Alternative Transaxillary Access for Transcatheter Aortic Valve Implantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Preoperative Planning and Operative Technique
3. Results
3.1. Baseline Characteristics
3.2. Periprocedural Outcomes
4. Discussion
4.1. Interpretation of the Results
4.2. Benefits of the Surgical Approach
5. Conclusions
5.1. Limitations
5.2. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKI | Acute Kidney Injury |
| BMI | Body Mass Index |
| CT | Computed Tomography |
| EUROSCORE II | European System for Cardiac Operative Risk Evaluation II |
| GFR | Glomerular Filtration Rate |
| mRS | Modified Rankin Scale |
| MSCT | Multislice Computed Tomography |
| PCI | Percutaneous Coronary Intervention |
| TAVI | Transcatheter Aortic Valve Implantation |
| TAx | Transaxillary |
| TIA | Transient Ischemic Attack |
| TF | Transfemoral |
| VARC | Valve Academic Research Consortium |
References
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Rev. Esp. Cardiol. Engl. Ed. 2022, 75, 524. [Google Scholar] [CrossRef] [PubMed]
- Abdelnour, M.W.; Patel, V.; Patel, P.M.; Kasel, A.M.; Frangieh, A.H. Alternative access in transcatheter aortic valve replacement—An updated focused review. Front. Cardiovasc. Med. 2024, 11, 1437626. [Google Scholar] [CrossRef] [PubMed]
