Study on Factors Affecting Toric Intraocular Lens Rotation Using Intraoperative OCT—Factors Influencing IOL Deployment and Proximity to Posterior Capsule After Insertion
Abstract
1. Introduction
2. Materials and Methods
2.1. T-IOL and Eyes
2.2. Equipment
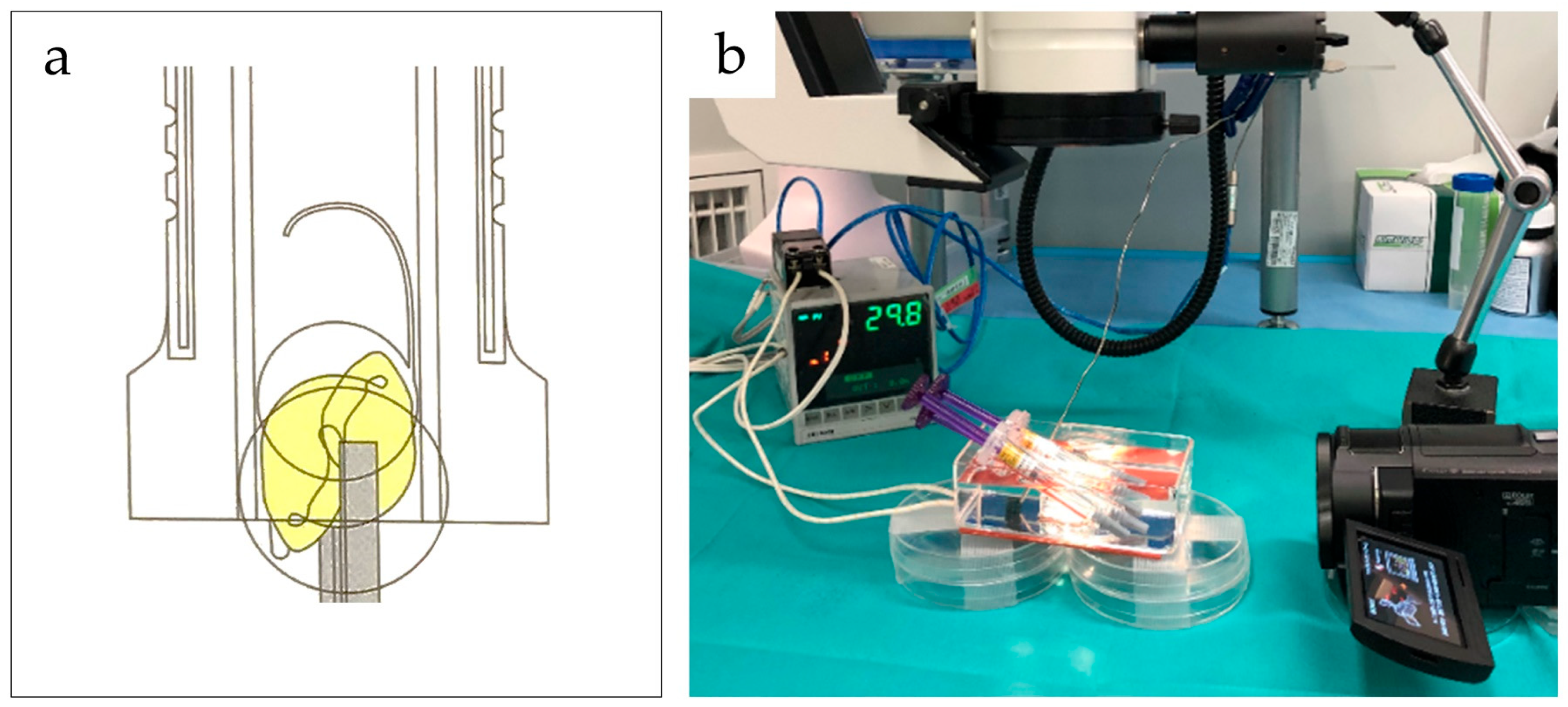
2.3. Experimental Procedure
2.3.1. Effects of Tacking Time and Temperature on IOL Deployment
2.3.2. Size Comparison Between Porcine and Human Lenses
2.3.3. Assessment of T-IOL Misalignment Due to Vibration
2.4. IOL Rotation Within One Week Postoperatively
2.5. Statistical Analysis
3. Results
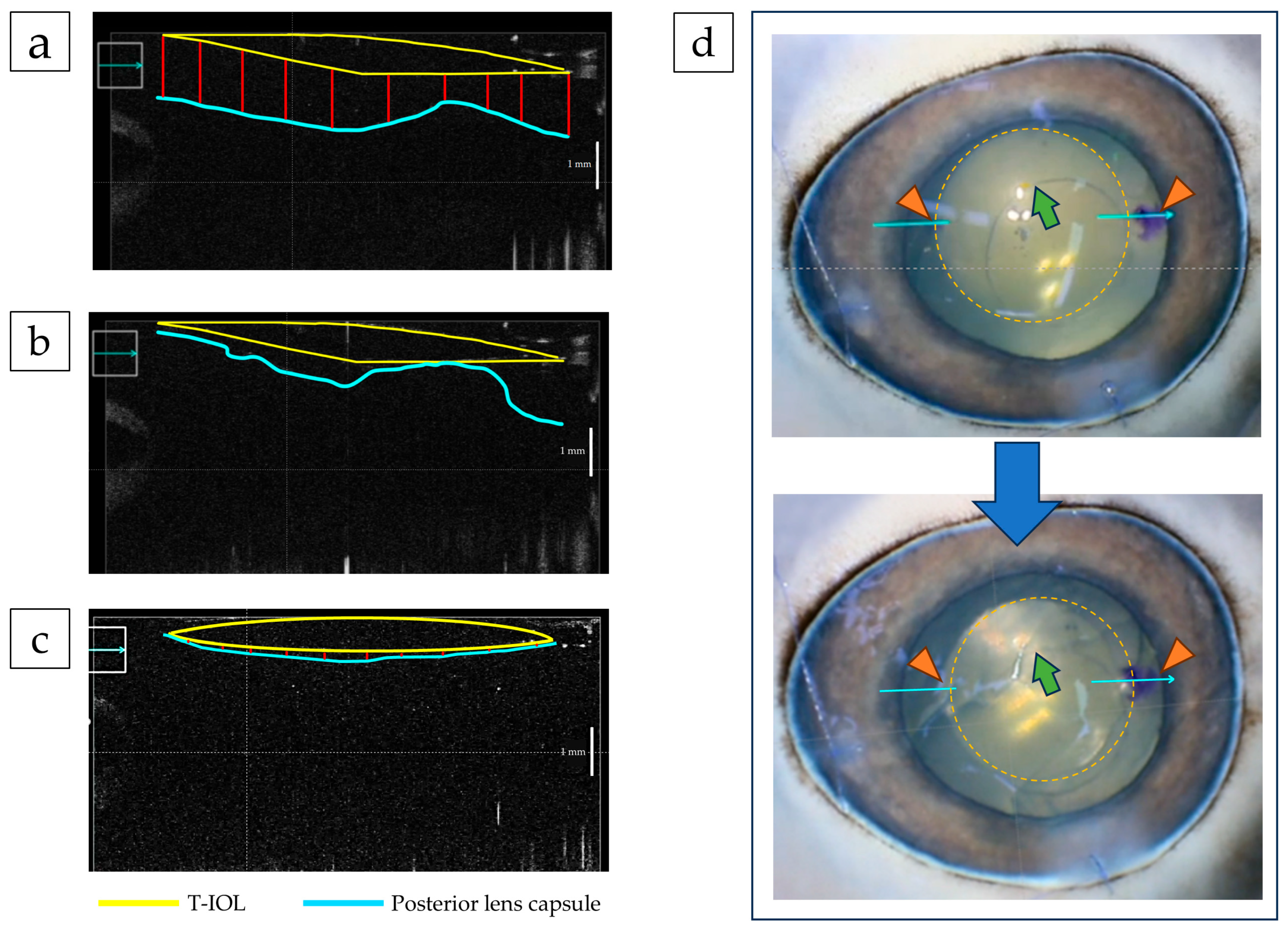
3.1. Factors Influencing T-IOL Rotation: IOL Deployment
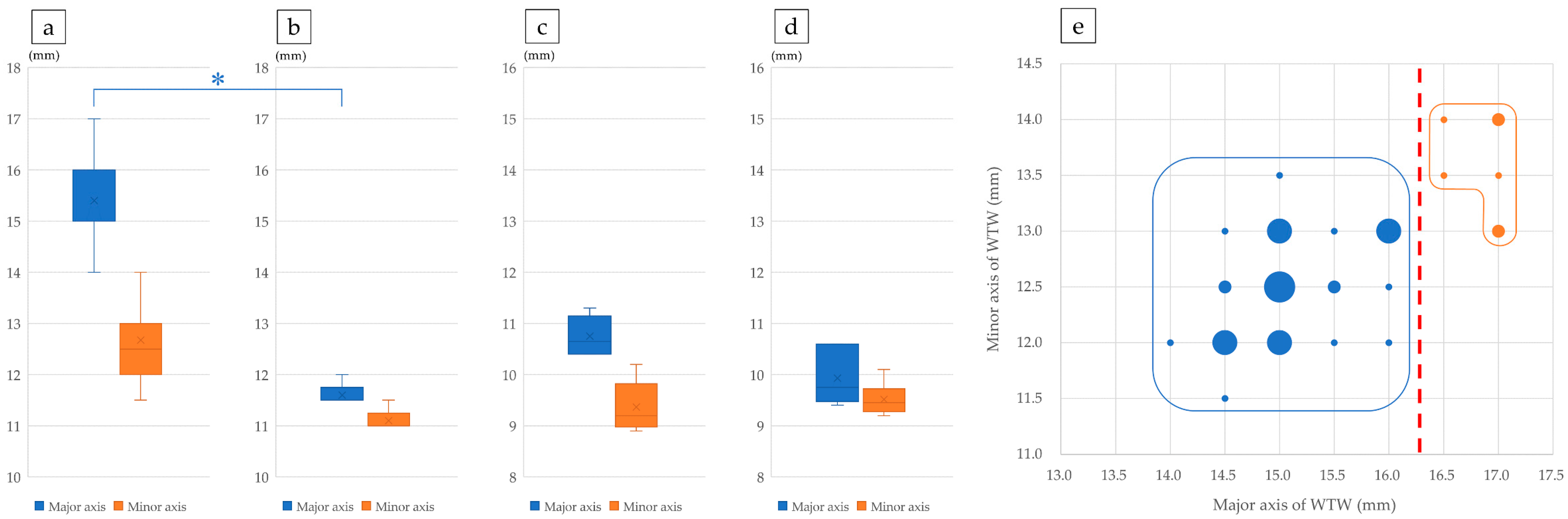
3.2. Factors Affecting T-IOL Rotation: Lens Size
3.3. Factors Influencing T-IOL Rotation: Proximity of Porcine Posterior Lens Capsule to IOL
3.4. Suppression of IOL Eccentricity by Temporary Low IOP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoffmann, P.C.; Hutz, W.W. Analysis of biometry and prevalence data for corneal astigmatism in 23,239 eyes. J. Cataract Refract. Surg. 2010, 36, 1479–1485. [Google Scholar] [CrossRef]
- Shimizu, K.; Misawa, A.; Suzuki, Y. Toric intraocular lenses: Correcting astigmatism while controlling axis shift. J. Cataract Refract. Surg. 1994, 20, 523–526. [Google Scholar] [CrossRef]
