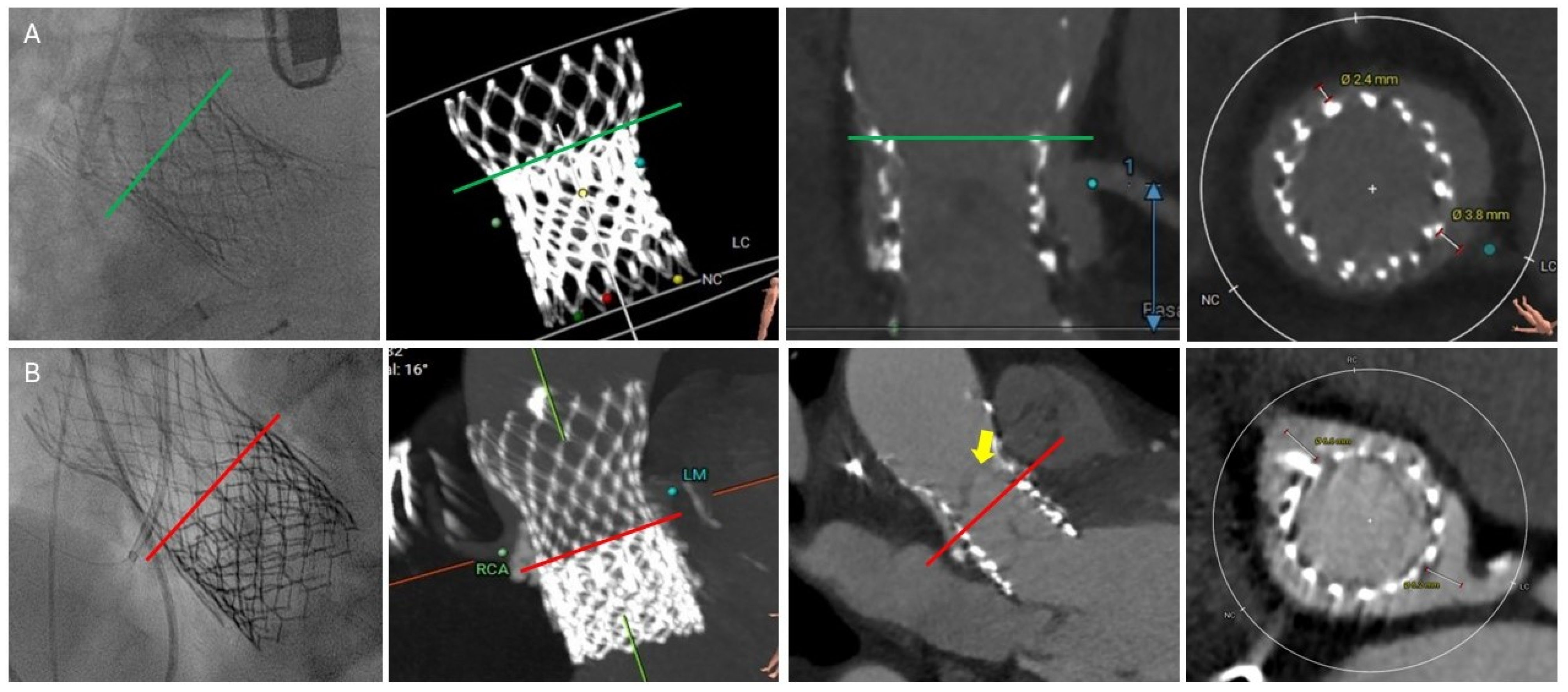
4. Role of Cardiac Computed Tomography Angiography (CTA) Imaging in Redo-TAVR Planning
4.1. Common CTA Terminology
4.2. Neoskirt
4.3. Neoskirt Plane (NSP)
4.4. Coronary Risk Plane (CRP)
4.5. Leaflet Overhang
5. Procedural Considerations for Redo-TAVR
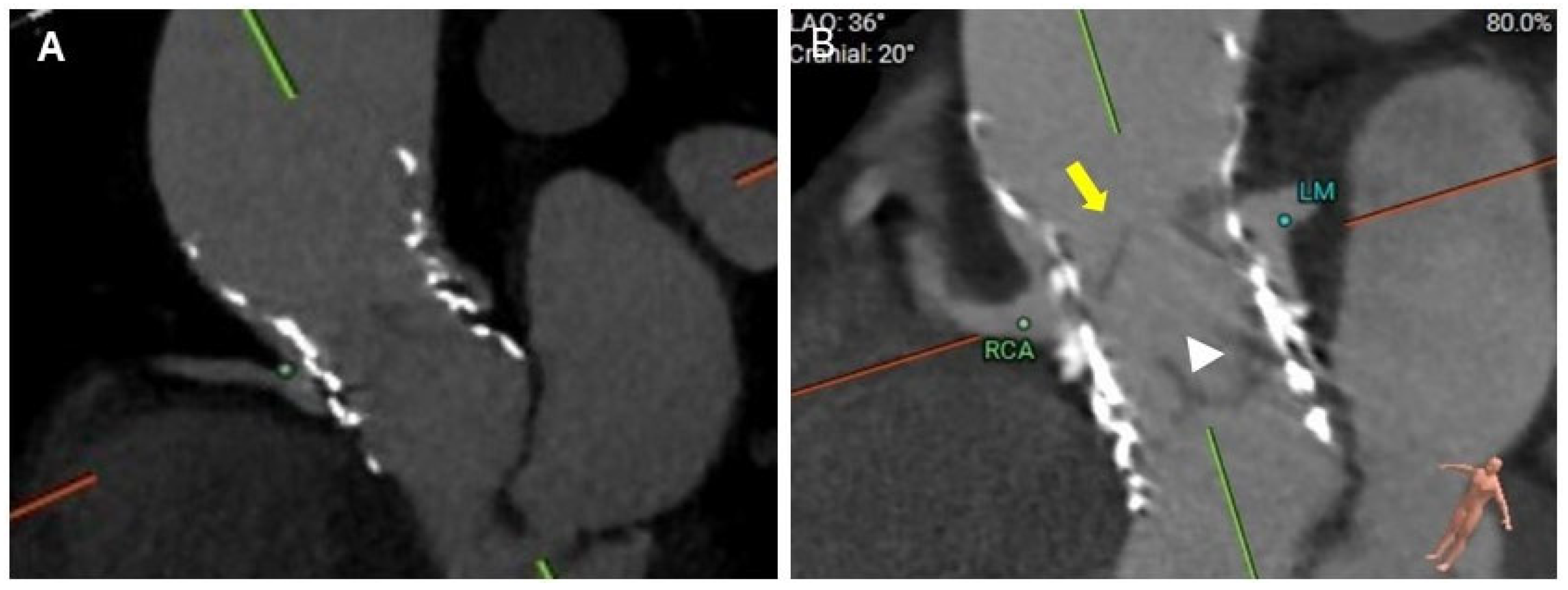
5.1. Coronary Occlusion Risk During Redo-TAVR and the Need for Protection
5.2. Patient Prosthesis Mismatch (PPM)
5.3. Stroke Risk During Redo-TAVR
5.4. Pacemaker After Redo-TAVR
6. Post-Procedural Considerations
Antithrombotic Therapy
7. Clinical Evidence for Redo-TAVR
Future Perspectives
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AS | Aortic stenosis |
| AR | Aortic regurgitation |
| BEV | balloon expandable valve |
| BVD | Bioprosthetic valve dysfunction |
| CRP | Coronary risk plane |
| CTA | Computed tomography angiography |
| HALT | Hypoattenuated leaflet thickening |
| NSP | Neoskirt plane |
| PPM | Patient prosthesis mismatch |
| PVL | Paravalvular leak |
| SAVR | Surgical aortic valve replacement |
| SEV | Self-expanding valve |
| SVD | Structural valve deterioration |
| TAVR | Transcatheter aortic valve replacement |
| THV | Transcatheter heart valve |
| VTA | Valve to aorta |
| VTC | Valve to coronary |
| VTJ | Valve to sinotubular junction |
References
- Cribier, A.; Eltchaninoff, H.; Bash, A.; Borenstein, N.; Tron, C.; Bauer, F.; Derumeaux, G.; Anselme, F.; Laborde, F.; Leon, M.B.; et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: First human case description. Circulation 2002, 106, 3006–3008. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.B.; Smith, C.R.; Mack, M.; Miller, C.; Moses, J.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 2010, 363, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.H.; Popma, J.J.; Reardon, M.J.; Yakubov, S.J.; Coselli, J.S.; Deeb, G.M.; Gleason, T.G.; Buchbinder, M.; Hermiller, J.; Kleiman, N.S.; et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N. Engl. J. Med. 2014, 370, 1790–1798. [Google Scholar] [CrossRef]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef]
- Reardon, M.J.; Van Mieghem, N.M.; Popma, J.J.; Kleiman, N.S.; Sondergaard, L.; Mumtaz, M.; Adams, D.H.; Deeb, G.M.; Maini, B.; Gada, H.; et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2017, 376, 1321–1331. [Google Scholar] [CrossRef]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef]
- Patel, S.P.; Garcia, S.; Sathananthan, J.; Tang, G.H.; Albaghdadi, M.S.; Pibarot, P.; Cubeddu, R.J. Structural Valve Deterioration in Transcatheter Aortic Bioprostheses: Diagnosis, Pathogenesis, and Treatment. Struct. Heart 2023, 7, 100155. [Google Scholar] [CrossRef]
