Hemoadsorption in Children with Cytokine Storm Using the Jafron HA330 and HA380 Cartridges
Abstract
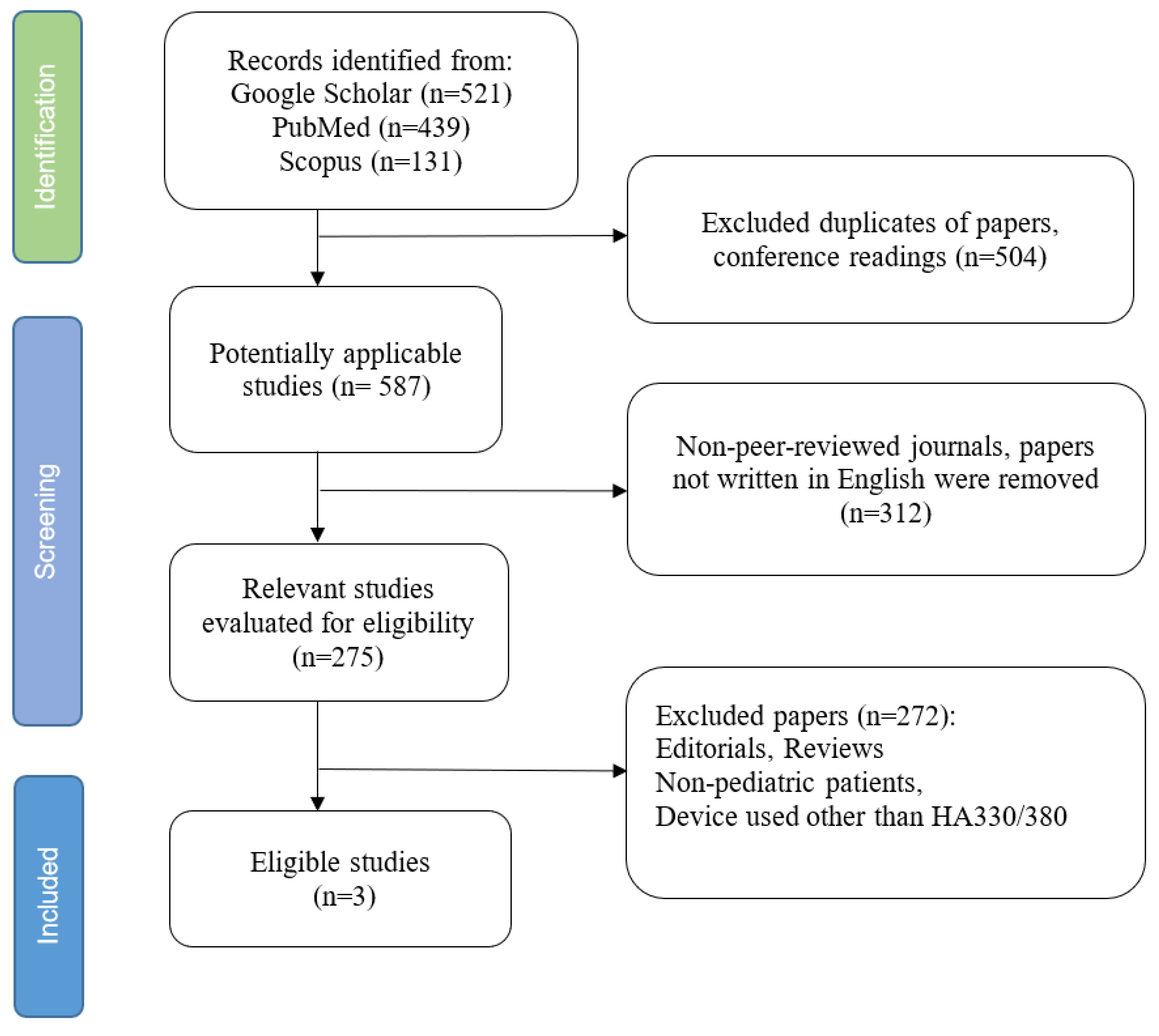
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
4.1. Main Findings
4.2. Interpretive Considerations and Clinical Nuances
4.3. Safety Considerations in Pediatrics
4.4. Limitations and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALL | Acute Lymphoblastic Leukemia |
| ALT | Alanine Aminotransferase |
| AML | Acute Myeloid Leukemia |
| AST | Aspartate Aminotransferase |
| BIS | Bispectral Index |
| CARE | CAse REport (reporting checklist) |
| CPB | Cardiopulmonary Bypass |
| COVID-19 | Coronavirus Disease 2019 |
| CRP | C-Reactive Protein |
| CRRT | Continuous Renal Replacement Therapy |
| CRS | Cytokine Release Syndrome |
| CVVHDF | Continuous Venovenous Hemodiafiltration |
| ECMO | Extracorporeal Membrane Oxygenation |
| FFP | Fresh Frozen Plasma |
| GI | Gastrointestinal |
| GGT | Gamma-Glutamyl Transferase |
| HIT | Heparin-Induced Thrombocytopenia |
| IL-1 | Interleukin-1 |
| IL-6 | Interleukin-6 |
| IQR | Interquartile Range |
| JBI | Joanna Briggs Institute |
| LOS | Length of Stay |
| MV-LOS | Mechanical Ventilation–Length of Stay |
| NOS | Newcastle–Ottawa Scale |
| NSS | Normal Saline Solution |
| PCT | Procalcitonin |
| PELOD-2 | Pediatric Logistic Organ Dysfunction-2 |
| PICU | Pediatric Intensive Care Unit |
| PICU-LOS | Pediatric Intensive Care Unit–Length of Stay |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PRISM-3 | Pediatric Risk of Mortality-3 |
| pSOFA | Pediatric Sequential Organ Failure Assessment |
| PSS | Phoenix Sepsis Score |
| RBC | Red Blood Cell |
| RASS | Richmond Agitation–Sedation Scale |
| RCTs | Randomized Controlled Trials |
| ROBINS-I | Risk Of Bias In Non-Randomized Studies of Interventions |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| SD | Standard Deviation |
| SIRS | Systemic Inflammatory Response Syndrome |
| TNF-α | Tumor Necrosis Factor-alpha |
| TDM | Therapeutic Drug Monitoring |
| VIS | Vasoactive Inotropic Score |
References
- Zanza, C.; Romenskaya, T.; Manetti, A.C.; Franceschi, F.; La Russa, R.; Bertozzi, G.; Maiese, A.; Savioli, G.; Volonnino, G.; Longhitano, Y. Cytokine Storm in COVID-19: Immunopathogenesis and Therapy. Medicina 2022, 58, 144. [Google Scholar] [CrossRef]
- Chousterman, B.G.; Swirski, F.K.; Weber, G.F. Cytokine storm and sepsis disease pathogenesis. Semin. Immunopathol. 2017, 39, 517–528. [Google Scholar] [CrossRef]
- Jarczak, D.; Nierhaus, A. Cytokine Storm-Definition, Causes, and Implications. Int. J. Mol. Sci. 2022, 23, 11740. [Google Scholar] [CrossRef]
- Doganyigit, Z.; Eroglu, E.; Akyuz, E. Inflammatory mediators of cytokines and chemokines in sepsis: From bench to bedside. Hum. Exp. Toxicol. 2022, 41, 9603271221078871. [Google Scholar] [CrossRef]