- Harloff, M.T.; Percy, E.D.; Hirji, S.A.; Yazdchi, F.; Shim, H.; Chowdhury, M.; Malarczyk, A.A.; Sobieszczyk, P.S.; Sabe, A.A.; Shah, P.B.; et al. A step-by-step guide to trans-axillary transcatheter aortic valve replacement. Ann. Cardiothorac. Surg. 2020, 9, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Herremans, A.; Stevesyns, D.T.; El Jattari, H.; Rosseel, M.; Rosseel, L. The Place of Transaxillary Access in Transcatheter Aortic Valve Implantation (TAVI) Compared to Alternative Routes—A Systematic Review Article. Rev. Cardiovasc. Med. 2023, 24, 150. [Google Scholar] [CrossRef] [PubMed]
- Van Wely, M.; Van Nieuwkerk, A.C.; Rooijakkers, M.; Van Der Wulp, K.; Gehlmann, H.; Verkroost, M.; Van Garsse, L.; Geuzebroek, G.; Baz, J.A.; Tchétché, D.; et al. Transaxillary versus transfemoral access as default access in TAVI: A propensity matched analysis. Int. J. Cardiol. 2024, 394, 131353. [Google Scholar] [CrossRef] [PubMed]
- Sündermann, S.H.; Dreger, H.; Hinkov, H.; Kempfert, J. The 10 Commandments for Transaxillary TAVI. Innov. Technol. Tech. Cardiothorac. Vasc. Surg. 2023, 18, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Généreux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; Blackstone, E.H.; et al. Valve Academic Research Consortium 3: Updated Endpoint Definitions for Aortic Valve Clinical Research. J. Am. Coll. Cardiol. 2021, 77, 2717–2746. [Google Scholar] [CrossRef] [PubMed]
- Ascher, E.; Haimovici, H. (Eds.) Haimovici’s Vascular Surgery, 6th ed.; Wiley-Blackwell: Chichester, UK, 2012; 1p. [Google Scholar]
