Global Longitudinal Strain as a Prognostic Biomarker for Asymptomatic Moderate to Severe Aortic Regurgitation with Preserved Ejection Fraction: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol Registration
2.2. Databases and Study Search
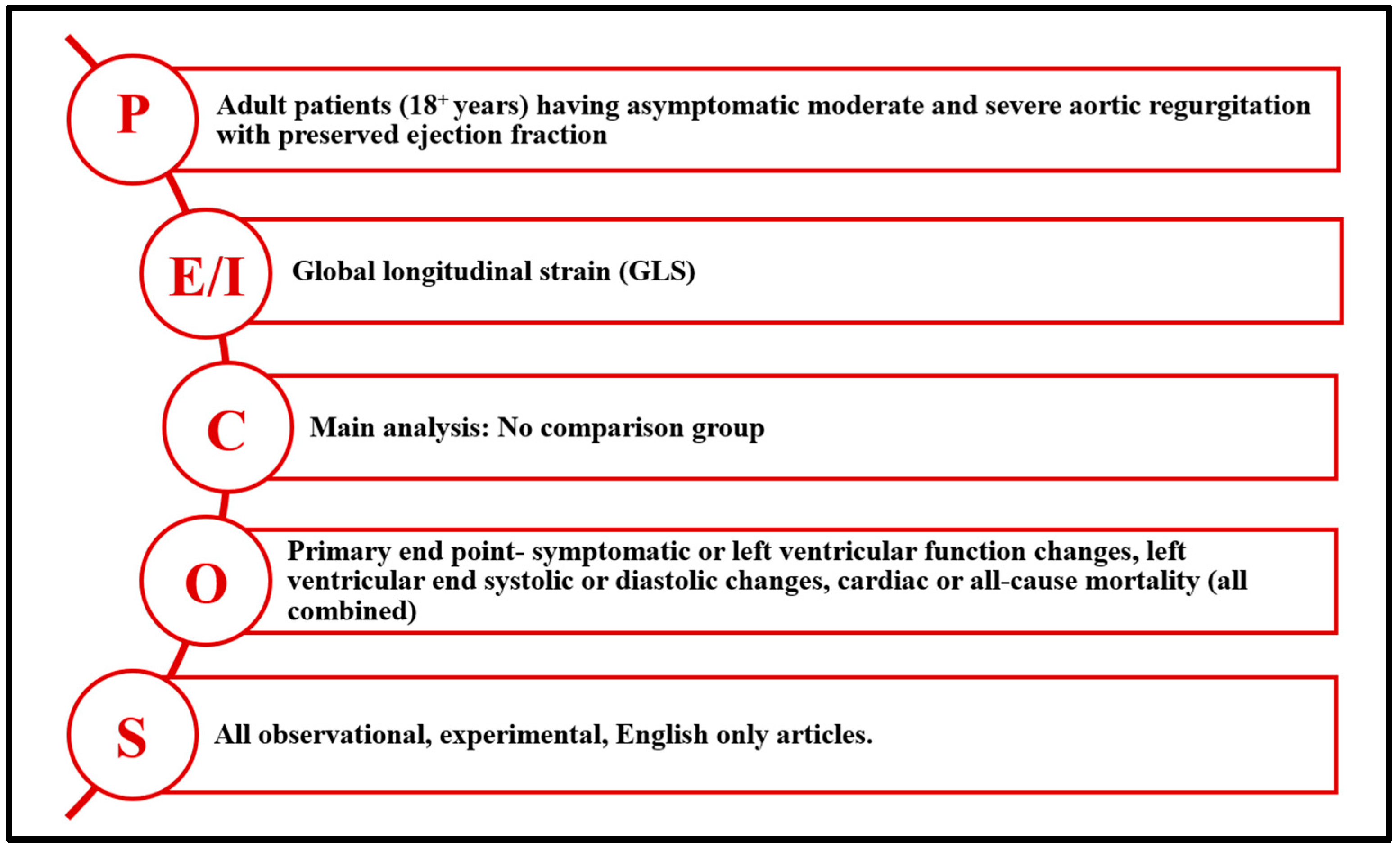
2.3. Eligibility Criteria
2.4. Selection Process
2.5. Data Extraction
2.6. Assessment of Bias Risk
2.7. Statistical Analysis
3. Results
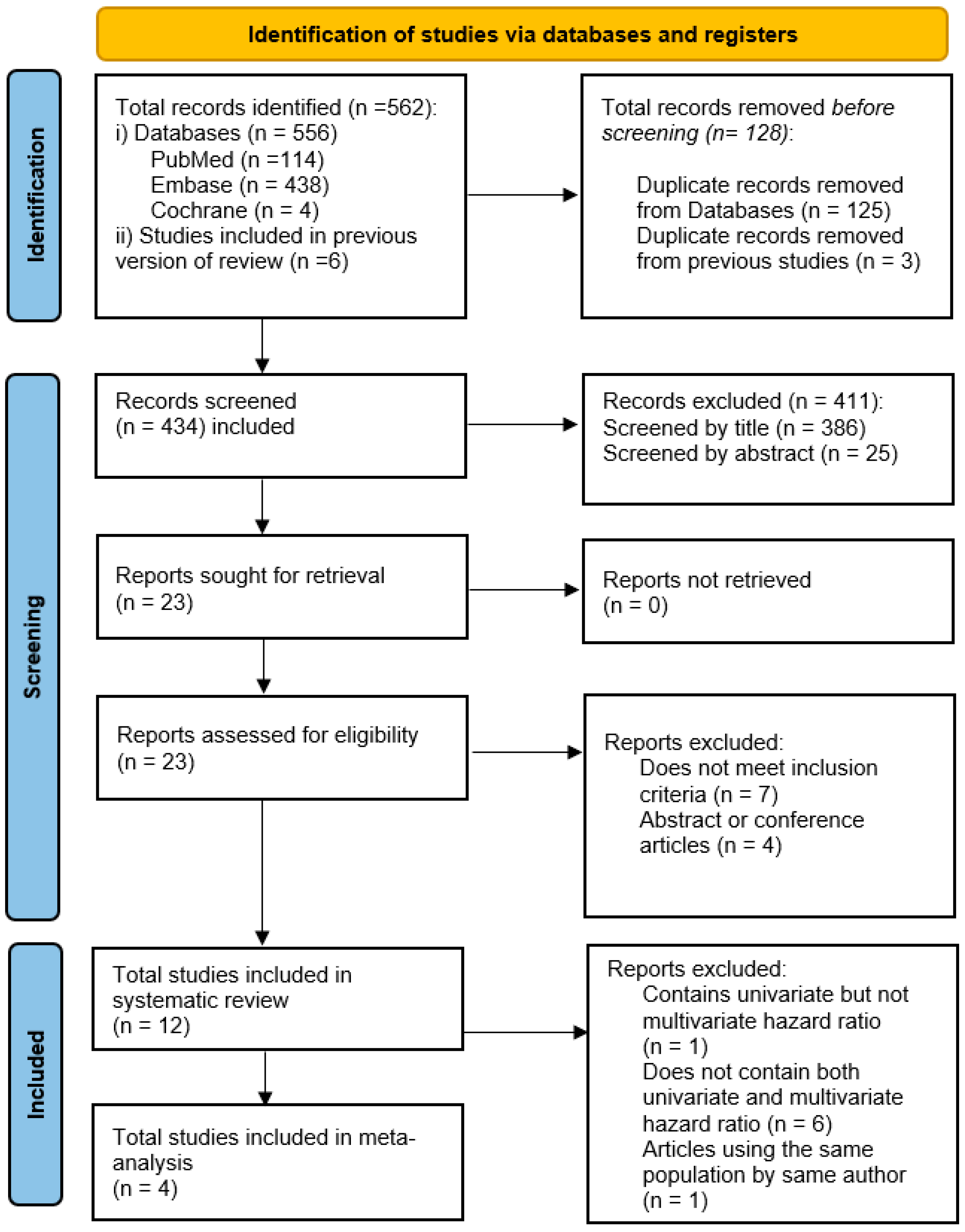
3.1. Study Screening
3.2. Study Quality
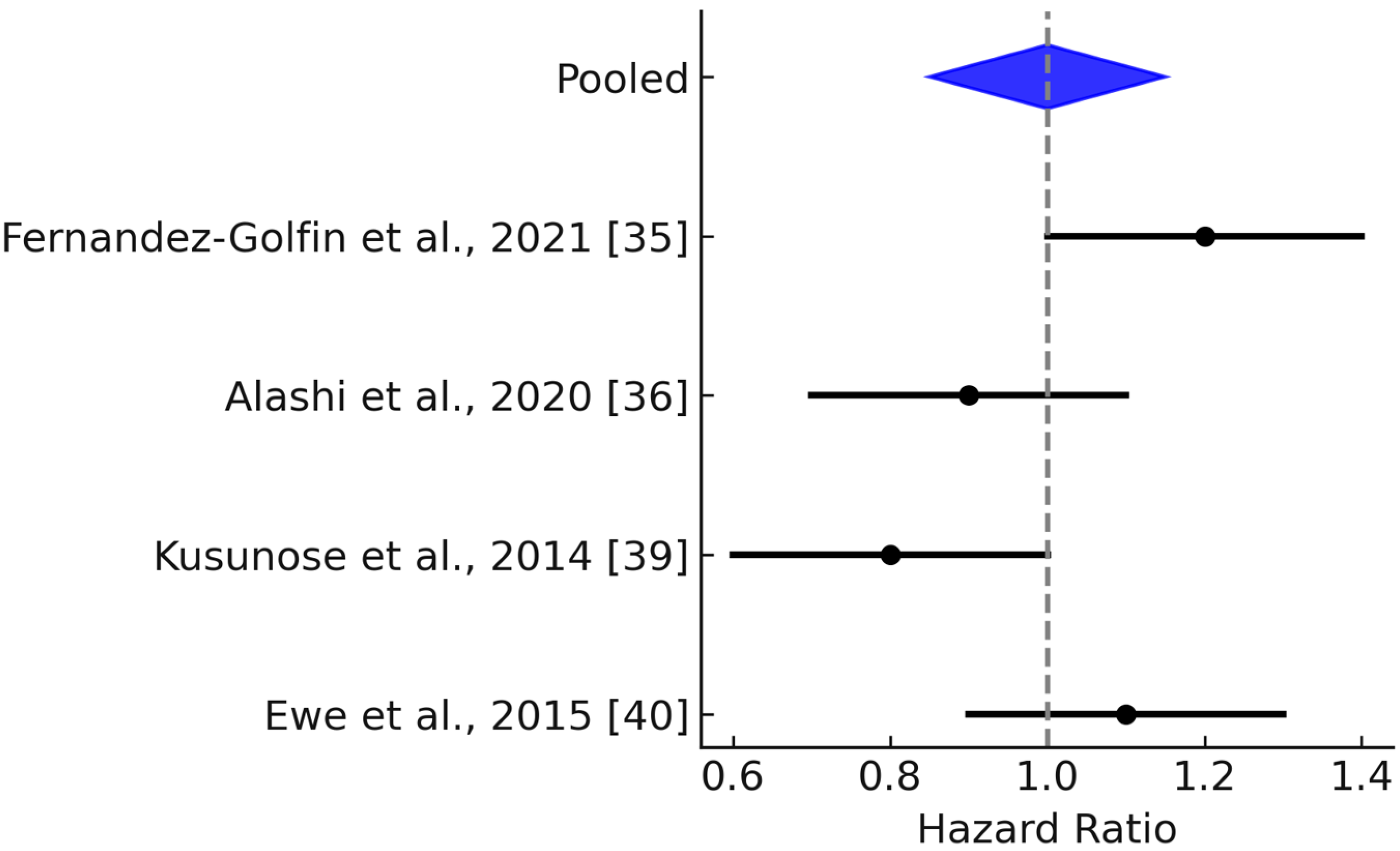
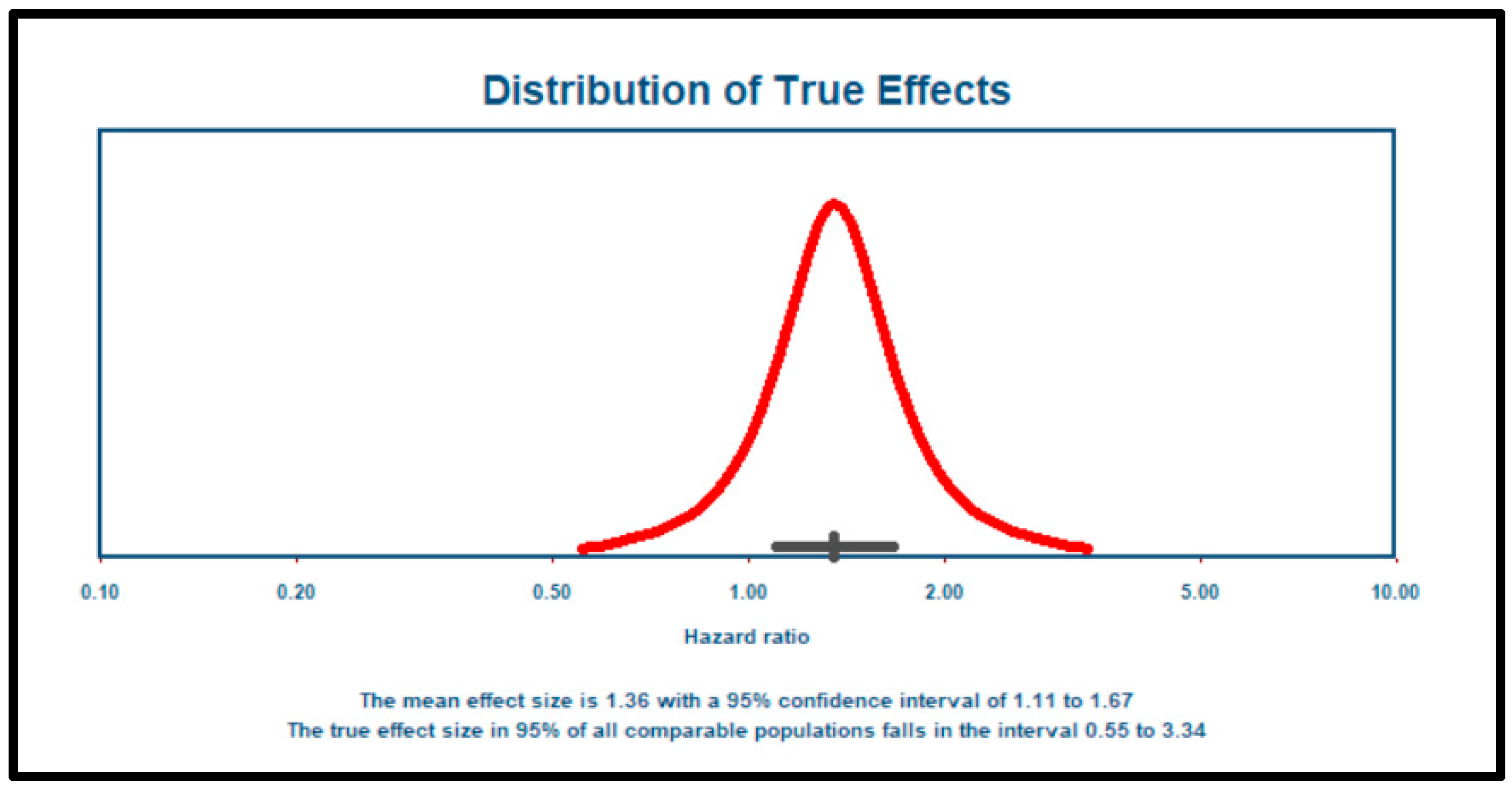
3.3. Meta-Analysis
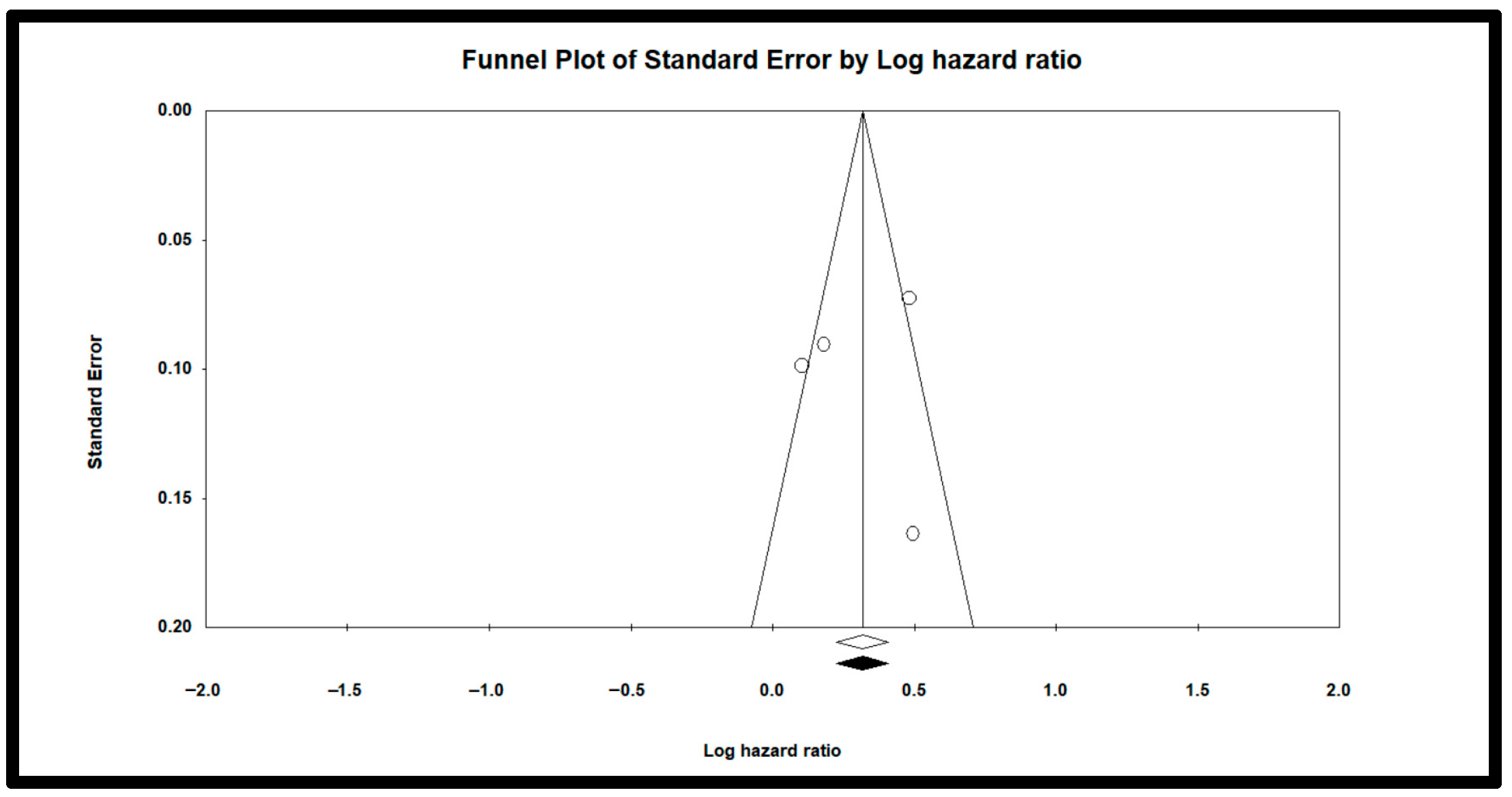
3.4. Publication Bias or Small-Study Effects
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A. Search Strategies (Search Date: 28 November 2024)
| Database | Query Used | Limit Criteria | Results | Totals |
|---|---|---|---|---|
| PubMed | ||||
| #1 | Aortic regurgitation | Inception—11/28/2024 | 31,031 | |
| #2 | Mixed aortic valve disease | Inception—11/28/2024 | 782 | |
| #3 | #1 OR #2 | Inception—11/28/2024 | 31,394 | |
| #4 | Global Longitudinal Strain | Inception—11/28/2024 | 7342 | |
| #5 | GLS | Inception—11/28/2024 | 5858 | |
| #6 | #4 OR #5 | Inception—11/28/2024 | 9861 | |
| #7 | #3 AND #6 | Inception—11/28/2024 | 114 | |
| Embase | ||||
| #1 | Aortic regurgitation | Inception—11/28/2024 | 57,763 | |
| #2 | Mixed aortic valve disease | Inception—11/28/2024 | 1272 | |
| #3 | #1 OR #2 | Inception—11/28/2024 | 58,275 | |
| #4 | Global Longitudinal Strain | Inception—11/28/2024 | 14,412 | |
| #5 | GLS | Inception—11/28/2024 | 12,609 | |