- Pace Napoleone, C.; Aidala, E.; Cascarano, M.T.; Deorsola, L.; Iannandrea, S.; Longobardo, A.; Bonaveglio, E.; Zanin, M.; Peruzzi, L. Hemoadsorption Contribution in Failing Fontan Pediatric Heart Transplantation. Cardiorenal Med. 2024, 14, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.S.; Carrol, E.D.; Carter, M.J.; Kissoon, N.; Ranjit, S.; Schlapbach, L.J. The burden and contemporary epidemiology of sepsis in children. Lancet Child. Adolesc. Health 2024, 8, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.L.; Fitzgerald, J.C. Pediatric Sepsis Diagnosis, Management, and Sub-phenotypes. Pediatrics 2024, 153, e2023062967. [Google Scholar] [CrossRef]
- Schlapbach, L.J.; Watson, R.S.; Sorce, L.R.; Argent, A.C.; Menon, K.; Hall, M.W.; Akech, S.; Albers, D.J.; Alpern, E.R.; Balamuth, F.; et al. International Consensus Criteria for Pediatric Sepsis and Septic Shock. JAMA 2024, 331, 665–674. [Google Scholar] [CrossRef]
- Gharamti, A.A.; Samara, O.; Monzon, A.; Montalbano, G.; Scherger, S.; DeSanto, K.; Chastain, D.B.; Sillau, S.; Montoya, J.G.; Franco-Paredes, C.; et al. Proinflammatory cytokines levels in sepsis and healthy volunteers, and tumor necrosis factor-alpha associated sepsis mortality: A systematic review and meta-analysis. Cytokine 2022, 158, 156006. [Google Scholar] [CrossRef] [PubMed]
- Shimazui, T.; Oami, T.; Shimada, T.; Tomita, K.; Nakada, T.A. Age-dependent differences in the association between blood interleukin-6 levels and mortality in patients with sepsis: A retrospective observational study. J. Intensive Care 2025, 13, 3. [Google Scholar] [CrossRef]
- Saravi, B.; Goebel, U.; Hassenzahl, L.O.; Jung, C.; David, S.; Feldheiser, A.; Stopfkuchen-Evans, M.; Wollborn, J. Capillary leak and endothelial permeability in critically ill patients: A current overview. Intensive Care Med. Exp. 2023, 11, 96. [Google Scholar] [CrossRef]
- Matsumoto, H.; Ogura, H.; Shimizu, K.; Ikeda, M.; Hirose, T.; Matsuura, H.; Kang, S.; Takahashi, K.; Tanaka, T.; Shimazu, T. The clinical importance of a cytokine network in the acute phase of sepsis. Sci. Rep. 2018, 8, 13995. [Google Scholar] [CrossRef]
- Kumar, N.R.; Balraj, T.A.; Kempegowda, S.N.; Prashant, A. Multidrug-Resistant Sepsis: A Critical Healthcare Challenge. Antibiotics 2024, 13, 46. [Google Scholar] [CrossRef]
- Ronco, C.; Bellomo, R. Hemoperfusion: Technical aspects and state of the art. Crit. Care 2022, 26, 135. [Google Scholar] [CrossRef]
