Rheological Theory Applied to Mechanical Ventilation in Acute Respiratory Distress Syndrome: A New Paradigm for Understanding and Preventing Ventilator-Induced Lung Injury
Abstract
1. Introduction
2. Evolution from Classical to Rheological VILI Models
- Biotrauma: inflammatory response secondary to mechanical damage [12].
3. New VILI Concepts Related to Rheological Theory
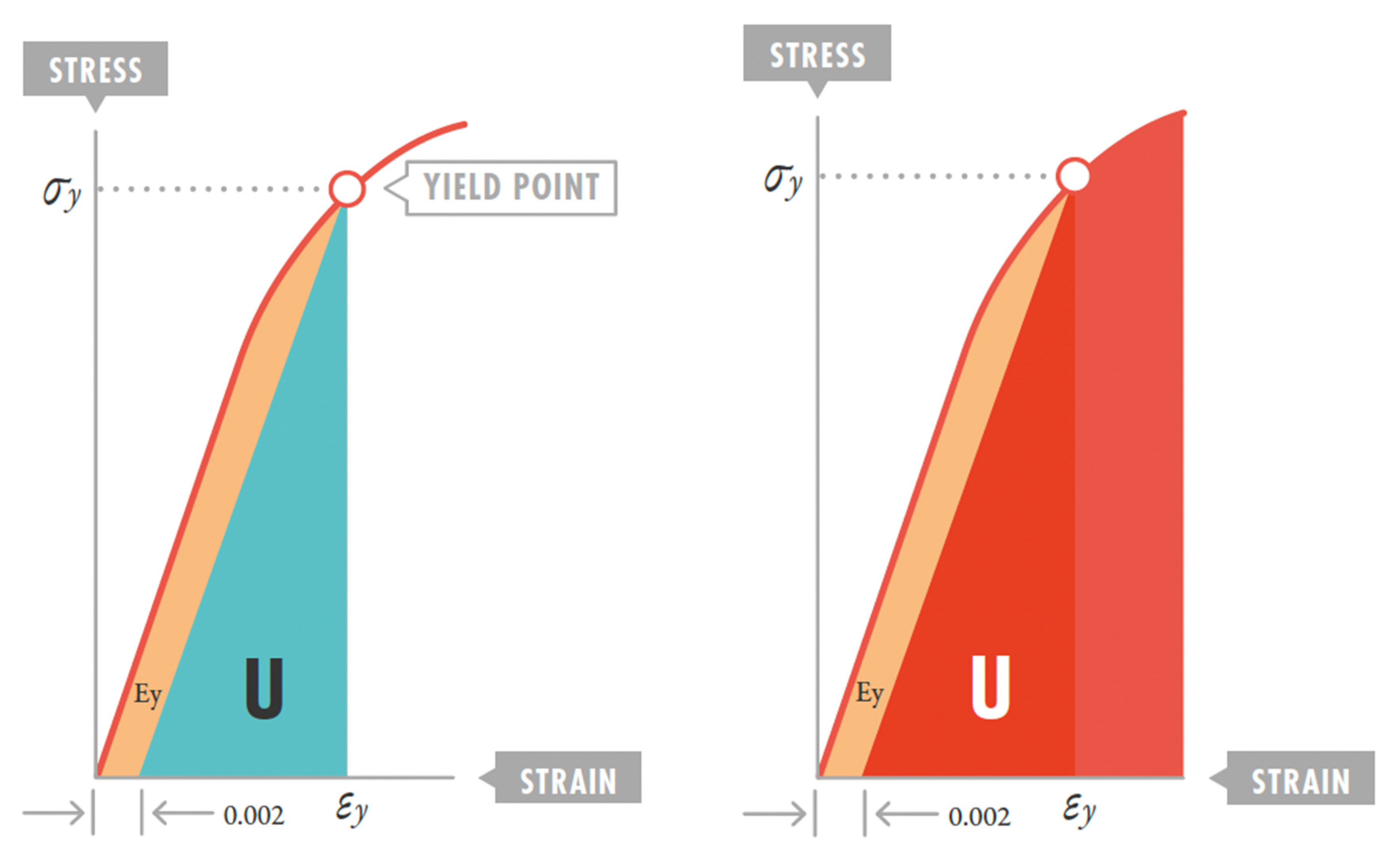
3.1. The Lung as an Elastic Solid
3.2. Strain Threshold and VILI Development
3.3. Stress and Strain Estimation with the Ventilator
3.4. Mechanical Power and Injury Threshold
- Giosa et al.’s [24] surrogate method: Proposed a more comprehensive calculation for volume-controlled ventilation, incorporating resistive and elastic components.MP = 4 × DP × RR.MP: mechanical power; RR: respiratory rate; DP: driving pressure.