- McGrath, D.; Lee, H.; Sun, C.; Kawabori, M.; Zhan, Y. Right transaxillary transcatheter aortic valve replacement is comparable to left despite challenges. Gen. Thorac. Cardiovasc. Surg. 2024, 72, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Dahle, T.G.; Kaneko, T.; McCabe, J.M. Outcomes Following Subclavian and Axillary Artery Access for Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2019, 12, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, U.; Deuschl, F.; Schofer, N.; Frerker, C.; Schmidt, T.; Kuck, K.H.; Kreidel, F.; Schirmer, J.; Mizote, I.; Reichenspurner, H.; et al. Safety and efficacy of the percutaneous transaxillary access for transcatheter aortic valve implantation using various transcatheter heart valves in 100 consecutive patients. Int. J. Cardiol. 2017, 232, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, A.; Sudo, M.; Al-Kassou, B.; Shamekhi, J.; Silaschi, M.; Wilde, N.; Sedaghat, A.; Becher, U.M.; Weber, M.; Sinning, J.-M.; et al. Percutaneous trans-axilla transcatheter aortic valve replacement. Heart Vessels 2022, 37, 1801–1807. [Google Scholar] [CrossRef] [PubMed]
- Muscoli, S.; Cammalleri, V.; Bonanni, M.; Prandi, F.R.; Sanseviero, A.; Massaro, G.; Di Luozzo, M.; Chiocchi, M.; Ascoli Marchetti, A.; Ippoliti, A.; et al. The Transaxillary Route as a Second Access Option in TAVI Procedures: Experience of a Single Centre. Int. J. Environ. Res. Public Health 2022, 19, 8649. [Google Scholar] [CrossRef] [PubMed]
- Mauri, V.; Reimann, A.; Stern, D.; Scherner, M.; Kuhn, E.; Rudolph, V.; Rosenkranz, S.; Eghbalzadeh, K.; Friedrichs, K.; Wahlers, T.; et al. Predictors of Permanent Pacemaker Implantation After Transcatheter Aortic Valve Replacement With the SAPIEN 3. JACC Cardiovasc. Interv. 2016, 9, 2200–2209. [Google Scholar] [CrossRef] [PubMed]