- Alwan, L.; Bernhard, B.; Brugger, N.; de Marchi, S.F.; Praz, F.; Windecker, S.; Pilgrim, T.; Grani, C. Imaging of Bioprosthetic Valve Dysfunction after Transcatheter Aortic Valve Implantation. Diagnostics 2023, 13, 1908. [Google Scholar] [CrossRef]
- Velho, T.R.; Pereira, R.M.; Fernandes, F.; Guerra, N.C.; Ferreira, R.; Nobre, A. Bioprosthetic Aortic Valve Degeneration: A Review from a Basic Science Perspective. Braz. J. Cardiovasc. Surg. 2022, 37, 239–250. [Google Scholar] [CrossRef]
- Fuchs, A.; De Backer, O.; Brooks, M.; de Knegt, M.C.; Bieliauskas, G.; Yamamoto, M.; Yanagisawa, R.; Hayashida, K.; Sondergaard, L.; Kofoed, K.F.; et al. Subclinical leaflet thickening and stent frame geometry in self-expanding transcatheter heart valves. EuroIntervention 2017, 13, e1067–e1075. [Google Scholar] [CrossRef]
- Kanjanauthai, S.; Pirelli, L.; Nalluri, N.; Kliger, C.A. Subclinical leaflet thrombosis following transcatheter aortic valve replacement. J. Interv. Cardiol. 2018, 31, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Braghiroli, J.; Kapoor, K.; Thielhelm, T.P.; Ferreira, T.; Cohen, M.G. Transcatheter aortic valve replacement in low risk patients: A review of PARTNER 3 and Evolut low risk trials. Cardiovasc. Diagn. Ther. 2020, 10, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Moumneh, M.B.; Damluji, A.A.; Heslop, A.W.; Sherwood, M.W. Structural heart disease review of TAVR in low-risk patients: Importance of lifetime management. Front. Cardiovasc. Med. 2024, 11, 1362791. [Google Scholar] [CrossRef]
- Dvir, D.; Bourguignon, T.; Otto, C.M.; Hahn, R.T.; Rosenhek, R.; Webb, J.G.; Treede, H.; Sarano, M.E.; Feldman, T.; Wijeysundera, H.C.; et al. Standardized Definition of Structural Valve Degeneration for Surgical and Transcatheter Bioprosthetic Aortic Valves. Circulation 2018, 137, 388–399. [Google Scholar] [CrossRef]
- Barrett, A.; Brown, J.A.; Smith, M.A.; Woodward, A.; Vavalle, J.P.; Kheradvar, A.; Griffith, B.E.; Fogelson, A.L. A model of fluid-structure and biochemical interactions for applications to subclinical leaflet thrombosis. Int. J. Numer. Method. Biomed. Eng. 2023, 39, e3700. [Google Scholar] [CrossRef]
- Yang, R.; Grober, A.F.; Riojas, R.; Ponna, V.; Shunk, K.A.; Zimmet, J.M.; Gustafson, J.; Ge, L.; Tseng, E.E. Midterm Durability and Structural Valve Degeneration of Transcatheter Aortic Valve Replacement in a Federal Facility. Innovations 2022, 17, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.V.; Goel, S.S.; Kleiman, N.S.; Reardon, M.J. Transcatheter Aortic Valve Implantation: Long-Term Outcomes and Durability. Methodist. Debakey Cardiovasc. J. 2023, 19, 15–25. [Google Scholar] [CrossRef]