- Bellomo, R.; Ankawi, G.; Bagshaw, S.M.; Baldwin, I.; Basu, R.; Bottari, G.; Cantaluppi, V.; Clark, W.; De Rosa, S.; Forni, L.G.; et al. Hemoadsorption: Consensus report of the 30th Acute Disease Quality Initiative workgroup. Nephrol. Dial. Transplant. 2024, 39, 1945–1964. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Han, M.; Yang, M.; Su, B. Hemoperfusion with the HA330/HA380 Cartridge in Intensive Care Settings: A State-Of-The-Art Review. Blood Purif. 2025, 54, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Supady, A.; Brodie, D.; Wengenmayer, T. Extracorporeal haemoadsorption: Does the evidence support its routine use in critical care? Lancet Respir. Med. 2022, 10, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Lai, P.C.; Lee, T.H.; Huang, Y.T. Blood Purification for Adult Patients With Severe Infection or Sepsis/Septic Shock: A Network Meta-Analysis of Randomized Controlled Trials. Crit. Care Med. 2023, 51, 1777–1789. [Google Scholar] [CrossRef]
- Chiewroongroj, S.; Ratanarat, R.; Naorungroj, T.; Teeratakulpisarn, N.; Theeragul, S. Efficacy of additional hemoperfusion in hospitalized patients with severe to critical COVID-19 disease. Sci. Rep. 2024, 14, 17651. [Google Scholar] [CrossRef]
- Borankulova, A.; Sazonov, V. Hemadsorption with CytoSorb in Infants with Sepsis: Non-Systematic Review of Cases. J. Clin. Med. 2024, 13, 6808. [Google Scholar] [CrossRef]
- Sazonov, V.; Tobylbayeva, Z.; Saparov, A.; Jubaniyazov, B.; Issakov, S.; Gaipov, A. New Therapeutic Approach to Reduce Methotrexate Toxicity after High-Dose Chemotherapy in a Child with Acute Lymphocytic Leukemia: Efficacy and Safety of Hemoadsorption with HA-230 Adsorber. Blood Purif. 2022, 51, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Siripanadorn, T.; Samransamruajkit, R. The Role of Blood Purification by HA330 as Adjunctive Treatment in Children with Septic Shock. Blood Purif. 2023, 52, 549–555. [Google Scholar] [CrossRef]
- Ryazanova, D.; Tobylbayeva, Z.; Mironova, O.; Kakenov, E.; Sazonov, V. Comparison of CytoSorb and Jafron HA330 Hemoadsorption Devices in Pediatric Oncological Patients with Sepsis: Retrospective Observational Study. J. Clin. Med. 2024, 13, 7694. [Google Scholar] [CrossRef]
- Berkley, A.; Ferro, A. Changes in C-reactive protein in response to anti-inflammatory therapy as a predictor of cardiovascular outcomes: A systematic review and meta-analysis. JRSM Cardiovasc. Dis. 2020, 9, 2048004020929235. [Google Scholar] [CrossRef]
- Schupp, T.; Weidner, K.; Rusnak, J.; Jawhar, S.; Forner, J.; Dulatahu, F.; Dudda, J.; Bruck, L.M.; Hoffmann, U.; Bertsch, T.; et al. C-reactive protein and procalcitonin during course of sepsis and septic shock. Ir. J. Med. Sci. 2024, 193, 457–468. [Google Scholar] [CrossRef]