- Bruno, F.; D’Ascenzo, F.; Vaira, M.P.; Elia, E.; Omedè, P.; Kodali, S.; Barbanti, M.; Rodès-Cabau, J.; Husser, O.; Sossalla, S.; et al. Predictors of pacemaker implantation after transcatheter aortic valve implantation according to kind of prosthesis and risk profile: A systematic review and contemporary meta-analysis. Eur. Heart J.-Qual. Care Clin. Outcomes 2021, 7, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Hokken, T.W.; Muhemin, M.; Okuno, T.; Veulemans, V.; Lopes, B.B.; Beneduce, A.; Vittorio, R.; Ooms, J.F.; Adrichem, R.; Neleman, T.; et al. Impact of membranous septum length on pacemaker need with different transcatheter aortic valve replacement systems: The INTERSECT registry. J. Cardiovasc. Comput. Tomogr. 2022, 16, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, K.; Kaleschke, G.; De Torres-Alba, F.; Martens, S.; Deschka, H. The Alternative Transaxillary Access for Transcatheter Aortic Valve Implantation. In Proceedings of the 54th annual congress of the German Society for Thoracic and Cardiovascular Surgery (DGTHG), Hamburg, Germany, 15–17 February 2025; The Thoracic and Cardiovascular Surgeon: Windsor, UK, 2025; pp. S1–S71. [Google Scholar]
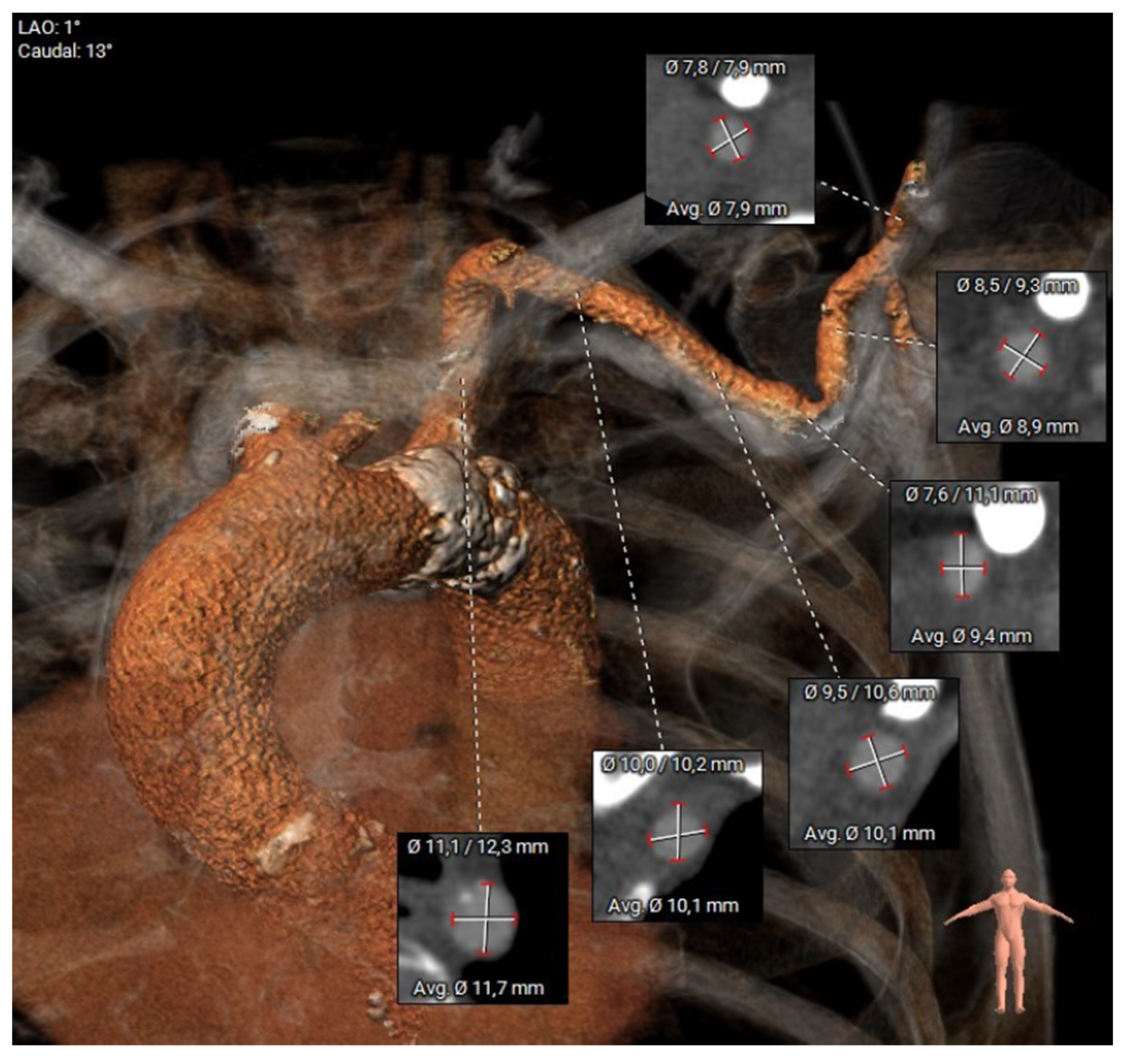
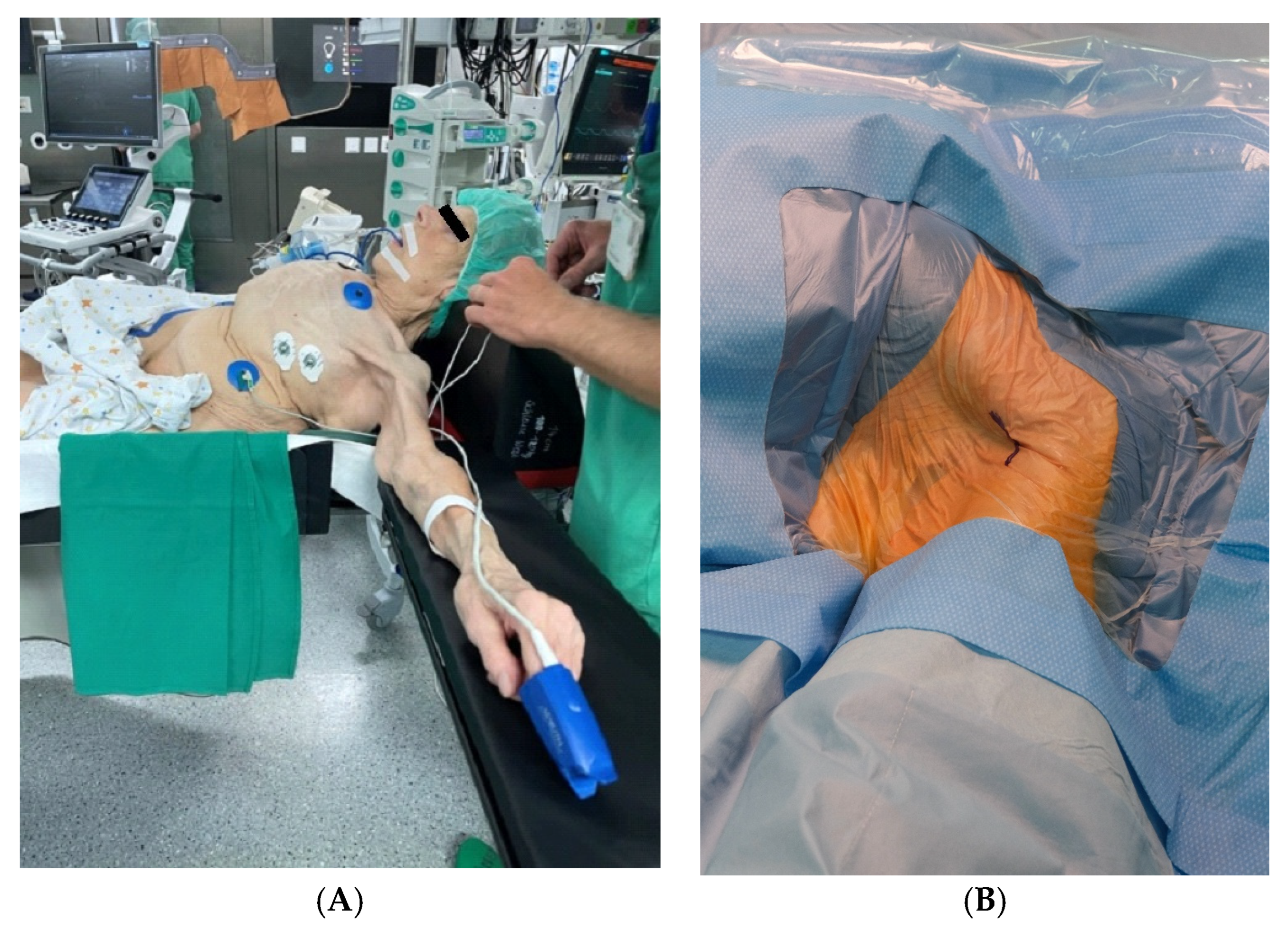
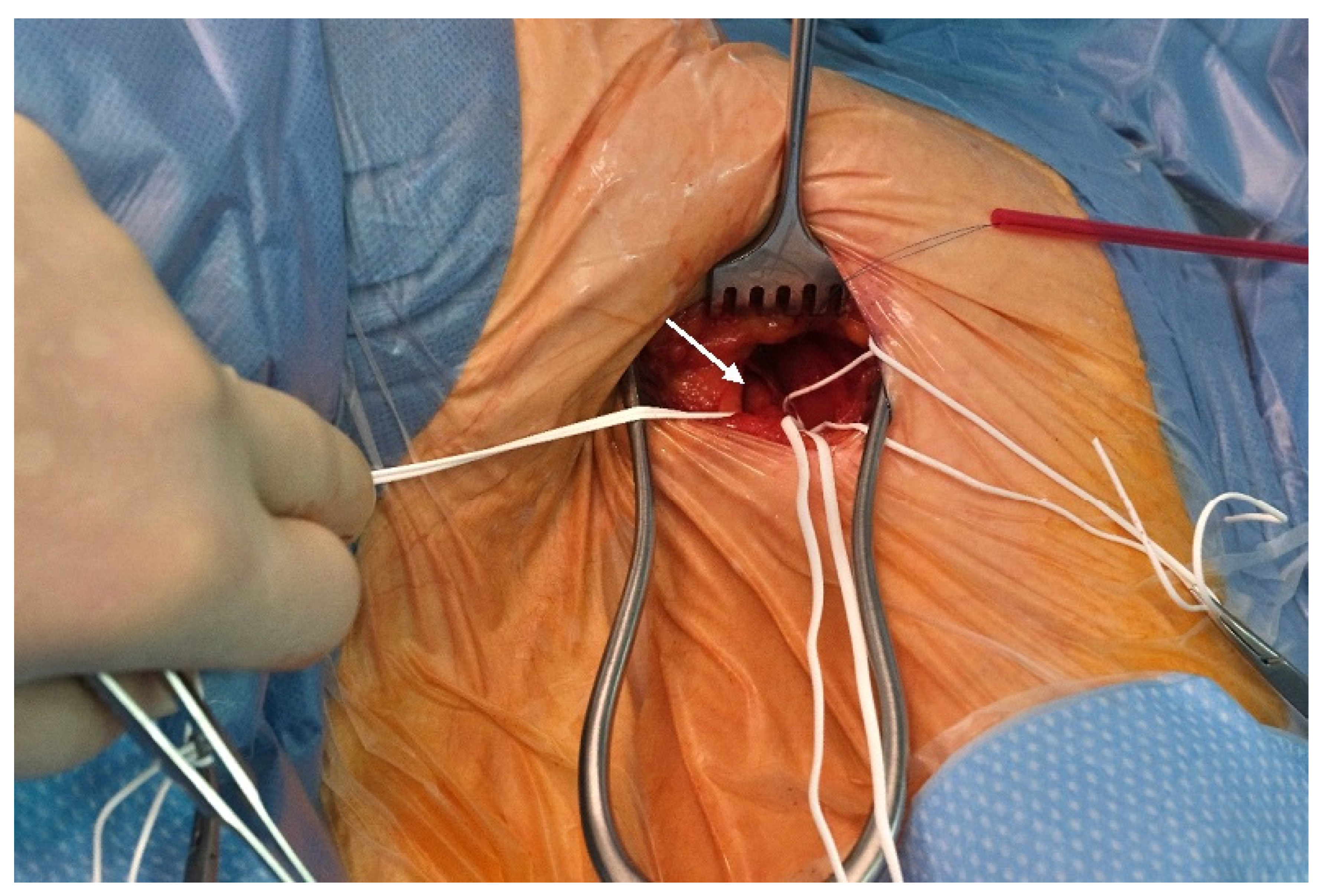
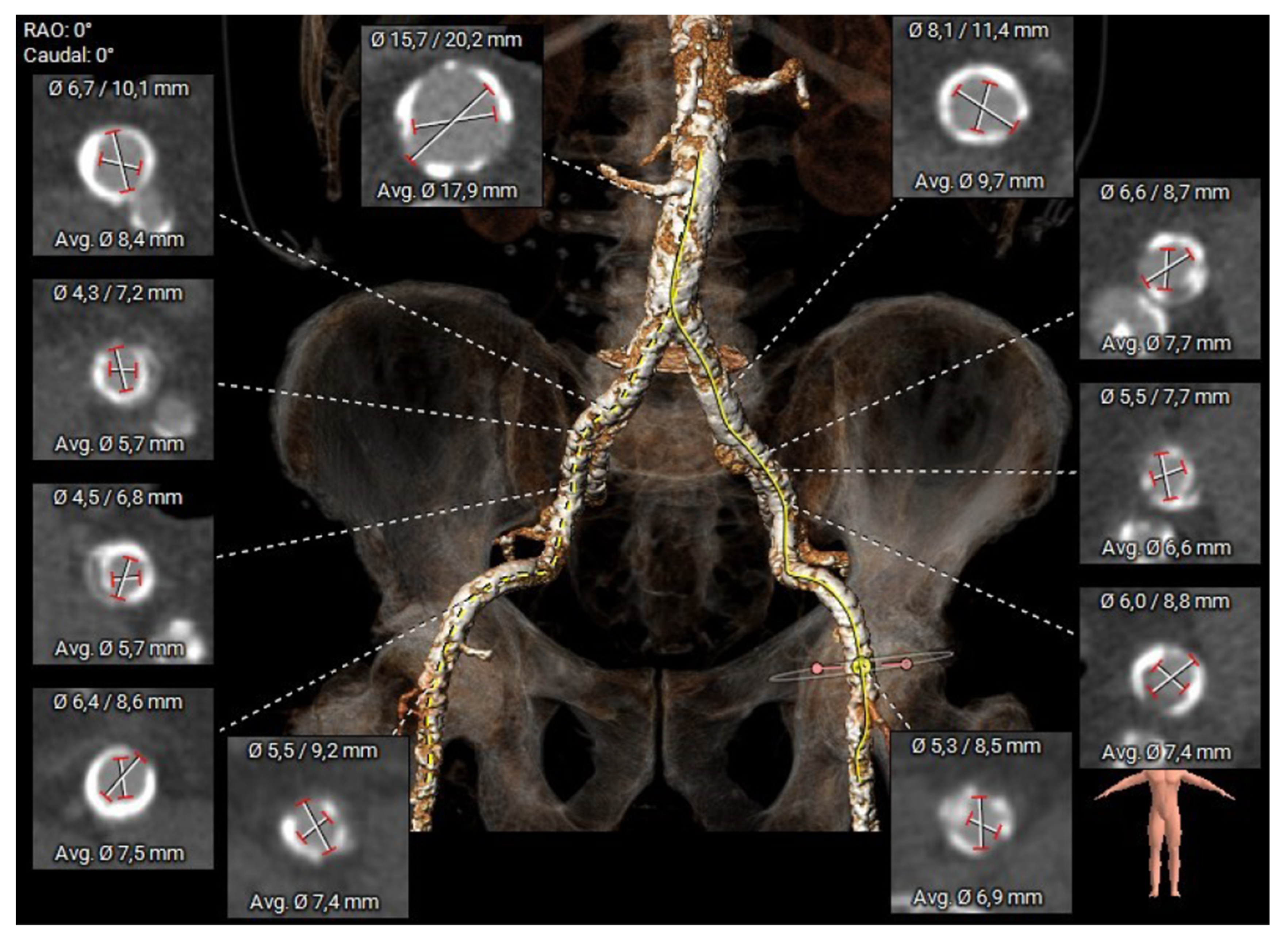
| Baseline Characteristics | Overall N = 24 (%, Mean ± SD) |
