Long COVID and Acute Stroke in the Emergency Department: An Analysis of Presentation, Reperfusion Treatment, and Early Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Definition of Long COVID
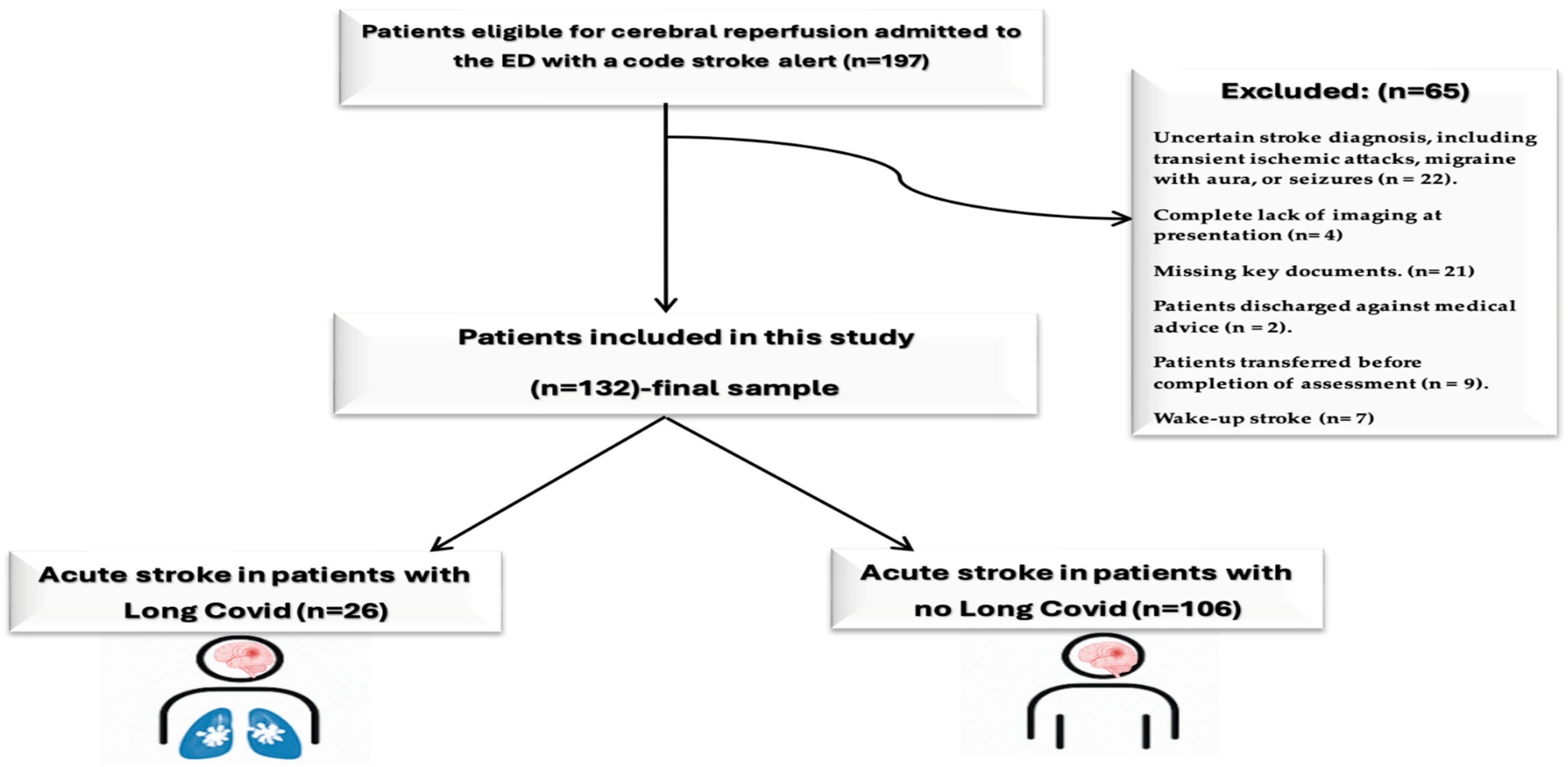
2.3. Study Population
2.4. Radiological Assessment
2.5. Statistical Analysis
3. Results
Demographic Characteristics
4. Discussion
5. Study Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fernández-de-Las-Peñas, C.; Raveendran, A.V.; Giordano, R.; Arendt-Nielsen, L. Long COVID or Post-COVID-19 Condition: Past, Present and Future Research Directions. Microorganisms 2023, 11, 2959. [Google Scholar] [CrossRef]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Post COVID-19 Condition (Long COVID) 2024. Available online: https://www.who.int/europe/news-room/fact-sheets/item/post-COVID-19-condition (accessed on 6 March 2025).