| #6 | #4 OR #5 | Inception—11/28/2024 | 18,851 | |
| #7 | #3 AND #6 | Inception—11/28/2024 | 438 | |
| Cochrane Library | ||||
| #1 | Aortic regurgitation | Title Abstract Keyword; Inception—11/28/2024 | 716 | |
| #2 | Mixed aortic valve disease | Title Abstract Keyword; Inception—11/28/2024 | 61 | |
| #3 | #1 OR #2 | Title Abstract Keyword; Inception—11/28/2024 | 762 | |
| #4 | Global Longitudinal Strain | Title Abstract Keyword; Inception—11/28/2024 | 828 | |
| #5 | GLS | Title Abstract Keyword; Inception—11/28/2024 | 618 | |
| #6 | #4 OR #5 | Title Abstract Keyword; Inception—11/28/2024 | 1052 | |
| #7 | #3 AND #6 | Title Abstract Keyword; Inception—11/28/2024 | 4 | |
| Previous Systematic Review | 6 | |||
| GRAND TOTAL | 562 |
References
- Gössl, M.; Stanberry, L.; Benson, G.; Steele, E.; Garberich, R.; Witt, D.; Cavalcante, J.; Sharkey, S.; Enriquez-Sarano, M. Burden of Undiagnosed Valvular Heart Disease in the Elderly in the Community: Heart of New Ulm Valve Study. JACC Cardiovasc. Imaging 2023, 16, 1118–1120. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e72–e227. [Google Scholar] [CrossRef] [PubMed]
- Aronow, W.S.; Ahn, C.; Kronzon, I.; Nanna, M. Prognosis of patients with heart failure and unoperated severe aortic valvular regurgitation and relation to ejection fraction. Am. J. Cardiol. 1994, 74, 286–288. [Google Scholar] [CrossRef] [PubMed]
- Dujardin, K.S.; Enriquez-Sarano, M.; Schaff, H.V.; Bailey, K.R.; Seward, J.B.; Tajik, A.J. Mortality and morbidity of aortic regurgitation in clinical practice: A long-term follow-up studies. Circulation 1999, 99, 1851–1857. [Google Scholar] [CrossRef]