- Nurmykhametova, Z.; Lesbekov, T.; Kaliyev, R.; Bekishev, B.; Jabayeva, N.; Novikova, S.; Faizov, L.; Vakhrushev, I.; Pya, Y. Preliminary report of extracorporeal blood purification therapy in patients receiving LVAD: Cytosorb or Jafron HA330. J. Extra Corpor. Technol. 2024, 56, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Waalders, N.J.B.; Kox, M.; Pickkers, P. Haemoadsorption to remove inflammatory mediators in sepsis: Past, present, and future. Intensive Care Med. Exp. 2025, 13, 38. [Google Scholar] [CrossRef]
- Sazonov, V.; Abylkassov, R.; Tobylbayeva, Z.; Saparov, A.; Mironova, O.; Poddighe, D. Case Series: Efficacy and Safety of Hemoadsorption With HA-330 Adsorber in Septic Pediatric Patients With Cancer. Front. Pediatr. 2021, 9, 672260. [Google Scholar] [CrossRef] [PubMed]
- Erkurt, M.A.; Sarici, A.; Ozer, A.B.; Kuku, I.; Bicim, S.; Aydogan, M.S.; Kose, A.; Memisoglu, F.; Ince, V.; Otan, E.; et al. The effect of HA330 hemoperfusion adsorbent method on inflammatory markers and end-organ damage levels in sepsis: A retrospective single center study. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 8112–8117. [Google Scholar] [CrossRef]
- Mitzner, S.; Kogelmann, K.; Ince, C.; Molnar, Z.; Ferrer, R.; Nierhaus, A. Adjunctive Hemoadsorption Therapy with CytoSorb in Patients with Septic/Vasoplegic Shock: A Best Practice Consensus Statement. J. Clin. Med. 2023, 12, 7199. [Google Scholar] [CrossRef]
- Mehta, Y.; Ansari, A.S.; Mandal, A.K.; Chatterjee, D.; Sharma, G.S.; Sathe, P.; Umraniya, P.V.; Paul, R.; Gupta, S.; Singh, V.; et al. Systematic review with expert consensus on use of extracorporeal hemoadsorption in septic shock: An Indian perspective. World J. Crit. Care Med. 2024, 13, 89026. [Google Scholar] [CrossRef]
- Bottari, G.; Ranieri, V.M.; Ince, C.; Pesenti, A.; Aucella, F.; Scandroglio, A.M.; Ronco, C.; Vincent, J.L. Use of extracorporeal blood purification therapies in sepsis: The current paradigm, available evidence, and future perspectives. Crit. Care 2024, 28, 432. [Google Scholar] [CrossRef]
- Gao, Y.; Huang, X.; Yang, Y.; Lei, Z.; Chen, Q.; Guo, X.; Tian, J.; Gao, X. Clinical analysis of AN69ST membrane continuous venous hemofiltration in the treatment of severe sepsis. Open Med. 2023, 18, 20230784. [Google Scholar] [CrossRef]
- Lesbekov, T.; Nurmykhametova, Z.; Kaliyev, R.; Kuanyshbek, A.; Faizov, L.; Bekishev, B.; Jabayeva, N.; Samalavicius, R.; Pya, Y. Hemadsorption in patients requiring V-A ECMO support: Comparison of Cytosorb versus Jafron HA330. Artif. Organs 2023, 47, 721–730. [Google Scholar] [CrossRef]
- Aidynbek, Z.; Kakenov, E.; Mironova, O.; Ydyrysheva, K.; Li, T.; Sazonov, V. Extracorporeal Membrane Oxygenation in the Management of Tumor Lysis Syndrome in Children: A Review of Cases. J. Clin. Med. 2025, 14, 2771. [Google Scholar] [CrossRef]
- Lorenzin, A.; de Cal, M.; Marcello, M.; Sorbo, D.; Copelli, S.; Ronco, C.; de Rosa, S.; Zanella, M. Vancomycin Adsorption during in vitro Model of Hemoperfusion with Mini-Module of HA380 Cartridge. Blood Purif. 2023, 52, 174–182. [Google Scholar] [CrossRef]