- Marza, A.M.; Petrica, A.; Buleu, F.N.; Mederle, O.A. Case Report: Massive Spontaneous Pneumothorax—A Rare Form of Presentation for Severe COVID-19 Pneumonia. Medicina 2021, 57, 82. [Google Scholar] [CrossRef] [PubMed]
- Fraiman, P.; Godeiro Junior, C.; Moro, E.; Cavallieri, F.; Zedde, M. COVID-19 and Cerebrovascular Diseases: A Systematic Review and Perspectives for Stroke Management. Front. Neurol. 2020, 11, 574694. [Google Scholar] [CrossRef] [PubMed]
- Zuin, M.; Mazzitelli, M.; Rigatelli, G.; Bilato, C.; Cattelan, A.M. Risk of ischemic stroke in patients recovered from COVID-19 infection: A systematic review and meta-analysis. Eur. Stroke J. 2023, 8, 915–922. [Google Scholar] [CrossRef]
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Buleu, F.; Popa, D.; Williams, C.; Tudor, A.; Sutoi, D.; Trebuian, C.; Ioan, C.C.; Iancu, A.; Cozma, G.; Marin, A.-M.; et al. Code Stroke Alert: Focus on Emergency Department Time Targets and Impact on Door-to-Needle Time across Day and Night Shifts. J. Pers. Med. 2024, 14, 596. [Google Scholar] [CrossRef]
- Popa, D.I.; Buleu, F.; Williams, C.; Tudor, A.; Sutoi, D.; Trebuian, C.I.; Ioan, C.C.; Forțofoiu, D.; Badalica-Petrescu, M.; Petre, I.; et al. Evaluating Thrombolysis Rates and Emergency Department Time Targets in Acute Ischemic Stroke: Need for Personalized Medicine. J. Pers. Med. 2024, 14, 955. [Google Scholar] [CrossRef]
- Popa, D.; Iancu, A.; Petrica, A.; Buleu, F.; Williams, C.G.; Sutoi, D.; Trebuian, C.; Tudor, A.; Mederle, O.A. Emergency Department Time Targets for Interhospital Transfer of Patients with Acute Ischemic Stroke. J. Pers. Med. 2023, 14, 13. [Google Scholar] [CrossRef]
- Talkington, G.M.; Kolluru, P.; Gressett, T.E.; Ismael, S.; Meenakshi, U.; Acquarone, M.; Solch-Ottaiano, R.J.; White, A.; Ouvrier, B.; Paré, K.; et al. Neurological sequelae of long COVID: A comprehensive review of diagnostic imaging, underlying mechanisms, and potential therapeutics. Front. Neurol. 2025, 15, 1465787. [Google Scholar] [CrossRef]
- Santoro, L.; Zaccone, V.; Falsetti, L.; Ruggieri, V.; Danese, M.; Miro, C.; Di Giorgio, A.; Nesci, A.; D’Alessandro, A.; Moroncini, G.; et al. Role of Endothelium in Cardiovascular Sequelae of Long COVID. Biomedicines 2023, 11, 2239. [Google Scholar] [CrossRef]
- Stamm, B.; Royan, R.; Giurcanu, M.; Messe, S.R.; Jauch, E.C.; Prabhakaran, S. Door-in-door-out times for interhospital transfer of patients with stroke. JAMA 2023, 330, 636–649. [Google Scholar] [CrossRef]
- Priority Action for Interventional Treatment of Patients with Acute Stroke. Standard Operating Procedure Regarding the Patient Track and Therapeutic Protocol in Romania. Available online: https://legislatie.just.ro/Public/DetaliiDocument/209994 (accessed on 28 March 2024).