- Becher et al.’s [25] method: Adapted calculations for pressure-controlled ventilation, emphasizing that MP estimates differ by mode.MP = 0.098 × RR × VT × [PEEP + (DP × ((Ti/60 × RR) + 1))/2]
4. Ventilatory Strategy in ARDS from a Rheological Perspective
4.1. Mechanical Power Adjustment by Ideal Weight or Compliance
- It was derived primarily from studies in animal models with homogeneous anatomy/structural features.
- Body size differences between patients were not accounted for.
- Differences in the proportion of functional “baby lung” available in each ARDS case were not adjusted.
- Differences in lung compliance between patients were not factored in.
- Normalized MP (J/min/kg) = Total MP (J/min)/Ideal weight (kg).
- Specific MP (J/min/L) = Total MP (J/min)/Static compliance (L/cmH2O)
- Patients with severe ARDS (lower compliance) would require lower absolute MP thresholds.
- Patients with preserved compliance could tolerate higher absolute MP values.
- Ventilatory strategy could be dynamically adjusted according to compliance evolution.
- It would allow more precise comparisons between patients with different characteristics.
- Clinical studies validating specific normalized MP thresholds.
- Practical methods to estimate baby lung volume at bedside.
- Algorithm development to automatically adjust ventilatory parameters according to normalized MP.
- Validation in specific populations (pediatric, obese, etc.).
4.2. VILI Development Dynamics and Recruitment. Optimal PEEP
- Hemodynamic tolerance assessment.
- Individual recruitability testing (P/F ratio response).
- Chest wall mechanics and intra-abdominal pressure.
- Underlying cardiac function.
- Real-time monitoring of compliance and driving pressure.
4.3. Importance of Respiratory Rate
4.4. Importance of Flow
4.5. Importance of Ventilatory Mode
- Late initiation timing (after conventional ventilation failure).
- Patient selection bias (most severe cases).
- Suboptimal HFOV settings in some centers.
- Learning curve effects in participating institutions.
4.6. Tidal Volume and Driving Pressure
4.7. Resilience Implications in ARDS Ventilatory Strategy
Clinical Implications of Resilience
- Safe energy threshold:
- 2.
- Personalization of mechanical ventilation:
- 3.
- VILI prevention:
- 4.
- PEEP optimization:
- 5.
- Permissive hypercapnia management:
- 6.
- Prone position ventilation:
- 7.
- Monitoring and biomarkers:
- 8.
- Non-invasive ventilation applications:
4.8. Self-Inflicted Lung Injury (SILI)
- Neuromuscular blockade in early severe ARDS (strong recommendation, high-quality evidence):
- Adequate sedation to minimize excessive respiratory drive (conditional recommendation, moderate-quality evidence).
- -
- Reduces metabolic demand and respiratory effort.
- -
- Must balance with delirium prevention.
- Ventilatory mode selection to minimize patient-ventilator asynchrony (Expert consensus).
- Esophageal pressure monitoring in selected cases (Expert consensus).
- Early consideration of ECMO in refractory cases (Conditional recommendation, Low-quality evidence).
5. Rheological Model Limitations
5.1. Regional Lung Variability Not Captured by the Model
5.2. Interaction Between Mechanical Ventilation and Inflammation Not Completely Explained
5.3. Challenges for Determining the “Baby Lung” Precisely at the Patient Bedside
6. Conclusions
- DP should be maintained below 15 cmH2O to avoid exceeding the lung elastic limit.
- Total MP should be limited to less than 12 J/min, adjusting tidal volume, respiratory rate, and flow.
- PEEP should be optimized to homogenize parenchyma and prevent stress multiplier formation.
- Inspiratory and expiratory flow control can reduce strain rate and minimize the viscous component of lung damage.