- Chaliki, H.P.; Mohty, D.; Avierinos, J.-F.; Scott, C.G.; Schaff, H.V.; Tajik, A.J.; Enriquez-Sarano, M. Outcomes after aortic valve replacement in patients with severe aortic regurgitation and markedly reduced left ventricular function. Circulation 2002, 106, 2687–2693. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.; Li, F.; Wang, Y.; Dong, N. Aortic valve replacement for severe aortic regurgitation in asymptomatic patients with normal ejection fraction and severe left ventricular dilatation. Interact. Cardiovasc. Thorac. Surg. 2016, 22, 425–430. [Google Scholar] [CrossRef]
- Yang, L.-T.; Enriquez-Sarano, M.; Michelena, H.I.; Nkomo, V.T.; Scott, C.G.; Bailey, K.R.; Oguz, D.; Ullah, M.W.; Pellikka, P.A. Predictors of Progression in Patients with Stage B Aortic Regurgitation. J. Am. Coll. Cardiol. 2019, 74, 2480–2492. [Google Scholar] [CrossRef]
- Siani, A.; Perone, F.; Costantini, P.; Rodolfi, S.; Muscogiuri, G.; Sironi, S.; Carriero, S.; Pavon, A.G.; van der Bilt, I.; van Rosendael, P.; et al. Aortic regurgitation: A multimodality approach. J. Clin. Ultrasound 2022, 50, 1041–1050. [Google Scholar] [CrossRef]
- Akinseye, O.A.; Pathak, A.; Ibebuogu, U.N. Aortic Valve Regurgitation: A Comprehensive Review. Curr. Probl. Cardiol. 2018, 43, 315–334. [Google Scholar] [CrossRef]
- Lee, J.K.; Franzone, A.; Lanz, J.; Siontis, G.C.; Stortecky, S.; Gräni, C.; Roost, E.; Windecker, S.; Pilgrim, T. Early Detection of Subclinical Myocardial Damage in Chronic Aortic Regurgitation and Strategies for Timely Treatment of Asymptomatic Patients. Circulation 2018, 137, 184–196. [Google Scholar] [CrossRef]