- Kim, H.Y. Statistical notes for clinical researchers: Chi-squared test and Fisher’s exact test. Restor. Dent. Endod. 2017, 42, 152–155. [Google Scholar] [CrossRef]
- González-Rubio, J.; Navarro-López, C.; López-Nájera, E.; López-Nájera, A.; Jiménez-Díaz, L.; Navarro-López, J.D.; Nájera, A. A Systematic Review and Meta-Analysis of Hospitalised Current Smokers and COVID-19. Int. J. Environ. Res. Public Health 2020, 17, 7394. [Google Scholar] [CrossRef] [PubMed]
- Klemperer, E.M.; West, J.C.; Peasley-Miklus, C.; Villanti, A.C. Change in Tobacco and Electronic Cigarette Use and Motivation to Quit in Response to COVID-19. Nicotine Tob. Res. 2020, 22, 1662–1663. [Google Scholar] [CrossRef] [PubMed]
- Patanavanich, R.; Glantz, S.A. Smoking Is Associated with COVID-19 Progression: A Meta-analysis. Nicotine Tob. Res. 2020, 22, 1653–1656. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-Las-Peñas, C.; Cuadrado, M.L.; Gómez-Mayordomo, V.; Torres-Macho, J.; Pellicer-Valero, O.J.; Martín-Guerrero, J.D.; Arendt-Nielsen, L. Headache as a COVID-19 onset symptom and post-COVID-19 symptom in hospitalized COVID-19 survivors infected with the Wuhan, Alpha, or Delta SARS-CoV-2 variants. Headache 2022, 62, 1148–1152. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Lechien, J.R.; Chiesa-Estomba, C.M.; Place, S.; Van Laethem, Y.; Cabaraux, P.; Mat, Q.; Huet, K.; Plzak, J.; Horoi, M.; Hans, S.; et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J. Intern. Med. 2020, 288, 335–344. [Google Scholar] [CrossRef]
- Carvalho, L.; Silva, P.A.D.; Rocha-Filho, P.A.S. Persistent headache and chronic daily headache after COVID-19: A prospective cohort study. Korean J. Pain 2024, 37, 247–255. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Taquet, M.; Dercon, Q.; Luciano, S.; Geddes, J.R.; Husain, M.; Harrison, P.J. Incidence, co-occurrence, and evolution of long-COVID features: A 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021, 18, e1003773. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiao, M.; Zhang, S.; Xia, P.; Cao, W.; Jiang, W.; Chen, H.; Ding, X.; Zhao, H.; Zhang, H.; et al. Coagulopathy and Antiphospholipid Antibodies in Patients with COVID-19. N. Engl. J. Med. 2020, 382, e38. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, E.; Vlok, M.; Venter, C.; Bezuidenhout, J.A.; Laubscher, G.J.; Steenkamp, J.; Kell, D.B. Persistent clotting protein pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc. Diabetol. 2021, 20, 172. [Google Scholar] [CrossRef]