- Capodanno, D.; Petronio, A.S.; Prendergast, B.; Eltchaninoff, H.; Vahanian, A.; Modine, T.; Lancellotti, P.; Sondergaard, L.; Ludman, P.F.; Tamburino, C.; et al. Standardized definitions of structural deterioration and valve failure in assessing long-term durability of transcatheter and surgical aortic bioprosthetic valves: A consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) endorsed by the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. J. Cardiothorac. Surg. 2017, 52, 408–417. [Google Scholar]
- Bapat, V.N.; Zaid, S.; Fukuhara, S.; Saha, S.; Vitanova, K.; Kiefer, P.; Squers, J.J.; Voisine, P.; Pirelli, L.; von Ballmoos, M.W.; et al. Surgical Explantation After TAVR Failure: Mid-Term Outcomes From the EXPLANT-TAVR International Registry. JACC Cardiovasc. Interv. 2021, 14, 1978–1991. [Google Scholar] [CrossRef]
- Tang, G.H.L.; Zaid, S.; Kleiman, N.S.; Goel, S.S.; Fukuhara, S.; Marin-Cuartas, M.; Kiefer, P.; Abdel-Wahab, M.; De Backer, O.; Sondergaard, L.; et al. Explant vs. Redo-TAVR After Transcatheter Valve Failure: Mid-Term Outcomes From the EXPLANTORREDO-TAVR International Registry. JACC Cardiovasc. Interv. 2023, 16, 927–941. [Google Scholar] [CrossRef]
- Bapat, V.N.; Fukui, M.; Zaid, S.; Okada, A.; Jilaihawi, H.; Rogers, T.; Khalique, O.; Cavalcante, J.L.; Landes, U.; Sathananthan, J.; et al. A Guide to Transcatheter Aortic Valve Design and Systematic Planning for a Redo-TAV (TAV-in-TAV) Procedure. JACC Cardiovasc. Interv. 2024, 17, 1631–1651. [Google Scholar] [CrossRef] [PubMed]
- Akodad, M.; Sellers, S.; Landes, U.; Meier, D.; Tang, G.H.; Gada, H.; Rogers, T.; Caskey, M.; Rutkin, B.; Puri, R.; et al. Balloon-Expandable Valve for Treatment of Evolut Valve Failure: Implications on Neoskirt Height and Leaflet Overhang. JACC Cardiovasc. Interv. 2022, 15, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Landes, U.; Webb, J.G.; De Backer, O.; Sondergaard, L.; Abdel-Wahab, M.; Crusius, L.; Kim, W.; Hamm, C.; Buzzatti, N.; Montorfano, M.; et al. Repeat Transcatheter Aortic Valve Replacement for Transcatheter Prosthesis Dysfunction. J. Am. Coll. Cardiol. 2020, 75, 1882–1893. [Google Scholar] [CrossRef]
- Testa, L.; Agnifili, M.; Van Mieghem, N.; Tchetche, D.; Asgar, A.W.; De Backer, O.; Latib, A.; Reimers, B.; Stefanini, G.; Trani, C.; et al. Transcatheter Aortic Valve Replacement for Degenerated Transcatheter Aortic Valves: The TRANSIT International Project. Circ. Cardiovasc. Interv. 2021, 14, e010440. [Google Scholar] [CrossRef] [PubMed]