|---|---|
| Age (years) | 77.9 ± 8 |
| Sex (female) | 7 (29.2%) |
| Weight (kg) | 74.7 ± 12.8 |
| Height (cm) | 171.0 ± 12.2 |
| BMI | 25.4 ± 3.5 |
| EuroScore II (%) | 7.4 ± 4.7 |
| Myocardial infarction | 3 (12.5%) |
| Prior PCI | 4 (16.7%) |
| Prior pacemaker | 2 (8.3%) |
| Prior stroke/TIA | 5 (20.8%) |
| History of smoking | 9 (37.5%) |
| Diabetes mellitus on insulin | 7 (29.2%) |
| Chronic pulmonary disease | 7 (29.2%) |
| Arterial hypertension | 18 (75%) |
| Peripheral vascular disease | 16 (66.7%) |
| Severe renal impairment (GFR < 30 mL/min/1.73 m2) | 3 (12.5%) |
| Creatinine (mg/dL) | 1.2 ± 0.8 |
| Malignant disease | 4 (16.7%) |
| Atrial fibrillation | 8 (33.3%) |
| Echocardiography | |
| Left-ventricular ejection fraction (%) | 52.3 ± 11.5 |
| Aortic valve area (cm2) | 0.7 ± 0.2 |
| Mean aortic valve gradient (mmHg) | 43.8 ± 10.5 |
| Peak aortic valve gradient (mmHg) | 73.3 ± 15.5 |
| Periprocedural Details | Overall N = 24 (%, Mean ± SD) |
|---|---|
| Procedure time (min) | 102.6 ± 46.1 |
| Fluoroscopy time (min) | 20.8 ± 14.7 |
| Contrast medium (mL) | 110.1 ± 42.0 |
| Pre-dilatation | 13 (54.2%) |
| Post dilatation | 5 (20.8%) |
| Sapien3 | 24 (100%) |
| Valve diameter (mm/mean) | 25.6 ± 2.4 |
| Valve size (mm) | |
| 23 | 9 |
| 26 | 9 |
| 29 | 6 |
| Clinical Outcomes | Overall N = 24 (%, Mean ± SD) |
|---|---|
| In-hospital-death | 1 (4.15%) |
| Device Intervention success | 22 (91.7%) |
| Conversion to surgery | 0 (0%) |
| Myocardial infarction | 1 (4.15%) |