- Al-Aly, Z.; Xie, Y.; Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021, 594, 259–264. [Google Scholar] [CrossRef]
- Qureshi, A.I.; Baskett, W.I.; Huang, W.; Shyu, D.; Myers, D.; Raju, M.; Lobanova, I.; Suri, M.F.K.; Naqvi, S.H.; French, B.R.; et al. Acute Ischemic Stroke and COVID-19: An Analysis of 27 676 Patients. Stroke 2021, 52, 905–912. [Google Scholar] [CrossRef]
- Siegler, J.E.; Zha, A.M.; Czap, A.L.; Ortega-Gutierrez, S.; Farooqui, M.; Liebeskind, D.S.; Desai, S.M.; Hassan, A.E.; Starosciak, A.K.; Linfante, I.; et al. Influence of the COVID-19 Pandemic on Treatment Times for Acute Ischemic Stroke: The Society of Vascular and Interventional Neurology Multicenter Collaboration. Stroke 2021, 52, 40–47. [Google Scholar] [CrossRef]
| Variables | Sample | p Value | ||
|---|---|---|---|---|
| Long COVID (n = 26) | No Long COVID (n = 106) | |||
| Demographics and Baseline Stroke Severity | ||||
| Age, years | 68.27 ± 12.82 69 (64–75.75) | 67.83 ± 13.19 70 (62–78) | 0.922 | |
| SBP, mmHg | 147.89 ± 17.46 150 (135.5–159) | 152.35 ± 21.27 150 (140–170) | 0.311 | |
| DBP, mmHg | 79.23 ± 15.34 80 (72.5–84.75) | 80.17 ± 13.68 80 (70–90) | 0.944 | |
| Gender | Male | 11 (42.3%) | 60 (56.6%) | 0.272 |
| Female | 15 (57.7%) | 46 (43.4%) | ||
| Shift | Day | 20 (76.9%) | 80 (75.5%) | 1.000 |
| Night | 6 (23.1%) | 26 (24.5%) | ||
| Arrival mode | Emergency Medical Services | 21 (80.8%) | 89 (83.9%) | 0.770 |
| Private car | 5 (19.2%) | 5 (21.7%) | ||
| NIHSS on admission | 13.62 ± 4.3 13 (11–15.75) | 12.11 ± 5.24 11 (8–16) | 0.162 | |
| NIHSS at 24H | 9.58 ± 5.46 8.5 (6.25–12.75) | 10.86 ± 7.45 10 (4–17) | 0.569 | |
| NIHSS Severity at admission to the ED | Severe 21–42 | 3 (11.5%) | 6 (5.7%) | 0.215 |
| Moderate to severe 16–20 | 3 (11.5%) | 24 (22.6%) | ||
| Moderate 5–15 | 20 (76.9%) | 69 (65.1%) | ||
| Mild 1–4 | 0 (0.0%) | 7 (6.6%) | ||
| TOAST Etiology | Stroke of Undetermined | 8 (30.8%) | 37 (34.9%) | 0.400 |