- In patients with spontaneous breathing, the additional effect of respiratory effort on total mechanical power should be considered, justifying muscle relaxant use in the most severe phases of disease.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mead, J.; Takishima, T.; Leith, D. Stress distribution in lungs: A model of pulmonary elasticity. J. Appl. Physiol. 1970, 28, 596–608. [Google Scholar] [CrossRef]
- Modesto I Alapont, V.; Carrascosa, M.A.; Villanueva, A.M. Stress, strain and mechanical power: Is material science the answer to prevent ventilator induced lung injury? Med. Intensiv. (Engl. Ed.) 2019, 43, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Modesto I Alapont, V.; Villanueva, A.M.; Carrascosa, M.A.; Díez García, P.; Nahir, N.N.U.; Calderini, E.; Cristopherson, M. Stress, strain y potencia mecánica. La ciencia para prevenir la lesión inducida por el ventilador (VILI). In Manual de Ventilación Mecánica Pediátrica y Neonatal, 6th ed.; Apéndice 1, e-book; Tesela Ediciones: Madrid, Spain, 2022. [Google Scholar]
- Lin, Y.H. Polymer Viscoelasticity: Basics, Molecular Theories and Simulations, 2nd ed.; World Scientific Publishing Co., Inc.: Singapore, 2011. [Google Scholar]
- Pilkey, W.D. Formulas for Stress, Strain and Structural Matrices, 2nd ed.; John, Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Cortés-Puentes, G.A.; Keenan, J.C.; Adams, A.B.; Parker, E.D.; Dries, D.J.; Marini, J.J. Impact of chest wall modifications and lung injury on the correspondence between airway and transpulmonary driving pressure. Crit. Care Med. 2015, 43, e287–e295. [Google Scholar] [CrossRef]
- Chiumello, D.; Carlesso, E.; Brioni, M.; Cressoni, M. Airway driving pressure and lung stress in ARDS patients. Crit. Care 2016, 20, 276. [Google Scholar] [CrossRef]
- Gattinoni, L.; Tonetti, T.; Cressoni, M.; Cadringher, P.; Herrmann, P.; Moerer, O.; Protti, A.; Gotti, M.; Chiurazzi, C.; Carlesso, E.; et al. Ventilator-related causes of lung injury: The mechanical power. Intensive Care Med. 2016, 42, 1567–1575. [Google Scholar] [CrossRef]
- Dreyfuss, D.; Saumon, G. Ventilator-induced lung injury: Lessons from experimental studies. Am. J. Respir. Crit. Care Med. 1998, 157, 294–323. [Google Scholar] [CrossRef]
- Acute Respiratory Distress Syndrome Network; Brower, R.G.; Matthay, M.A.; Morris, A.; Schoenfeld, D.; Thompson, T.B.; Wheeler, A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1301–1308. [Google Scholar]
- Amato, M.B.P.; Barbas, C.S.V.; Medeiros, D.M.; de P Schettino, G.; Filho, G.L.; Kairalla, R.A.; Deheinzelin, D.; Morais, C.; de O Fernandez, E.; Takagaki, T.Y.; et al. Beneficial effects of the “open lung approach” with low distending pressures in acute respiratory distress syndrome: A prospective randomized study on mechanical ventilation. Am. J. Respir. Crit. Care Med. 1995, 152, 1835–1846. [Google Scholar] [CrossRef]
- Slutsky, A.; Tremblay, L. Multiple system organ failure: Is mechanical ventilation a contributing factor? Am. J. Respir. Crit. Care Med. 1998, 157, 1721–1725. [Google Scholar] [CrossRef] [PubMed]
- Slutsky, A.S.; Ranieri, V.M. Ventilator-induced lung injury. N. Engl. J. Med. 2013, 369, 2126–2136. [Google Scholar] [CrossRef] [PubMed]
- González-López, A.; García-Prieto, E.; Batalla-Solís, E.; Amado-Rodríguez, L.; Avello, N.; Blanch, L.; Muñíz-Albaiceta, G. Lung strain and biological response in mechanically ventilated patients. Intensive Care Med. 2012, 38, 240–247. [Google Scholar] [CrossRef]
- Rahaman, U. Mathematics of ventilator-induced lung injury. Indian J. Crit. Care Med. 2017, 21, 521–524. [Google Scholar] [CrossRef]
- Laffey, J.G.; Bellani, G.; Pham, T.; Fan, E.; Madotto, F.; Bajwa, E.K.; Brochard, L.; Clarkson, K.; Esteban, A.; Gattinoni, L.; et al. Potentially modificable factors contributing to outcome from acute respiratory distress syndrome: The LUNG SAFE study. Intensive Care Med. 2016, 42, 1865–1876. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.B.P.; Meade, M.O.; Slutsky, A.S.; Brochard, L.; Costa, E.L.V.; Schoenfeld, D.A.; Stewart, T.E.; Briel, M.; Talmor, D.; Mercat, A.; et al. Driving pressure and survival in the Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2015, 372, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Protti, A.; Votta, E.; Gattinoni, L. Which is the most important strain in the pathogenesis of ventilator-induced lung injury: Dynamic or static? Curr. Opin. Crit. Care 2014, 20, 33–38. [Google Scholar] [CrossRef]