- Smedsrud, M.K.; Pettersen, E.; Gjesdal, O.; Svennevig, J.L.; Andersen, K.; Ihlen, H.; Edvardsen, T. Detection of left ventricular dysfunction by global longitudinal systolic strain in patients with chronic aortic regurgitation. J. Am. Soc. Echocardiogr. 2011, 24, 1253–1259. [Google Scholar] [CrossRef]
- Abou, R.; van der Bijl, P.; Bax, J.J.; Delgado, V. Global longitudinal strain: Clinical use and prognostic implications in contemporary practice. Heart 2020, 106, 1438–1444. [Google Scholar] [CrossRef] [PubMed]
- Klaeboe, L.G.; Edvardsen, T. Echocardiographic assessment of left ventricular systolic function. J. Echocardiogr. 2019, 17, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Stokke, T.M.; Hasselberg, N.E.; Smedsrud, M.K.; Sarvari, S.I.; Haugaa, K.H.; Smiseth, O.A.; Edvardsen, T.; Remme, E.W. Geometry as a Confounder When Assessing Ventricular Systolic Function: Comparison Between Ejection Fraction and Strain. J. Am. Coll. Cardiol. 2017, 70, 942–954. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, C.F.; Nigri, M.; Higuchi, M.L.; Pomerantzeff, P.M.; Spina, G.S.; Sampaio, R.O.; Tarasoutchi, F.; Grinberg, M.; Rochitte, C.E. Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. J. Am. Coll. Cardiol. 2010, 56, 278–287. [Google Scholar] [CrossRef]
- Li, C.-M.; Li, C.; Bai, W.-J.; Zhang, X.-L.; Tang, H.; Qing, Z.; Li, R. Value of three-dimensional speckle-tracking in detecting left ventricular dysfunction in patients with aortic valvular diseases. J. Am. Soc. Echocardiogr. 2013, 26, 1245–1252. [Google Scholar] [CrossRef]