- Damlin, A.; Meduri, C.; Manouras, A.; Verouhis, D.; Linder, R.; Ruck, A.; Settergren, M. BASILICA Procedure Prior to Valve-in-Valve TAVR in a Supra-Annular TAV Prosthesis. JACC Case Rep. 2023, 11, 101777. [Google Scholar] [CrossRef]
- Khan, J.M.; Bruce, C.G.; Babaliaros, V.C.; Greenbaum, A.B.; Rogers, T.; Lederman, R.J. TAVR Roulette: Caution Regarding BASILICA Laceration for TAVR-in-TAVR. JACC Cardiovasc Interv. 2020, 13, 787–789. [Google Scholar] [CrossRef]
- Dvir, D.; Leon, M.B.; Abdel-Wahab, M.; Unbehaun, A.; Kodali, S.; Tchetche, D.; Pibarot, P.; Leipsic, J.; Blanke, P.; Gerckens, U.; et al. First-in-Human Dedicated Leaflet Splitting Device for Prevention of Coronary Obstruction in Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2023, 16, 94–102. [Google Scholar] [CrossRef]
- Mew, C.; Dahiya, A.; Chong, A.A.; Hayman, S.M.; Moore, P.T.; Harrop, D.L.; Reyaldeen, R.; Cole, C.M.; Ross, J.D.; Roberts, S.; et al. First-in-human: Leaflet laceration with balloon mediated annihilation to prevent coronary obstruction with radiofrequency needle (LLAMACORN) for valve-in-valve transcatheter aortic valve replacement. Catheter. Cardiovasc. Interv. 2024, 104, 1079–1085. [Google Scholar] [CrossRef]
- Tang, G.H.; Spencer, J.; Rogers, T.; Grubb, K.J.; Gleason, P.; Gada, H.; Mahoney, P.; Dauerman, H.L.; Forrest, J.K.; Reardon, M.J.; et al. Feasibility of coronary access following redo-TAVR for Evolut failure: A computed tomography simulation study. Circ. Cardiovasc. Interv. 2023, 16, e013238. [Google Scholar] [CrossRef]
- Gallinoro, E.; Scarsini, R.; Ancona, M.B.; Paolisso, P.; Portolan, L.; Springhetti, P.; Mora, F.D.; Mainardi, A.; Belmonte, M.; Moroni, F.; et al. Effect of TAVI on epicardial functional indices and their relationship to coronary microvascular function. Circ. Cardiovasc. Interv. 2025, 18, e014940. [Google Scholar] [CrossRef]
- Lønborg, J.; Jabbari, R.; Sabbah, M.; Veien, K.T.; Niemela, M.; Freeman, P.; Linder, R.; Ioanes, D.; Terkelsen, C.J.; Kajander, O.A.; et al. PCI in patients undergoing transcatheter aortic valve implantation. N. Engl. J. Med. 2024, 391, 2189–2200. [Google Scholar] [CrossRef]
- Duncan, A.; Moat, N.; Simonato, M.; de Weger, A.; Kempfert, J.; Eggebrecht, H.; Walton, A.; Hellig, F.; Kornowski, R.; Spargias, K.; et al. Outcomes Following Transcatheter Aortic Valve Replacement for Degenerative Stentless Versus Stented Bioprostheses. JACC Cardiovasc. Interv. 2019, 12, 1256–1263. [Google Scholar] [CrossRef]