- Gattinoni, L.; Carlesso, E.; Cadringher, P.; Caironi, P.; Valenza, F.; Polli, F.; Tallarini, F.; Cozzi, P.; Cressoni, M.; Colombo, A.; et al. Lung stress and strain during mechanical ventilation for acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2008, 178, 346–355. [Google Scholar] [CrossRef]
- Protti, A.; Cressoni, M.; Santini, A.; Langer, T.; Mietto, C.; Febres, D.; Chierichetti, M.; Coppola, S.; Conte, G.; Gatti, S.; et al. Lung stress and strain during mechanical ventilation: Any safe threshold? Am. J. Respir. Crit. Care Med. 2011, 183, 1354–1362. [Google Scholar] [CrossRef]
- Chiumello, D.; Chidini, G.; Calderini, E.; Colombo, A.; Crimella, F.; Brioni, M. Respiratory mechanics and lung stress/strain in children with acute respiratory distres syndrome. Ann. Intensive Care 2016, 6, 11. [Google Scholar] [CrossRef]
- Gibson, C.; Roberts, F. Anaesthesia data. In Oxford Handbook of Anesthesia, 4th ed.; Allman, K., Ed.; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Cressoni, M.; Chiurazzi, C.; Gotti, M.; Amini, M.; Brioni, M.; Algieri, I.; Cammaroto, A.; Rovati, C.; Massari, D.; Bacile di Castiglione, C.; et al. Lung inhomogeneities and time-course of ventilator-induced mechanical injuries. Anesthesiology 2015, 123, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Giosa, L.; Busana, M.; Pasticci, I.; Bonifazi, M.; Macri, M.M.; Romitti, F.; Vassalli, F.; Chiumello, D.; Quintel, M.; Marini, J.J.; et al. Mechanical power at a glance: A simple surrogate for volume-controlled ventilation. Intensive Care Med. Exp. 2019, 7, 61. [Google Scholar] [CrossRef]
- Becher, T.; van der Staay, M.; Schädler, D.; Frerichs, I.; Weiler, N. Calculation of mechanical power for pressure-controlled ventilation. Intensive Care Med. 2019, 45, 1321–1323. [Google Scholar] [CrossRef] [PubMed]
- Neto, A.S.; Deliberato, R.O.; Johnson, A.E.W.; Bos, L.D.; Amorim, P.; Moreto, P.S.; Carnieli, C.D.; Cordioli, R.L.; Domingos Correa, T.; Pollard, T.J.; et al. Mechanical power of ventilation is associated with mortality in critically ill patients: An analysis of patients in two observational cohorts. Intensive Care Med. 2018, 44, 1914–1922. [Google Scholar] [CrossRef]
- Coppola, S.; Caccioppola, A.; Froio, S.; Formenti, P.; De Giorgis, V.; Galanti, V.; Consonni, D.; Chiumello, D. Effect of mechanical power on intensive care mortality in ARDS patients. Crit. Care 2020, 24, 246. [Google Scholar] [CrossRef]
- Costa, E.L.V.; Slutsky, A.S.; Brochard, L.J.; Brower, R.; Serpa-Neto, A.; Cavalcanti, A.B.; Mercat, A.; Meade, M.; Morais, C.C.A.; Goligher, E.; et al. Ventilatory variables and mechanical power in patients with acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2021, 204, 303–311. [Google Scholar] [CrossRef]
- Ito, Y.; Takeuchi, M.; Inata, Y.; Kyogoku, M.; Hotz, J.C.; Bhalla, A.K.; Newth, C.J.L.; Khemani, R.G. Normalization to Predicted Body Weight May Underestimate Mechanical Energy in Pediatric Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2022, 205, 1360–1363. [Google Scholar] [CrossRef]
- Tonetti, T.; Vasques, F.; Rapetti, F.; Maiolo, G.; Collino, F.; Romitti, F.; Camporota, L.; Cressoni, M.; Cadringher, P.; Quintel, M.; et al. Driving pressure and mechanical power: New targets for VILI prevention. Ann. Transl. Med. 2017, 5, 286. [Google Scholar] [CrossRef]
- Ilia, S.; Geromarkaki, E.; Briassoulis, P.; Bourmpaki, P.; Tavladaki, T.; Miliaraki, M.; Briassoulis, G. Effects of increasing PEEP on lung stress and strain in children with and without ARDS. Intensive Care Med. 2019, 45, 1315–1317. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Marini, J.J.; Pesenti, A.; Quintel, M.; Mancebo, J.; Brochard, L. The “baby lung” became an adult. Intensive Care Med. 2016, 42, 663–675. [Google Scholar] [CrossRef]