- Kessel, L.; Andresen, J.; Tendal, B.; Erngaard, D.; Flesner, P.; Hjortdal, J. Toric intraocular lenses in the correction of astigmatism during cataract surgery: A systematic review and meta-analysis. Ophthalmology 2016, 123, 275–286. [Google Scholar] [CrossRef]
- Hirnschall, N.; Hoffmann, P.C.; Draschl, P.; Maedel, S.; Findl, O. Evaluation of factors influencing the remaining astigmatism after toric intraocular lens implantation. J. Refract. Surg. 2014, 30, 394–400. [Google Scholar] [CrossRef]
- Visser, N.; Bauer, N.J.; Nuijts, R.M. Toric intraocular lenses: Historical overview, patient selection, IOL calculation, surgical techniques, clinical outcomes, and complications. J. Cataract Refract. Surg. 2013, 39, 624–637. [Google Scholar] [CrossRef]
- Jampaulo, M.; Olson, M.D.; Miller, K.M. Long-term Staar toric intraocular lens rotational stability. Am. J. Ophthalmol. 2008, 146, 550–553. [Google Scholar] [CrossRef]
- Shah, G.D.; Praveen, M.R.; Vasavada, A.R.; Vasavada, V.A.; Rampal, G.; Shastry, L.R. Rotational stability of a toric intraocular lens: Influence of axial length and alignment in the capsular bag. J. Cataract Refract. Surg. 2012, 38, 54–59. [Google Scholar] [CrossRef]
- Potvin, R.; Kramer, B.A.; Hardten, D.R.; Berdahl, J.P. Toric intraocular lens orientation and residual refractive astigmatism: An analysis. Clin. Ophthalmol. 2016, 10, 1829–1836. [Google Scholar] [CrossRef]