- Purwowiyoto, S.L.; Halomoan, R. Highlighting the role of global longitudinal strain assessment in valvular heart disease. Egypt. Heart J. 2022, 74, 46. [Google Scholar] [CrossRef]
- deCampos, D.; Teixeira, R.; Saleiro, C.; Botelho, A.; Gonçalve, L. Global longitudinal strain in chronic asymptomatic aortic regurgitation: Systematic review. Echo Res. Pract. 2020, 7, 39–48. [Google Scholar] [CrossRef]
- Liao, H.; Yang, S.; Yu, S.; Hu, X.; Meng, X.; Wu, K. Prognostic Value of Left Ventricular Global Longitudinal Strain for Major Adverse Cardiovascular Events in Patients with Aortic Valve Disease: A Meta-Analysis. Cardiology 2024, 149, 277–285. [Google Scholar] [CrossRef]
- Gherbesi, E.; Gianstefani, S.; Angeli, F.; Ryabenko, K.; Bergamaschi, L.; Armillotta, M.; Guerra, E.; Tuttolomondo, D.; Gaibazzi, N.; Squeri, A.; et al. Myocardial strain of the left ventricle by speckle tracking echocardiography: From physics to clinical practice. Echocardiography 2024, 41, e15753. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- McGowan, J.; Sampson, M.; Salzwedel, D.M.; Cogo, E.; Foerster, V.; Lefebvre, C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J. Clin. Epidemiol. 2016, 75, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.L.; Whaley, P.; Thayer, K.A.; Schünemann, H.J. Identifying the PECO: A framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ. Int. 2018, 121 Pt 1, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- National Heart, Lung, and Blood Institute. Study Quality Assessment Tools. 2024. Available online: https://internet-prod.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 13 January 2025).