- Ilia, S.; Geromarkaki, E.; Briassoulis, P.; Bourmpaki, P.; Tavladaki, T.; Miliaraki, M.; Briassolis, G. Longitudinal PEEP Responses Differ Between Children with ARDS and at Risk for ARDS. Respir. Care 2021, 66, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Chiumello, D.; Gotti, M.; Guanziroli, M.; Formenti, M.; Pasticci, I.; Mistraletti, G.; Busana, M. Bedside calculation of mechanical power during volume- and pressure-controlled mechanical ventilation. Crit. Care 2020, 24, 417. [Google Scholar] [CrossRef]
- Protti, A.; Andreis, D.T.; Monti, M.; Santini, A.; Sparacino, C.C.; Langer, T.; Votta, E.; Gatt, S.; Lombardi, L.; Leopardi, O.; et al. Lung stress and strain during mechanical ventilation: Any difference between statics and dynamics? Crit. Care Med. 2013, 41, 1046–1055. [Google Scholar] [CrossRef]
- Modesto I Alapont, V.; Aguar-Carrascosa, M.; Medina Villanueva, A. Clinical implications of the rheological theory in the prevention of ventilator-induced lung injury. Is mechanical power the solution? Med. Intensiv. 2019, 43, 373–381. [Google Scholar] [CrossRef]
- Gordo-Vidal, F.; Gómez-Tello, V.; Palencia-Herrejón, E.; Latour-Pérez, J.; Sánchez-Artola, B.; Díaz-Alersi, R. PEEP alta frente a PEEP convencional en el síndrome de distrés respiratorio agudo. Revisión sistemática y metaanálisis. Med. Intensiv. 2007, 31, 491–501. [Google Scholar] [CrossRef]
- Farías, J.A.; Frutos, F.; Esteban, A.; Casado Flores, J.; Retta, A.; Baltodano, A.; Alía, I.; Hatzis, T.; Olazarri, F.; Petros, A.; et al. What is the daily practice of mechanical ventilation in pediatric intensive care units? A multicenter study. Intensive Care Med. 2004, 30, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.; Barbas, C.S.V.; Medeiros, D.M.; Magaldi, R.B.; Schettino, G.P.; Lorenzi Filho, G.; Kairalla, R.A.; Deheinzelin, D.; Muñoz, C.; Oliveira, R.; et al. Effect of a Protective-Ventilation Strategy on Mortality in the Acute Respiratory Distress Syndrome. N. Engl. J. Med. 1998, 338, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, V.M.; Suter, P.M.; Tortorella, C.; De Tullio, R.; Dayer, J.M.; Brienza, A.; Bruno, F.; Slutsky, A.S. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: A randomized controlled trial. JAMA 1999, 282, 54–61. [Google Scholar] [CrossRef]
- Villar, J.; Kacmarek, R.M.; Pérez-Méndez, L.; Aguirre-Jaime, A. A high positive end- expiratory pressure, low tidal volume ventilatory syndrome: A randomized, controlled trial. Crit. Care Med. 2006, 34, 1311–1318. [Google Scholar] [CrossRef]
- Caramez, M.P.; Kacmarek, R.M.; Helmy, M.; Miyoshi, E.; Malhotra, A.; Amato, M.B.P.; Scott Harris, R. A comparison of methods to identify open-lung PEEP. Intensive Care Med. 2009, 35, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Modesto I Alapont, V.; Villanueva, A.M.; Del Villar Guerra, P.; Camilo, C.; Fernández-Ureña, S.; Gordo-Vidal, F.; Khemani, R. OLA strategy for ARDS: Its effect on mortality depends on achieved recruitment (PaO2/FIO2) and mechanical power. Systematic review and meta-analysis with meta-regression. Med. Intensiv. (Engl. Ed.) 2021, 45, 516–531. [Google Scholar] [CrossRef]
- Brower, R.G.; Morris, A.; Maclntyre, N.; Matthay, M.A.; Hayden, D.; Thompson, T.; Clemmer, T.; Lanken, P.N.; Schoenfeld, D.; ARDS Clinical Trials Network; et al. Effects of recruitment maneuvers in patients with acute lung injury and acute respiratory distress syndrome ventilated with high positive end-expiratory pressure. Crit. Care Med. 2003, 31, 2592–2597. [Google Scholar] [CrossRef]
- Meade, M.O.; Cook, D.J.; Guyatt, G.H.; Slutsky, A.S.; Arabi, Y.M.; Cooper, D.J.; Davies, A.R.; Hand, L.E.; Zhou, Q.; Thabane, L.; et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: A randomized controlled trial. JAMA 2008, 299, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Mercat, A.; Richard, J.C.; Vielle, B.; Jaber, S.; Osman, D.; Diehl, J.L.; Lefrant, J.Y.; Prat, G.; Richecoeur, J.; Nieszkowska, A.; et al. Expiratory Pressure (Express) Study Group. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: A randomized controlled trial. JAMA 2008, 299, 646–655. [Google Scholar] [CrossRef]