- Emesz, M.; Dexl, A.K.; Krall, E.M.; Bachernegg, A.; Moussa, S.; Jell, G.; Grabner, G.; Arlt, E.M. Randomized controlled clinical trial to evaluate different intraocular lenses for the surgical compensation of low to moderate-to-high regular corneal astigmatism during cataract surgery. J. Cataract Refract. Surg. 2015, 41, 2683–2694. [Google Scholar] [CrossRef]
- Minami, K.; Yaguchi, S.; Bissen-Miyajima, H. Temperature-controlled porcine eye holder for observing intraocular temperature during cataract surgery. Sci. Rep. 2023, 13, 4331. [Google Scholar] [CrossRef]
- Ruiz-Ederra, J.; Garcia, M.; Hernandez, M.; Urcola, H.; Hernandez-Barbachano, E.; Araiz, J.; Vecino, E. The pig eye as a novel model of glaucoma. Exp. Eye Res. 2005, 81, 561–569. [Google Scholar] [CrossRef]
- Fernandez-Bueno, I.; Pastor, J.C.; Gayoso, M.J.; Alcalde, I.; Garcia, M.T. Muller and macrophage-like cell interactions in an organotypic culture of porcine neuroretina. Mol. Vis. 2008, 14, 2148–2156. [Google Scholar]
- Sanchez, I.; Martin, R.; Ussa, F.; Fernandez-Bueno, I. The parameters of the porcine eyeball. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 475–482. [Google Scholar] [CrossRef]
- Takaku, R.; Nakano, S.; Iida, M.; Oshika, T. Influence of frosted haptics on rotational stability of toric intraocular lenses. Sci. Rep. 2021, 11, 15099. [Google Scholar] [CrossRef]