- Almarzooq, Z.I.; Kazi, D.S.; Wang, Y.; Chung, M.; Tian, W.; Strom, J.B.; Baron, S.; Yeh, R.W. Outcomes of stroke events during transcatheter aortic valve implantation. EuroIntervention 2022, 18, e335–e344. [Google Scholar] [CrossRef]
- Carroll, J.D.; Mack, M.J.; Vemulapalli, S.; Herrmann, H.C.; Gleason, T.G.; Hanzel, G.; Deeb, G.M.; Thourani, V.H.; Cohen, D.J.; Desai, N.; et al. STS-ACC TVT Registry of Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2020, 76, 2492–2516. [Google Scholar] [CrossRef]
- Barbanti, M.; Webb, J.G.; Tamburino, C.; Mieghem, N.M.; Makkar, R.; Piazza, N.; Latib, A.; Sinning Jwon-Keun, K.; Bleiziffer, S.; Bedogni, F.; et al. Outcomes of Redo Transcatheter Aortic Valve Replacement for the Treatment of Postprocedural and Late Occurrence of Paravalvular Regurgitation and Transcatheter Valve Failure. Circ. Cardiovasc. Interv. 2016, 9, e003930. [Google Scholar] [CrossRef]
- Percy, E.D.; Harloff, M.T.; Hirji, S.; McGurk, S.; Yazdchi, F.; Newell, P.; Malarczyk, A.; Sabe, A.; Landes, U.; Webb, J.; et al. Nationally Representative Repeat Transcatheter Aortic Valve Replacement Outcomes: Report From the Centers for Medicare and Medicaid Services. JACC Cardiovasc. Interv. 2021, 14, 1717–1726. [Google Scholar] [CrossRef]
- Kapadia, S.R.; Makkar, R.; Leon, M.; Abdel-Wahab, M.; Waggoner, T.; Massberg, S.; Rottbauer, W.; Horr, S.; Sondergaard, L.; Karha, J.; et al. Cerebral Embolic Protection during Transcatheter Aortic-Valve Replacement. N. Engl. J. Med. 2022, 387, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Kharbanda, R.K.; Kennedy, J.; Jamal, Z.; Dodd, M.; Evans, R.; Bal, K.; Perkins, A.D.; Blackman, D.J.; Hildick-Smith, D.; Banning, A.P.; et al. Routine Cerebral Embolic Protection during Transcatheter Aortic-Valve Implantation. N. Engl. J. Med. 2025, 392, 2403–2412. [Google Scholar] [CrossRef] [PubMed]
- Aktuerk, D.; Mirsadraee, S.; Quarto, C.; Davies, S.; Duncan, A. Leaflet thrombosis after valve-in-valve transcatheter aortic valve implantation: A case series. Eur. Heart J. Case Rep. 2020, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Frerker, C.; Alessandrini, H.; Schliter, M.; Kreidel, F.; Schafer, U.; Thielsen, T.; Kuck, K.; Jose, J.; Holy, E.W.; et al. Redo TAVI: Initial experience at two German centres. EuroIntervention 2016, 12, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Dvir, D.; Webb, J.G.; Bleiziffer, S.; Pasic, M.; Waksman, R.; Kodali, S.; Barbanti, M.; Latib, A.; Schaefer, U.; Rodes-Cabau, J.; et al. Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA 2014, 312, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. J. Am. Coll. Cardiol. 2021, 77, e25–e197. [Google Scholar] [CrossRef]