| Stroke of Other Determined | 2 (7.7%) | 9 (8.5%) | ||
| Small-Vessel Occlusion | 2 (7.7%) | 17 (16.0%) | ||
| Large-Artery Atherosclerosis | 5 (19.2%) | 8 (7.5%) | ||
| Cardioembolism | 9 (34.6%) | 35 (33.0%) | ||
| Variables | Sample | p Value | |
|---|---|---|---|
| Long COVID (n = 26) | No Long COVID (n = 106) | ||
| Medical History and Risk factors | |||
| Hypertension | 18 (69.2%) | 74 (69.8%) | 1.000 |
| Diabetes mellitus | 12 (46.2%) | 39 (36.8%) | 0.380 |
| Atrial Fibrillation | 6 (23.1%) | 14 (13.2%) | 0.581 |
| Dyslipidemia | 11 (42.3%) | 38 (35.8%) | 0.651 |
| Previously stroke | 2 (7.7%) | 9 (8.5%) | 1.000 |
| Current smoking | 1 (3.8%) | 24 (22.6%) | 0.027 * |
| Current alcohol consumption | 6 (23.1%) | 20 (18.9%) | 0.593 |
| Neurological symptoms on admission | |||
| Hemiparesis | 9 (34.6%) | 31 (29.2%) | 0.637 |
| Aphasia | 3 (11.5%) | 26 (24.5%) | 0.192 |
| Dysarthria | 5 (19.2%) | 18 (17.0%) | 0.777 |
| Headache | 5 (19.2%) | 6 (5.7%) | 0.040 * |
| Facial drooping | 11 (42.3%) | 21 (19.8%) | 0.022 * |
| Sudden vision problems | 1 (3.8%) | 12 (11.3%) | 0.462 |
| Loss of consciousness | 1 (3.8%) | 9 (8.5%) | 0.686 |
| Variables | Sample | p Value | |
|---|---|---|---|
| Long COVID (n = 26) | No Long COVID (n = 106) | ||
| Emergency Department time targets | |||
| Onset-to-ED-door time (minutes) | 188.85 ± 45.02 190 (157.5–220) | 194.39 ± 53.67 200 (151.25–230) | 0.696 |
| Door-to-physician time (minutes) | 5.81 ± 2.93 4.5 (4–7.5) | 5.29 ± 2.98 5 (4–7) | 0.465 |
| Door-to-CT time (minutes) | 21.39 ± 19.67 14.5 (12–21.25) | 22.38 ± 18.96 16 (11.25–20.75) | 0.624 |
| Door-in-door-out time (minutes) | 50.42 ± 18.55 45 (40–53.5) | 49.41 ± 18.34 45 (39–51.75) | 0.639 |
| Variables | Sample | p Value | |
|---|---|---|---|
| Long COVID (n = 26) | No Long COVID (n = 106) | ||
| Laboratory results | |||
| Hemoglobin, mg/dL | 13.52 ± 1.74 13.55 (12.425–14.8) | 13.26 ± 1.51 13.45 (12.2–14.48) | 0.554 |
| Platelets count, ×109 μL | 218.27 ± 69.39 204.5 (162.5–246) | 221.58 ± 73.59 223.5 (175.3–251.8) | 0.593 |
| Blood sugar level, mg/dL | 126.15 ± 30.02 118.5 (106.75–137.75) | 134.38 ± 47.44 119.5 (100.5–153) | 0.823 |
| INR | 0.86 ± 0.06 0.9 (0.8–0.9) | 0.89 ± 0.08 0.9 (0.8–0.9) | 0.093 |