- Inoue, Y.; Takehara, H.; Oshika, T. Axis misalignment of toric intraocular lens: Placement error and postoperative rotation. Ophthalmology 2017, 124, 1424–1425. [Google Scholar] [CrossRef]
- Eom, Y.; Lee, J.S.; Rhim, J.W.; Kang, S.Y.; Song, J.S.; Kim, H.M. A simple method to shorten the unfolding time of prehydrated hydrophobic intraocular lens. Can. J. Ophthalmol. 2014, 49, 382–387. [Google Scholar] [CrossRef]
- Kodera, S.; Hirata, A.; Miura, F.; Rashed, E.A.; Hatsusaka, N.; Yamamoto, N.; Kubo, E.; Sasaki, H. Model-based approach for analyzing prevalence of nuclear cataracts in elderly residents. Comput. Biol. Med. 2020, 126, 104009. [Google Scholar] [CrossRef]
- Yamamoto, N.; Takeda, S.; Hatsusaka, N.; Hiramatsu, N.; Nagai, N.; Deguchi, S.; Nakazawa, Y.; Takata, T.; Kodera, S.; Hirata, A.; et al. Effect of a lens protein in low-temperature culture of novel immortalized human lens epithelial cells (iHLEC-NY2). Cells 2020, 9, 2670. [Google Scholar] [CrossRef]
- Rocher, E.E.; Mukherjee, R.; Pitingolo, J.; Levenshus, E.; Alexander, G.; Park, M.; Acharya, R.; Khan, S.; Shuff, J.; Aguirre, A.; et al. Intraocular lens unfurling time exponentially decays with increased solution temperature. Clin. Ophthalmol. 2023, 17, 2471–2481. [Google Scholar] [CrossRef]
- Wendt, M.; Bockhorst, K.; He, L.; Glasser, A. Accuracy and resolution of in vitro imaging based porcine lens volumetric measurements. Exp. Eye Res. 2011, 93, 741–752. [Google Scholar] [CrossRef]