- Song, D.; Jin, Y.; Zhang, Y.; Zhou, Z. Heparin-induced thrombocytopenia in extracorporeal membrane oxygenation-supported patients: A systematic review and meta-analysis. Thromb. J. 2024, 22, 55. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, R.; Verbrugghe, W.; Dams, K.; Roelant, E.; Couttenye, M.M.; Devroey, D.; Jorens, P. Regional Citrate Anticoagulation in Continuous Renal Replacement Therapy: Is Metabolic Fear the Enemy of Logic? A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Life 2023, 13, 1198. [Google Scholar] [CrossRef]
- Hu, J.; Wang, C.; Bai, K.; Liu, C. Clinical application of regional citrate anticoagulation for membrane-based therapeutic plasma exchange in children with liver failure. Front. Pediatr. 2023, 11, 1206999. [Google Scholar] [CrossRef] [PubMed]
- Faybik, P.; Hetz, H.; Mitterer, G.; Krenn, C.G.; Schiefer, J.; Funk, G.C.; Bacher, A. Regional citrate anticoagulation in patients with liver failure supported by a molecular adsorbent recirculating system. Crit. Care Med. 2011, 39, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Y.; Xu, S.Y.; Yin, L.; Yang, T.; Jin, K.; Zhang, Q.B.; Sun, F.; Tan, D.Y.; Xin, T.Y.; Chen, Y.G.; et al. Management of regional citrate anticoagulation for continuous renal replacement therapy: Guideline recommendations from Chinese emergency medical doctor consensus. Mil. Med. Res. 2023, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; He, J.; Xiao, Z.; Zhou, X.; Zhang, X. Citrate and low-dose heparin combined anticoagulation in pediatric continuous renal replacement therapy. Sci. Rep. 2024, 14, 13504. [Google Scholar] [CrossRef] [PubMed]
- Botan, E.; Durak, A.; Gün, E.; Gurbanov, A.; Balaban, B.; Kahveci, F.; Özen, H.; Uçmak, H.; Gençay, A.G.; Kendirli, T. Comparison of Citrate and Heparin for Continuous Renal Replacement Therapy in Pediatric Intensive Units. Turk. J. Pediatr. Emerg. Intensive Care Med. 2023, 10, 198–204. [Google Scholar] [CrossRef]
| Authors | Siripanadorn and Samransamruajkit (2023) [22] | Napoleone et al. (2024) [5] | Ryazanova et al. (2024) [23] |
|---|---|---|---|
| Study design | Prospective observational | Single pediatric CPB case report | Retrospective, oncology cohort |
| Number of patients, n | 12 | 1 | 10 |
| Age, years (Median) | 9.5 | 10 | 6.5 |
| Sex (M/F) | M: 8; F: 4 | M | M: 4; F: 6 |
| Comorbidities /Inflammatory response caused by (underlying disease) | COVID-19; GI and hepatobiliary infection; Invasive pulmonary aspergillosis | Single ventricle/SIRS caused by surgical intervention | ALL; Sarcoma; AML; Pure red cell aplasia |
| Severity score before the procedure (If reported) | PELOD-2: 9.67 ± 3.90; Median = 9.5 PRISM-3: 18.08 ± 3.90; Median = 16.5 | Not reported | pSOFA: 16.6 ± 3.16 |