- Briel, M.; Meade, M.; Mercat, A.; Brower, R.G.; Talmor, D.; Walter, S.D.; Slutsky, A.S.; Pullenayegum, E.; Zhou, Q.; Cook, D.; et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: Systematic review and meta-analysis. JAMA 2010, 303, 865–873. [Google Scholar] [CrossRef]
- Putensen, C.; Theuerkauf, N.; Zinserling, J.; Wrigge, H.; Pelosi, P. Meta-analysis: Ventilation strategies and outcomes of the acute respiratory distress syndrome and acute lung injury. Ann. Intern. Med. 2009, 151, 566–576. [Google Scholar] [CrossRef]
- Hotchkiss, J.R.; Blanch, L.; Murias, G.; Adams, A.B.; Olson, D.A.; Wangensteen, O.D.; Leo, P.H.; Marini, J.J. Effects of decreased respiratory frequency on ventilator-induced lung injury. Am. J. Respir. Crit. Care Med. 2000, 161, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Retamal, J.; Borges, J.B.; Bruhn, A.; Feinstein, R.; Hedenstierra, G.; Suarez-Sipmann, F.; Larsson, A. Open lung approach ventilation abolishes the negative effects of respiratory rate in experimental lung injury. Acta Anaesthesiol. Scand. 2016, 60, 1131–1141. [Google Scholar] [CrossRef]
- Protti, A.; Maraffi, T.; Milesi, M.; Votta, E.; Santini, A.; Pugni, P.; Andreis, D.T.; Nocosia, F.; Zannin, E.; Gatti, S.; et al. Role of Strain rate in the pathogenesis of Ventilator-Induced Lung Edema. Crit. Care Med. 2016, 44, e838–e845. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Fujino, Y.; Uchiyama, A.; Matsuura, N.; Mashimo, T.; Nishimura, M. Effects of peak inspiratory flow on development of ventilator-induced lung injury in rabbits. Anesthesiology 2004, 101, 722–728. [Google Scholar] [CrossRef]
- Fujita, Y.; Maeda, Y.; Fujino, Y.; Uchiyama, A.; Mashimo, T.; Nishimura, M. Effect of peak inspiratory flow on gas echange, pulmonary mechaniccs and lung histology in rabbits with injured lungs. J. Anesth. 2006, 20, 96–101. [Google Scholar] [CrossRef]
- Fujita, Y.; Fujino, Y.; Uchiyama, A.; Mashimo, T.; Nishimura, M. High peak inspiratory flow can aggravate ventilator-induced lung injury. Med. Sci. Monit. 2007, 13, BR95–BR100. [Google Scholar]
- Schmidt, J.; Wenzel, C.; Spassov, S.; Borgmann, S.; Lin, Z.; Wollborn, J.; Weber, J.; Haberstroh, J.; Meckel, S.; Eiden, S.; et al. Flow-controlled ventilation attenuates lung injury in a porcine model of acute respiratory distress syndrome: A preclinical randomized controlled study. Crit. Care Med. 2020, 48, e241–e248. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, N.D.; Cook, D.J.; Guyatt, G.H.; Mehta, S.; Hand, L.; Austin, P.; Zhou, Q.; Matte, A.; Walter, S.D.; Lamontagne, F.; et al. High-frequency oscillation in early acute respiratory distress syndrome. N. Engl. J. Med. 2013, 368, 795–805. [Google Scholar] [CrossRef]
- Young, D.; Lamb, S.E.; Shah, S.; MacKenzie, I.; Tunnicliffe, W.; Lall, R.; Rowan, K.; Cuthbertson, B.H.; OSCAR Study Group. High-frequency oscillation for acute respiratory distress syndrome. N. Engl. J. Med. 2013, 368, 806–813. [Google Scholar] [CrossRef]
- Villar, J.; Kacmarek, R.M.; Hedenstierna, G. From ventilator-induced lung injury to physician-induced lung injury: Why the reluctance to use small tidal volumes? Acta Anaesthesiol. Scand. 2004, 48, 267–271. [Google Scholar] [CrossRef]
- Slutsky, A.S. Consensus conference on mechanical ventilation January 28–30, 1993 at Northbrook, Illinois, USA. Part II. Intensive Care Med. 1994, 20, 150–162. [Google Scholar] [CrossRef]
- Eisner, M.D.; Thompson, T.; Hudson, L.D.; Luce, J.M.; Hayden, D.; Schoenfeld, D.; Matthay, M.A.; Acute Respiratory Distress Syndrome Network. Efficacy of low tidal volume ventilation in patients with different clinical risk factors for acute lung injury and the acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2001, 164, 231–236. [Google Scholar] [CrossRef]