- Oshika, T.; Fujita, Y.; Hirota, A.; Inamura, M.; Inoue, Y.; Miyata, K.; Miyoshi, T.; Nakano, S.; Nishimura, T.; Sugita, T. Comparison of incidence of repositioning surgery to correct misalignment with three toric intraocular lenses. Eur. J. Ophthalmol. 2020, 30, 680–684. [Google Scholar] [CrossRef]
- Osawa, R.; Oshika, T.; Sano, M.; Yuguchi, T.; Kaiya, T. Rotational stability of modified toric intraocular lens. PLoS ONE 2021, 16, e0247844. [Google Scholar] [CrossRef]
- Hoffmann, P.; Potvin, R.; Anello, R.D.; Hengerer, F.; Auffarth, G.; Guldenfels, Y.; Bertelmann, E.; Ruiz Mesa, R.; Fischinger, I.; Krawczyk, S.; et al. Comparing rotational stability over time between four monofocal toric intraocular lenses. Clin. Ophthalmol. 2025, 19, 1345–1355. [Google Scholar] [CrossRef]
- Jaitli, A.; Roy, J.; Chatila, A.; Liao, J.; Tang, L. Effect of time and temperature-dependent changes of IOL material properties on IOL: Lens capsule interactions. Exp. Eye Res. 2021, 211, 108726. [Google Scholar] [CrossRef]
- Felipe, A.; Artigas, J.M.; Diez-Ajenjo, A.; Garcia-Domene, C.; Alcocer, P. Residual astigmatism produced by toric intraocular lens rotation. J. Cataract Refract. Surg. 2011, 37, 1895–1901. [Google Scholar] [CrossRef]
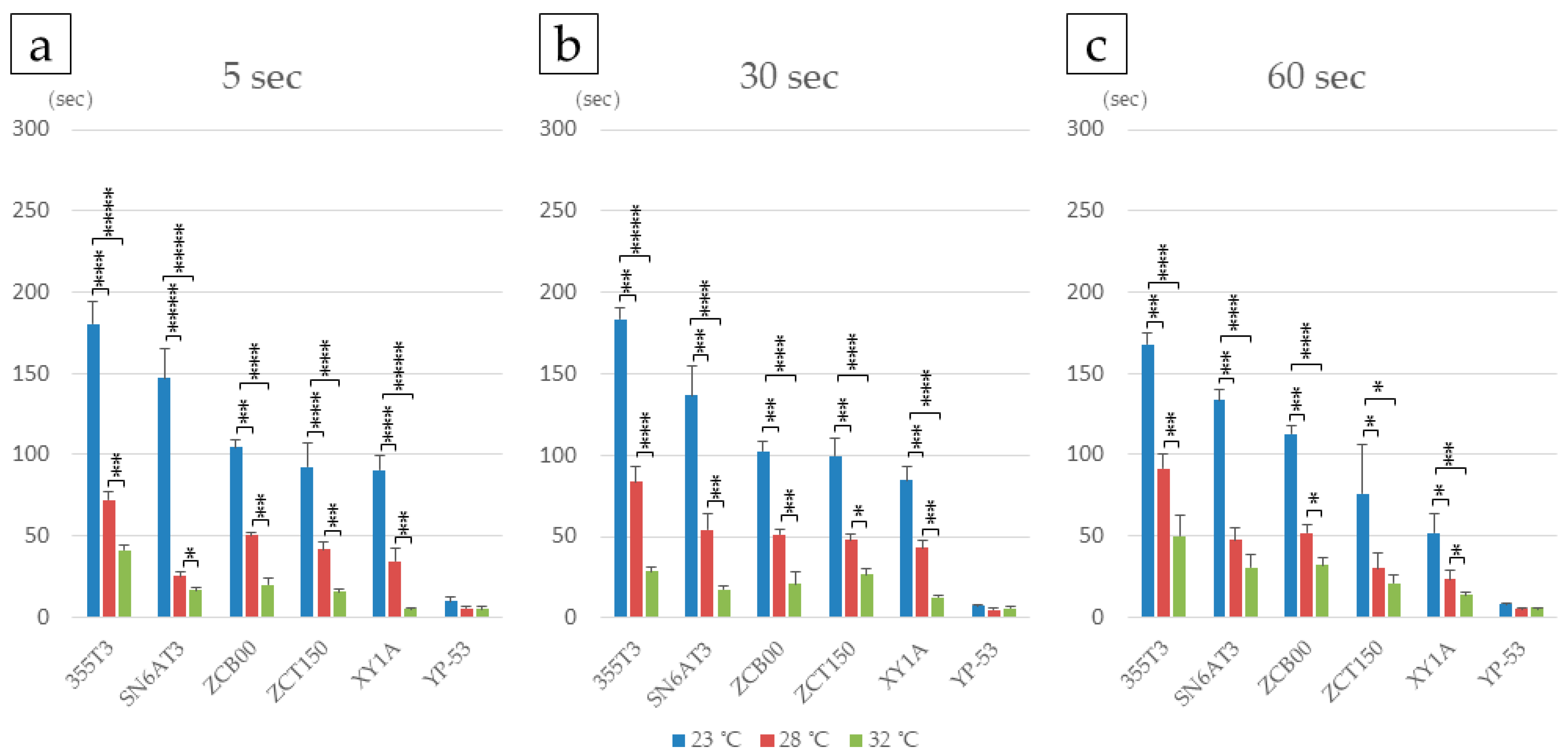
| Lens | Tacking Duration | 23 °C | 28 °C | 32 °C |