- Li, M.; Gao, Q.; Yu, T. Kappa statistic considerations in evaluating inter-rater reliability between two raters: Which, when and context matters. BMC Cancer 2023, 23, 799. [Google Scholar] [CrossRef] [PubMed]
- IntHout, J.; Ioannidis, J.P.A.; Rovers, M.M.; Goeman, J.J. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 2016, 6, e010247. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials revisited. Contemp. Clin. Trials 2015, 45 Pt A, 139–145. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Sterne, J.A.; Egger, M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J. Clin. Epidemiol. 2001, 54, 1046–1055. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Sutton, A.J.; Ioannidis, J.P.A.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomized controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.P.T.; Altman, D.G. Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Martín, A.G.; Sequeiros, M.A.; Gómez, A.G.G.; Díaz, L.M.R.; Ruiz, J.M.M.; Baydés, R.H.; Mur, J.L.M.; Zamorano, J.L.; Fernández-Golfín, C. Prognostic value of diastolic function parameters in significant aortic regurgitation: The role of the left atrial strain. J. Echocardiogr. 2022, 20, 216–223. [Google Scholar] [CrossRef]
- Kočková, R.; Línková, H.; Hlubocká, Z.; Mědílek, K.; Tuna, M.; Vojáček, J.; Skalský, I.; Černý, Š.; Malý, J.; Hlubocký, J.; et al. Multiparametric Strategy to Predict Early Disease Decompensation in Asymptomatic Severe Aortic Regurgitation. Circ. Cardiovasc. Imaging 2022, 15, e014901. [Google Scholar] [CrossRef]
- Fernández-Golfín, C.; Hinojar-Baydes, R.; González-Gómez, A.; Monteagudo, J.M.; Esteban, A.; Alonso-Salinas, G.; Fernández, M.A.; García-Martín, A.; Santoro, C.; Pascual-Izco, M.; et al. Prognostic implications of cardiac magnetic resonance feature tracking derived multidirectional strain in patients with chronic aortic regurgitation. Eur. Radiol. 2021, 31, 5106–5115. [Google Scholar] [CrossRef]
- Alashi, A.; Khullar, T.; Mentias, A.; Gillinov, A.M.; Roselli, E.E.; Svensson, L.G.; Popovic, Z.B.; Griffin, B.P.; Desai, M.Y. Long-Term Outcomes After Aortic Valve Surgery in Patients with Asymptomatic Chronic Aortic Regurgitation and Preserved LVEF: Impact of Baseline and Follow-Up Global Longitudinal Strain. JACC Cardiovasc. Imaging 2020, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Verseckaite, R.; Mizariene, V.; Montvilaite, A.; Auguste, I.; Bieseviciene, M.; Laukaitiene, J.; Jonkaitiene, R.; Jurkevicius, R. The predictive value of left ventricular myocardium mechanics evaluation in asymptomatic patients with aortic regurgitation and preserved left ventricular ejection fraction. A long-term speckle-tracking echocardiographic study. Echocardiography 2018, 35, 1277–1288. [Google Scholar] [CrossRef] [PubMed]
- Alashi, A.; Mentias, A.; Abdallah, A.; Feng, K.; Gillinov, A.M.; Rodriguez, L.L.; Johnston, D.R.; Svensson, L.G.; Popovic, Z.B.; Griffin, B.P.; et al. Incremental Prognostic Utility of Left Ventricular Global Longitudinal Strain in Asymptomatic Patients with Significant Chronic Aortic Regurgitation and Preserved Left Ventricular Ejection Fraction. JACC Cardiovasc. Imaging 2018, 11, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Kusunose, K.; Agarwal, S.; Marwick, T.H.; Griffin, B.P.; Popović, Z.B. Decision making in asymptomatic aortic regurgitation in the era of guidelines: Incremental values of resting and exercise cardiac dysfunction. Circ. Cardiovasc. Imaging 2014, 7, 352–362. [Google Scholar] [CrossRef]
- Ewe, S.H.; Haeck, M.L.; Ng, A.C.; Witkowski, T.G.; Auger, D.; Leong, D.P.; Abate, E.; Marsan, N.A.; Holman, E.R.; Schalij, M.J.; et al. Detection of subtle left ventricular systolic dysfunction in patients with significant aortic regurgitation and preserved left ventricular ejection fraction: Speckle tracking echocardiographic analysis. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 992–999. [Google Scholar] [CrossRef]