- Praz, F.; Borger, M.A.; Lanz, J.; Marin-Cuartas, M.; Abreu, A.; Adamo, M.; Marsan, N.A.; Barili, F.; Bonaros, N.; Cosyns, B.; et al. 2025 ESC/EACTSguidelines for the management of valvular heart disease. Eur. Heart J. 2025, ehaf194. [Google Scholar] [CrossRef] [PubMed]


| Study (Year) | Number of Patients | Index THV | Mode of Failure | 2nd THV | Time Between Index TAVR and Redo-TAVR | All-Cause Mortality | Permanent Pacemaker | Coronary Obstruction | Stroke (30 Days) |
|---|---|---|---|---|---|---|---|---|---|
| Schmidt et al. (2016) [41] | 19 | SEV = 84% BEV = 16% | AR = 84% AS = 16% | SEV = 37% BEV = 63% | 644 (191–1831) days | 33% at 1 yr | 11% | None | 5% |
| Barbanti et al. (2016) [36] | 50 | SEV = 76% BEV = 24% | AR = 76% AS = 18% Mixed = 6% | SEV = 60% BEV = 40% | 812 ± 750 days | 14.9% at 2 years | 8.6% | 2% | 2% |
| Landes et al. (2020) [24] | 212 | SEV = 61.4% BEV = 38.6% | AR = 45% AS = 30% Mixed = 25% | SEV = 50% BEV = 50% | 2 days to 11.6 yrs | 2.9% at 30 days | 9.6% | 0.9% | 1.4% |
| Percy et al. (2020) [37] | 617 | N/A | N/A | N/A | 154 (58–537) days | 22% at 1 yr | 4.2% | N/A | 1.8% |
| Testa et al. (2021) [25] | 172 | SEV = 65% BEV = 35% | AR = 56% AS = 33% Mixed = 11% | SEV = 61% BEV = 39% | 268–1323 days (reported by valve type) | 10% at 1 yr | 4.1% | 0% | 3.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guddeti, R.R.; Bashir, H.; Seshiah, P.; El-Hangouche, N.; Kereiakes, D.J.; Garcia, S. Redo-Transcatheter Aortic Valve Replacement: Current Evidence and Procedural Considerations. J. Clin. Med. 2025, 14, 6608. https://doi.org/10.3390/jcm14186608
Guddeti RR, Bashir H, Seshiah P, El-Hangouche N, Kereiakes DJ, Garcia S. Redo-Transcatheter Aortic Valve Replacement: Current Evidence and Procedural Considerations. Journal of Clinical Medicine. 2025; 14(18):6608. https://doi.org/10.3390/jcm14186608
Chicago/Turabian StyleGuddeti, Raviteja R., Hanad Bashir, Puvi Seshiah, Nadia El-Hangouche, Dean J. Kereiakes, and Santiago Garcia. 2025. "Redo-Transcatheter Aortic Valve Replacement: Current Evidence and Procedural Considerations" Journal of Clinical Medicine 14, no. 18: 6608. https://doi.org/10.3390/jcm14186608
APA StyleGuddeti, R. R., Bashir, H., Seshiah, P., El-Hangouche, N., Kereiakes, D. J., & Garcia, S. (2025). Redo-Transcatheter Aortic Valve Replacement: Current Evidence and Procedural Considerations. Journal of Clinical Medicine, 14(18), 6608. https://doi.org/10.3390/jcm14186608