- Fan, E.; Del Sorbo, L.; Goligher, E.C.; Hodgson, C.L.; Munshi, L.; Wakey, A.J.; Adhikari, N.K.J.; Amato, M.B.P.; Branson, R.; Brower, R.G.; et al. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical Ventilation in Adult Patients with Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2017, 195, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Emeriaud, G.; López-Fernández, Y.M.; Iyer, N.P.; Bembea, M.M.; Agulnik, A.; Barbaro, R.P.; Baudin, F.; Bhalla, A.; Brunow de Carvalho, W.; Carroll, C.L.; et al. Executive Summary of the Second International Guidelines for the Diagnosis and Management of Pediatric Acute Respiratory Distress Syndrome (PALICC-2). Pediatr. Crit. Care Med. 2023, 24, 143–168. [Google Scholar] [CrossRef] [PubMed]
- Kneyber, M.C.J.; de Luca, D.; Calderini, E.; Jarreau, P.H.; Javouhey, E.; López Herce, J.; Hammer, J.; Macrae, D.; Markhorst, D.G.; Medina, A.; et al. Recommendations for mechanical ventilation of critically ill children from the Paediatric Mechanical Ventilation Consensus Conference (PEMVECC). Intensive Care Med. 2017, 43, 1764–1780. [Google Scholar] [CrossRef]
- Lista, G.; Castoldi, F.; Fontana, P.; Reali, R.; Reggiani, A.; Bianchi, S.; Compagnoni, G. Lung inflammation in preterm infants with respiratory distress syndrome: Effects of ventilation with different tidal volumes. Pediatr. Pulmonol. 2006, 41, 357–363. [Google Scholar] [CrossRef]
- Eichacker, P.Q.; Gerstenberger, E.P.; Banks, S.M.; Cui, X.; Natanson, C. Meta-analysis of acute lung injury and acute respiratory distress syndrome trials testing low tidal volumes. Am. J. Respir. Crit. Care Med. 2002, 166, 1510–1514. [Google Scholar] [CrossRef]
- Gillies, D.; Wells, D.; Bhandari, A.P. Positioning for acute respiratory distress in hospitalised infants and children. Cochrane Database Syst. Rev. 2012, 7, CD003645. [Google Scholar] [CrossRef]
- Rotta, A.T.; Gunnarsson, B.; Fuhrman, B.P.; Hernan, L.J.; Steinhorn, D.M. Comparison of lung protective ventilation strategies in a rabbit model of acute lung injury. Crit. Care Med. 2001, 29, 2176–2184. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.R.; Barbas, C.S.; Medeiros, D.M.; Magaldi, R.B.; Lorenzi Filho, G.; Kairalla, R.A.; Deheinzelin, D.; Muñoz, C.; Kaufmann, M.; Ferreira, M.; et al. Temporal hemodynamic effects of permissive hypercapnia associated with ideal PEEP in ARDS. Am. J. Respir. Crit. Care Med. 1997, 156, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
- Marini, J.J.; Rocco, P.R.M.; Gattinoni, L. Static and Dynamic Contributors to Ventilator-induced Lung Injury in Clinical Practice. Pressure, Energy, and Power. Am. J. Respir. Crit. Care Med. 2020, 201, 767–774. [Google Scholar] [CrossRef]
- Cressoni, M.; Gotti, M.; Chiurazzi, C.; Massari, D.; Algieri, I.; Amini, M.; Cammaroto, A.; Brioni, M.; Montaruli, C.; Niklla, K. Mechanical Power and development of Ventilator-induced Lung Injury. Anesthesiology 2016, 124, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Bellani, G.; Grassi, A.; Sosio, S.; Gatti, S.; Kavanagh, B.P.; Pesenti, A.; Foti, G. Driving pressure is associated with outcome during assisted ventilation in acute respiratory distress syndrome. Anesthesiology 2019, 131, 594–604. [Google Scholar] [CrossRef]
- Fanelli, V.; Ranieri, M.V.; Mancebo, J.; Moerer, O.; Quintel, M.; Morley, S.; Moran, I.; Parrilla, F.; Costamagna, A.; Gaudiosi, M.; et al. Feasibility and safety of low-flow extracorporeal carbon dioxide removal to facilitate ultra-protective ventilation in patients with moderate acute respiratory distress syndrome. Crit. Care 2016, 20, 36. [Google Scholar] [CrossRef]
- Brochard, L.; Slutsky, A.; Pesenti, A. Mechanical Ventilation to Minimize Progression of Lung Injury in Acute Respiratory Failure. Am. J. Respir. Crit. Care Med. 2017, 195, 438–442. [Google Scholar] [CrossRef]
- Chiumello, D.; Pristine, G.; Slutsky, A.S. Mechanical ventilation affects local and systemic cytokines in an animal model of acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1999, 160, 109–116. [Google Scholar] [CrossRef]