- Reil, J.-C.; Reil, G.-H.; Hecker, N.; Sequeira, V.; Borer, J.S.; Stierle, U.; Lavall, D.; Marquetand, C.; Busch, C.; Patzelt, J.; et al. Reduced left ventricular contractility, increased diastolic operant stiffness and high energetic expenditure in patients with severe aortic regurgitation without indication for surgery. Interact. Cardiovasc. Thorac. Surg. 2021, 32, 29–38. [Google Scholar] [CrossRef]
- Gao, L.; Lin, Y.; Ji, M.; Wu, W.; Li, H.; Qian, M.; Zhang, L.; Xie, M.; Li, Y. Clinical Utility of Three-Dimensional Speckle-Tracking Echocardiography in Heart Failure. J. Clin. Med. 2022, 11, 6307. [Google Scholar] [CrossRef]
- Suzuki, S.; Amano, M.; Nakagawa, S.; Irie, Y.; Moriuchi, K.; Okada, A.; Kitai, T.; Amaki, M.; Kanzaki, H.; Nishimura, K.; et al. Outcomes of Watchful Waiting Strategy and Predictors of Postoperative Prognosis in Asymptomatic or Equivocally Symptomatic Chronic Severe Aortic Regurgitation with Preserved Left Ventricular Function. J. Am. Heart Assoc. 2024, 13, e036292. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Wang, S.; Zhang, L.; Li, Y.; Cheng, L.; Wang, J.; Yang, Y.; Wang, D.; Zhang, Y.; Xie, Y.; et al. Left Ventricular Remodeling and Its Progression in Asymptomatic Patients with Chronic Aortic Regurgitation: Evaluation by Speckle-Tracking Echocardiography. J. Am. Soc. Echocardiogr. 2021, 34, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Stens, N.A.; van Iersel, O.; Rooijakkers, M.J.; van Wely, M.H.; Nijveldt, R.; Bakker, E.A.; Rodwell, L.; Pedersen, A.L.; Poulsen, S.H.; Kjønås, D.; et al. Prognostic Value of Preprocedural LV Global Longitudinal Strain for Post-TAVR-Related Morbidity and Mortality: A Meta-Analysis. JACC Cardiovasc. Imaging 2023, 16, 332–341. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, Y.; Wang, Y.; Tian, W.; Li, Z.; Ge, L.; Wang, G.; Chen, Z. Prognostic Value of CT-Derived Myocardial Biomarkers: Extracellular Volume Fraction and Strain in Patients with Severe Aortic Stenosis Undergoing Transcatheter Aortic Valve Replacement: A Systematic Review and Meta-analysis. Acad. Radiol. 2024, 31, 4352–4364. [Google Scholar] [CrossRef]
- Canessa, M.; Thamman, R.; Americo, C.; Soca, G.; Dayan, V. Global Longitudinal Strain Predicts Survival and Left Ventricular Function After Mitral Valve Surgery: A Meta-analysis. Semin. Thorac. Cardiovasc. Surg. 2021, 33, 337–342. [Google Scholar] [CrossRef]
- Kaur, S.; Jain, V.; Sadana, D.; Gillinov, A.M.; Desai, M.Y.; Griffin, B.P.; Xu, B. Prognostic Utility of Left Ventricular Global Longitudinal Strain in Surgery for Primary Mitral Regurgitation: A Systematic Review. JACC Cardiovasc. Imaging 2020, 13, 1838–1840. [Google Scholar] [CrossRef]
- Farsalinos, K.E.; Daraban, A.M.; Ünlü, S.; Thomas, J.D.; Badano, L.P.; Voigt, J.-U. Head-to-head comparison of global longitudinal strain measurements among nine different vendors: The EACVI/ASE inter-vendor comparison study. J. Am. Soc. Echocardiogr. 2015, 28, 1171–1181.e2. [Google Scholar] [CrossRef]
- Rösner, A.; Barbosa, D.; Aarsæther, E.; Kjønås, D.; Schirmer, H.; D’hooge, J. The influence of frame rate on two-dimensional speckle-tracking strain measurements: A study on silico-simulated models and images recorded in patients. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1137–1147. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Nicolosi, G.L.; Granato, A.; Bonanomi, A.; Rigamonti, E.; Lombardo, M. Influence of chest wall conformation on reproducibility of main echocardiographic indices of left ventricular systolic function. Minerva Cardiol. Angiol. 2024, 72, 111–124. [Google Scholar] [CrossRef]
- Nielsen, M.Ø.; Ljoki, A.; Zerahn, B.; Jensen, L.T.; Kristensen, B. Reproducibility and Repeatability in Focus: Evaluating LVEF Measurements with 3D Echocardiography by Medical Technologists. Diagnostics 2024, 14, 1729. [Google Scholar] [CrossRef]