|---|---|---|---|---|
| 355T3 | 5 s | 180.2 (200.0/166.4) | 72.2 (76.0/66.0) | 41.1 (44.9/38.5) |
| 30 s | 183.4 (190.2/173.0) | 83.4 (96.0/71.6) | 28.5 (32.2/24.7) | |
| 60 s | 167.8 (174.6/161.0) | 91.3 (100.2/78.8) | 50.0 (65.9/34.9) | |
| SN6AT3 | 5 s | 147.2 (165.0/129.4) | 25.2 (29.0/23.0) | 16.6 (17.5/15.1) |
| 30 s | 136.7 (150.1/118.3) | 54.3 (67.4/47.2) | 17.6 (19.5/15.6) | |
| 60 s | 133.6 (140.0/127.2) | 47.5 (54.5/37.9) | 30.4 (37.6/19.2) | |
| ZCB00 | 5 s | 104.3 (110.0/98.0) | 50.3 (53.0/48.0) | 20.0 (25.0/15.1) |
| 30 s | 102.3 (110.2/95.0) | 51.0 (55.4/48.2) | 21.0 (29.6/14.4) | |
| 60 s | 112.7 (120.0/108.1) | 51.7 (55.0/45.2) | 31.7 (35.3/25.0) | |
| ZCT150 | 5 s | 92.3 (112.2/77.0) | 41.4 (50.5/37.2) | 15.3 (18.1/14.0) |
| 30 s | 99.7 (113.0/86.2) | 48.0 (53.1/42.3) | 26.7 (34.0/21.9) | |
| 60 s | 75.8 (116.5/65.2) | 29.7 (42.3/18.2) | 20.9 (27.3/16.5) | |
| XY1A | 5 s | 90.3 (101.5/80.4) | 34.5 (45.3/25.0) | 5.0 (6.0/4.0) |
| 30 s | 84.7 (96.1/78.5) | 43.0 (50.0/37.9) | 12.7 (14.0/10.9) | |
| 60 s | 51.7 (67.6/38.8) | 23.4 (30.9/18.2) | 13.5 (16.2/11.5) | |
| YP-53 | 5 s | 9.8 (11.6/6.1) | 5.4 (6.7/4.6) | 4.7 (6.8/3.3) |
| 30 s | 7.1 (8.7/6.2) | 5.1 (6.6/3.9) | 5.7 (7.5/4.3) | |