| Stroke (moderate disability/Rankin) | 1 (4.15%) |
| New permanent pacemaker | 4 (16.7%) |
| Major bleeding (Type 3, gastrointestinal) | 3 (12.5%) |
| Major vascular complications | 1 (4.15%) |
| Acute kidney injury (>AKI Stage 2) | 2 (8.3%) |
| Dialysis | 1 (4.15%) |
| Mean aortic valve gradient (mmHg) (at discharge) | 13.0 ± 3.1 |
| Peak aortic valve gradient (mmHg) (at discharge) | 22.9 ± 3.4 |
| Length of hospital stay (days) | 7.3 ± 5.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wisniewski, K.; Kaleschke, G.; De-Torres-Alba, F.; Martens, S.; Deschka, H. Alternative Transaxillary Access for Transcatheter Aortic Valve Implantation. J. Clin. Med. 2025, 14, 5127. https://doi.org/10.3390/jcm14145127
Wisniewski K, Kaleschke G, De-Torres-Alba F, Martens S, Deschka H. Alternative Transaxillary Access for Transcatheter Aortic Valve Implantation. Journal of Clinical Medicine. 2025; 14(14):5127. https://doi.org/10.3390/jcm14145127
Chicago/Turabian StyleWisniewski, Konrad, Gerrit Kaleschke, Fernando De-Torres-Alba, Sven Martens, and Heinz Deschka. 2025. "Alternative Transaxillary Access for Transcatheter Aortic Valve Implantation" Journal of Clinical Medicine 14, no. 14: 5127. https://doi.org/10.3390/jcm14145127
APA StyleWisniewski, K., Kaleschke, G., De-Torres-Alba, F., Martens, S., & Deschka, H. (2025). Alternative Transaxillary Access for Transcatheter Aortic Valve Implantation. Journal of Clinical Medicine, 14(14), 5127. https://doi.org/10.3390/jcm14145127