| Author, Year | Study Design | Sample Size | Characteristics of the Entire Sample | Outcomes (Number) | Quality Rating | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age M (SD) or [Range] | Male n (%) | Bicuspid Valve n (%) | LVESD (mm)/ LVESDi (cm/m2) M (SD) | LVEDD (mm)/ LVEDDi (cm/m2) M (SD) | Dev. of SX (n) | Reduced LVEF (n) | Aortic Valve Interv. (n) | CV Mortality (n) | All-Cause Mortality (n) | ||||
| Garcia Martin et al., 2022 [33] | OCS | 126 | 70.1 (17.2) | 75 (59.5%) | 25 (20.5%) | 32.5 (6.4)/ NR | 52.5 (7.4)/ NR | 5 | NR | 25 | 4 | NR | Medium |
| Kočková et al., 2022 [34] | OCS | 127 | 45 (14) | 107 (84%) | 90 (71%) | 37 (5)/ NR | 58 (6)/ NR | 34 | 7 | 41 | NR | NR | Good |
| Fernandez-Golfin et al., 2021 [35] | OCS | 109 | * 57.42 (15.8) | 74 (68%) | NR | NR/ NR | NR/ NR | 9 | 4 | 14 | 0 | 1 | Good |
| Alashi et al., 2020 [36] | OCS | 865 | 52 (15) | 684 (79%) | NR | NR/ 1.9 (0.4) | NR/ 2.8 (0.5) | NR | NR | NR | 94 | 105 | Good |
| Verseckaite et al., 2018 [37] | OCS | 127 | * 46.6 (15.2) | 88 (69%) | NR | * 34.84 (5.5)/NR | * 51.68 (5.8) /NR | 4 | 12 | NR | NR | NR | Good |
| Alashi et al., 2017 [38] | OCS | 1063 | 53 (16) | 813 (77%) | 383 (36%) | 35 (6)/ 1.7 (0.3) | 54 (8)/ 2.7 (0.5) | NR | NR | NR | 135 | 146 | Good |
| Kusunose et al., 2014 [39] | OCS | 159 | 50 (15) | 124 (80%) | 70 (45%) | 37 (6)/ NR | 57 (7)/ NR | 41 | 28 | 50 | 0 | 0 | Good |
| Ewe et al., 2015 [40] | CSS | 129 | * 54.5 (16.5) | 82 (64%) | 40 (31%) | * 35 (7)/ NR | * 56 (8)/ NR | 21 | 5 | 26 | NR | NR | Good |
| Reil et al., 2020 [41] | CCS | 80 | * 57.5 (15.1) | 66 (83%) | 26 (43%) | NR/ * 2.02 (0.4) | NR/ NR | NR | NR | NR | NR | NR | Good |
| Li et al., 2013 [42] | CSS | 107 | * 52.3 (15.5) | 59 (55%) | NR | NR/ NR | NR/ NR | NR | NR | NR | NR | NR | Good |
| Suzuki et al., 2024 [43] | OCS | 210 | 65 [46–73] | 148 (71%) | NR | 41 (6)/ NR | 61 (7)/ NR | 4 | NR | 33 | 0 | 3 | Good |
| Zeng et al., 2021 [44] | OCS | 176 | * 56.5 (12.8) | 70 (40%) | NR | NR/ NR | NR/ NR | NR | NR | NR | NR | NR | Good |
| Study (Year) | GLS Cutoff Value | Sensitivity (%) | Specificity (%) | Outcome Predicted | Modeling Approach |
|---|---|---|---|---|---|
| Alashi et al., 2020 [36] | NA | NA | NA | Adverse outcomes (composite) | Continuous (per 1% worsening, absolute value) |
| Verseckaite et al., 2018 [37] | –18.5% | 83 | 84 | Reduced LVEF | Categorical (cutoff) |
| Ewe et al., 2015 [40] | –17.4% | 77 | 57 | Symptom onset/adverse outcome | Categorical (cutoff) |
| Reil et al., 2020 [41] | NA | NA | NA | Surgical indication/subgroup outcomes | Continuous (absolute values) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-R.; Shaikh, T.; Taylor, S.; Wang, S.; Nguyen, D.; Khetarpal, B.K.; Namazi, A.; Goel, V.S.; Sagaribay, R.; Batra, K. Global Longitudinal Strain as a Prognostic Biomarker for Asymptomatic Moderate to Severe Aortic Regurgitation with Preserved Ejection Fraction: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 6534. https://doi.org/10.3390/jcm14186534
Kim M-R, Shaikh T, Taylor S, Wang S, Nguyen D, Khetarpal BK, Namazi A, Goel VS, Sagaribay R, Batra K. Global Longitudinal Strain as a Prognostic Biomarker for Asymptomatic Moderate to Severe Aortic Regurgitation with Preserved Ejection Fraction: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(18):6534. https://doi.org/10.3390/jcm14186534
Chicago/Turabian StyleKim, Myung-Rho, Taha Shaikh, Spencer Taylor, Shawn Wang, Darren Nguyen, Banveet Kaur Khetarpal, Ali Namazi, Vidhani S. Goel, Roberto Sagaribay, and Kavita Batra. 2025. "Global Longitudinal Strain as a Prognostic Biomarker for Asymptomatic Moderate to Severe Aortic Regurgitation with Preserved Ejection Fraction: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 18: 6534. https://doi.org/10.3390/jcm14186534
APA StyleKim, M.-R., Shaikh, T., Taylor, S., Wang, S., Nguyen, D., Khetarpal, B. K., Namazi, A., Goel, V. S., Sagaribay, R., & Batra, K. (2025). Global Longitudinal Strain as a Prognostic Biomarker for Asymptomatic Moderate to Severe Aortic Regurgitation with Preserved Ejection Fraction: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(18), 6534. https://doi.org/10.3390/jcm14186534