| 60 s | 7.6 (8.7/7.0) | 4.9 (5.7/3.7) | 5.6 (5.9/5.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ichikawa, K.; Tokiwa, S.; Tanaka, Y.; Toda, H.; Kato, Y.; Sakai, Y.; Ichikawa, K.; Yamamoto, N. Study on Factors Affecting Toric Intraocular Lens Rotation Using Intraoperative OCT—Factors Influencing IOL Deployment and Proximity to Posterior Capsule After Insertion. J. Clin. Med. 2025, 14, 6599. https://doi.org/10.3390/jcm14186599
Ichikawa K, Tokiwa S, Tanaka Y, Toda H, Kato Y, Sakai Y, Ichikawa K, Yamamoto N. Study on Factors Affecting Toric Intraocular Lens Rotation Using Intraoperative OCT—Factors Influencing IOL Deployment and Proximity to Posterior Capsule After Insertion. Journal of Clinical Medicine. 2025; 14(18):6599. https://doi.org/10.3390/jcm14186599
Chicago/Turabian StyleIchikawa, Kei, Seiji Tokiwa, Yoshiki Tanaka, Hiroto Toda, Yukihito Kato, Yukihiro Sakai, Kazuo Ichikawa, and Naoki Yamamoto. 2025. "Study on Factors Affecting Toric Intraocular Lens Rotation Using Intraoperative OCT—Factors Influencing IOL Deployment and Proximity to Posterior Capsule After Insertion" Journal of Clinical Medicine 14, no. 18: 6599. https://doi.org/10.3390/jcm14186599
APA StyleIchikawa, K., Tokiwa, S., Tanaka, Y., Toda, H., Kato, Y., Sakai, Y., Ichikawa, K., & Yamamoto, N. (2025). Study on Factors Affecting Toric Intraocular Lens Rotation Using Intraoperative OCT—Factors Influencing IOL Deployment and Proximity to Posterior Capsule After Insertion. Journal of Clinical Medicine, 14(18), 6599. https://doi.org/10.3390/jcm14186599