| Hemoadsorption therapy initiation time after diagnosing sepsis | Within 24 h | Not reported | Not reported |
| Device/Circuit | HA330/CRRT, ECMO in 1 patient | HA380/CPB | HA330/CVVHDF |
| Priming | NSS; 5% albumin in two cases | Fresh frozen plasma (FFP) 200 mL, albumin 20% 50 mL, and ringer acetate 800 mL | NSS; 2 patients were replaced to red blood cell suspension |
| Inflammation markers levels before hemoadsorption | Median (Interquartile Range (IQR) IL-6: 206.4 (111–412.5) pg/mL; CRP: 87.6 (16.3–135.2) mg/L; PCT: 8.4 (1.3–21.6) pg/mL | IL-6: 146 pg/mL; CRP: 169 mg/L; PCT: 10.88 ng/mL | (mean ± SD) IL-6: 539.89 ± 475.02 pg/mL; CRP: 288.17 ± 99.34 mg/L; PCT: 245.10 ± 115.73 ng/mL |
| Number of sessions, n/Hemoadsorption duration time | 24 sessions: 2 sessions per patient/2–4 h per patient | 1 session/218 min (3 h 38 min) | 11 sessions; 1 session per patient; one pt had 2 sessions/4 h per patient |
| Inflammation marker levels after hemoadsorption | (median (IQR) IL-6: 37.4 (6.4–146.8) pg/mL; CRP: 25.2 (6.8–120.4) mg/L; PCT: 1.6 (1.1–8.8) pg/mL | IL-6: 21 pg/mL; CRP: 95.5 mg/L; PCT: 6.16 ng/mL | (mean ± SD) IL-6: 107.7 ± 52.21 pg/mL; CRP: 105.63 ± 49.32 mg/L; PCT: 11.89 ± 13.18 ng/mL |
| Reduction rate, % | IL-6: −81.89; CRP: −71.23; PCT: −80.95 | IL-6: −85.62; CRP: −43.49; PCT: −43.38 | IL-6: −68.02 ± 19.78; CRP: −60.596 ± 19.51; PCT: −73.06 ± 23.79 |
| Severity score after the procedure (If applicable) | PELOD-2: ~2 (median) PRISM-3: ~5.5 (median) | Not reported | pSOFA: 3.5 ± 1.17 |
| Hospital stay metrics (PICU-LOS/MV-LOS), days (mean ± SD) | PICU-LOS: 15.58 ± 11.08 MV-LOS: 8.75 ± 9.37 | PICU-LOS: 15 MV-LOS: N/A | PICU-LOS: 15.7 ± 4.69 MV-LOS: 2–3 |
| Outcome (Discharged/Fatal) | Discharged: 10; Fatal: 2 | Discharged | Discharged: 9; Fatal: 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azenova, K.; Sazonov, V. Hemoadsorption in Children with Cytokine Storm Using the Jafron HA330 and HA380 Cartridges. J. Clin. Med. 2025, 14, 6359. https://doi.org/10.3390/jcm14186359
Azenova K, Sazonov V. Hemoadsorption in Children with Cytokine Storm Using the Jafron HA330 and HA380 Cartridges. Journal of Clinical Medicine. 2025; 14(18):6359. https://doi.org/10.3390/jcm14186359
Chicago/Turabian StyleAzenova, Kamila, and Vitaliy Sazonov. 2025. "Hemoadsorption in Children with Cytokine Storm Using the Jafron HA330 and HA380 Cartridges" Journal of Clinical Medicine 14, no. 18: 6359. https://doi.org/10.3390/jcm14186359
APA StyleAzenova, K., & Sazonov, V. (2025). Hemoadsorption in Children with Cytokine Storm Using the Jafron HA330 and HA380 Cartridges. Journal of Clinical Medicine, 14(18), 6359. https://doi.org/10.3390/jcm14186359