- Beitler, J.R.; Sands, S.A.; Loring, S.H.; Owens, R.L.; Malhotra, A.; Spragg, R.G.; Matthay, M.A.; Thompson, B.T.; Talmor, D. Quantifying unintended exposure to high tidal volumes from breath stacking dyssynchrony in ARDS: The BREATHE criteria. Intensive Care Med. 2016, 42, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Papazian, L.; Forel, J.M.; Gacouin, A.; Penot-Ragon, C.; Perrin, G.; Loundou, A.; Jaber, S.; Arnal, J.M.; Perez, D.; Seghboyan, J.M.; et al. Neuromuscular blockers in early acute respiratory distress syndrome. N. Engl. J. Med. 2010, 363, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.T.N.; Patolia, S.; Guervilly, C. Neuromuscular blockade in acute respiratory distress syndrome: A systematic review and meta-analysis of randomized controlled trials. J. Intensive Care 2020, 8, 12. [Google Scholar] [CrossRef] [PubMed]


| Concept | Physical Definition | Pulmonary Application | Units |
|---|---|---|---|
| Stress | Force per unit area (f/A) | Transpulmonary pressure (PTP) | cmH2O |
| Strain | Relative deformation (dX − dX0)/dX0 | Tidal volume/Functional residual capacity (VT/FRC) | Dimensionless |
| Strain rate | Deformation velocity | Flow/FRC | s−1 |
| Young’s Modulus (EY) | Proportionality constant between stress and strain | Specific lung elastance (ESL) | cmH2O |
| Driving pressure (DP) | Difference between plateau pressure and PEEP | Clinical approximation to pulmonary stress | cmH2O |
| Mechanical power (MP) | Energy per unit time | Energy delivered to the respiratory system per minute | J/min |
| Resilience | Maximum energy storable without permanent deformation | Energy threshold to prevent VILI | J/m3 |
| Key Clinical Implications: Mechanical Power (MP) Normalization |
|---|
| Traditional approach
Absolute MP threshold: 12 J/min (derived from animal studies).│
│• Limitation: Does not account for patient size or lung function. |
Proposed normalizations:
|
Clinical impact:
|
| Evidence level: Preliminary. Requires prospective validation. |
| Parameter | Recommendation | Rheological Justification |
|---|---|---|
| Driving pressure (DP) | <15 cmH2O | Maintains strain < 1 (elastic limit) |
| Tidal volume | Adjusted for DP < 15 cmH2O Adjusted for Pplat = 28–32 cmH2O | Limits stress and strain |
| PEEP | PEEP titration to maximize homogeneity and recover pulmonary FRC | Reduces stress multipliers Reduces strain Reduces strain rate |
| Respiratory rate | Lowest possible allowing adequate ventilation | Limits mechanical power |
| Inspiratory flow | Moderate, avoiding high peaks | Reduces strain rate |
| Mechanical power | <12 J/min | Below the injury threshold |
| Inspiratory time | Prolonged (lower flow) | Reduces strain rate |
| Flow pattern | Constant and square | Optimizes stress distribution Decreases the strain rate |
| Expiratory flow control | Consider if available | Reduces expiratory strain rate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina, A.; del Villar Guerra, P.; Valle Ortiz, J.R.; Modesto I Alapont, V. Rheological Theory Applied to Mechanical Ventilation in Acute Respiratory Distress Syndrome: A New Paradigm for Understanding and Preventing Ventilator-Induced Lung Injury. J. Clin. Med. 2025, 14, 6544. https://doi.org/10.3390/jcm14186544
Medina A, del Villar Guerra P, Valle Ortiz JR, Modesto I Alapont V. Rheological Theory Applied to Mechanical Ventilation in Acute Respiratory Distress Syndrome: A New Paradigm for Understanding and Preventing Ventilator-Induced Lung Injury. Journal of Clinical Medicine. 2025; 14(18):6544. https://doi.org/10.3390/jcm14186544
Chicago/Turabian StyleMedina, Alberto, Pablo del Villar Guerra, Juan Ramón Valle Ortiz, and Vicent Modesto I Alapont. 2025. "Rheological Theory Applied to Mechanical Ventilation in Acute Respiratory Distress Syndrome: A New Paradigm for Understanding and Preventing Ventilator-Induced Lung Injury" Journal of Clinical Medicine 14, no. 18: 6544. https://doi.org/10.3390/jcm14186544
APA StyleMedina, A., del Villar Guerra, P., Valle Ortiz, J. R., & Modesto I Alapont, V. (2025). Rheological Theory Applied to Mechanical Ventilation in Acute Respiratory Distress Syndrome: A New Paradigm for Understanding and Preventing Ventilator-Induced Lung Injury. Journal of Clinical Medicine, 14(18), 6544. https://doi.org/10.3390/jcm14186544