| Partial thromboplastin time, seconds | 33.39 ± 2.16 33 (31.5–35) | 33.01 ± 2.83 33.5 (31–35) | 0.878 |
| Association Variables | Sample | p Value | ||
|---|---|---|---|---|
| Long COVID (n = 26) | No Long COVID (n = 106) | |||
| Treatment | ||||
| Treatment | No treatment | 16 (61.5%) | 75 (70.8%) | 0.205 |
| Intravenous thrombolysis | 2 (7.7%) | 14 (13.2%) | ||
| Mechanical thrombectomy | 8 (30.8%) | 17 (16.0%) | ||
| Outcomes | ||||
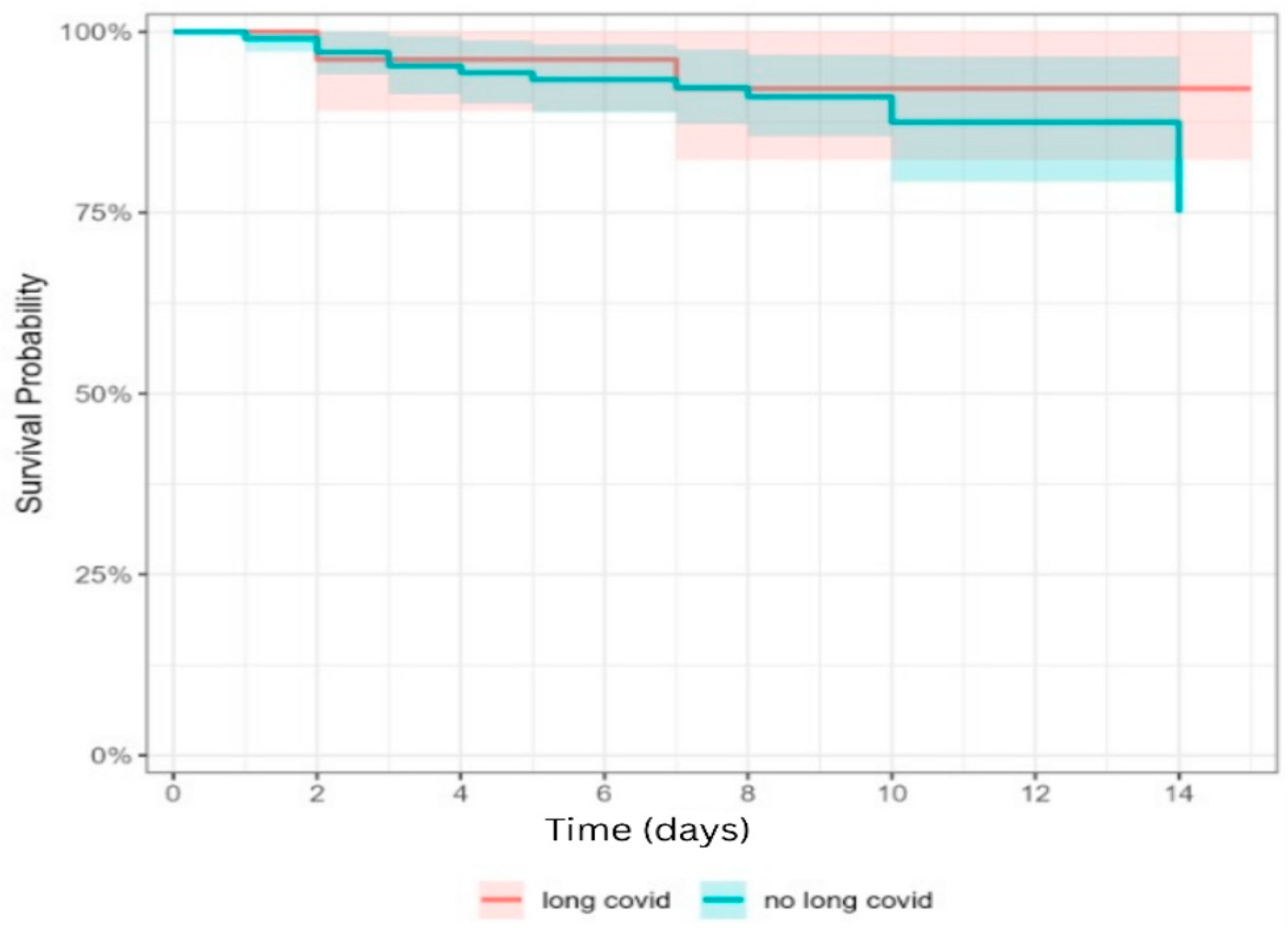
| Discharge | 24 (92.3%) | 95 (90.1%) | 1.000 | |
| Hospitalization days | 8.27 ± 2.24 8.5 (7–9) | 8.38 ± 2.73 8 (7–9) | 0.956 | |
| Hemorrhagic transformation | 7 (26.9%) | 15 (14.2%) | 0.143 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popa, D.-I.; Buleu, F.; Iancu, A.; Tudor, A.; Williams, C.G.; Militaru, M.; Levai, C.M.; Buleu, T.; Ciolac, L.; Militaru, A.G.; et al. Long COVID and Acute Stroke in the Emergency Department: An Analysis of Presentation, Reperfusion Treatment, and Early Outcomes. J. Clin. Med. 2025, 14, 6514. https://doi.org/10.3390/jcm14186514
Popa D-I, Buleu F, Iancu A, Tudor A, Williams CG, Militaru M, Levai CM, Buleu T, Ciolac L, Militaru AG, et al. Long COVID and Acute Stroke in the Emergency Department: An Analysis of Presentation, Reperfusion Treatment, and Early Outcomes. Journal of Clinical Medicine. 2025; 14(18):6514. https://doi.org/10.3390/jcm14186514
Chicago/Turabian StylePopa, Daian-Ionel, Florina Buleu, Aida Iancu, Anca Tudor, Carmen Gabriela Williams, Marius Militaru, Codrina Mihaela Levai, Tiberiu Buleu, Livia Ciolac, Anda Gabriela Militaru, and et al. 2025. "Long COVID and Acute Stroke in the Emergency Department: An Analysis of Presentation, Reperfusion Treatment, and Early Outcomes" Journal of Clinical Medicine 14, no. 18: 6514. https://doi.org/10.3390/jcm14186514
APA StylePopa, D.-I., Buleu, F., Iancu, A., Tudor, A., Williams, C. G., Militaru, M., Levai, C. M., Buleu, T., Ciolac, L., Militaru, A. G., & Mederle, O. A. (2025). Long COVID and Acute Stroke in the Emergency Department: An Analysis of Presentation, Reperfusion Treatment, and Early Outcomes. Journal of Clinical Medicine, 14(18), 6514. https://doi.org/10.3390/jcm14186514







