Endotoxin’s Impact on Organism: From Immune Activation to Tolerance and Beyond
Abstract
1. Introduction
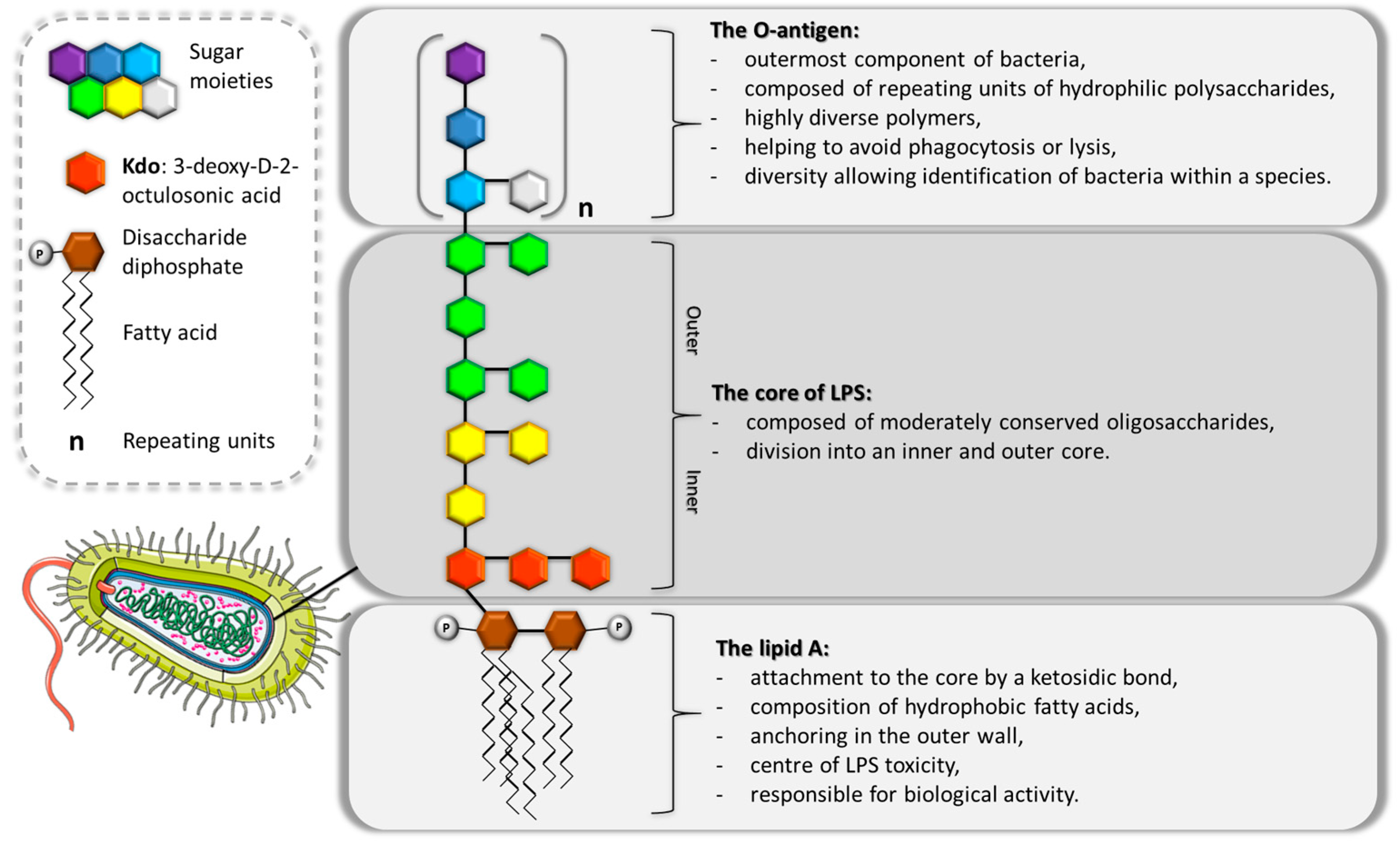
2. Endotoxin—A Pivotal Pro-Inflammatory Factor in Gram-Negative Bacterial Pathogenesis
3. Structural Modifications of Endotoxin Act as a Defence Mechanism for Bacteria, Shielding Them from the Immune System’s Attacks
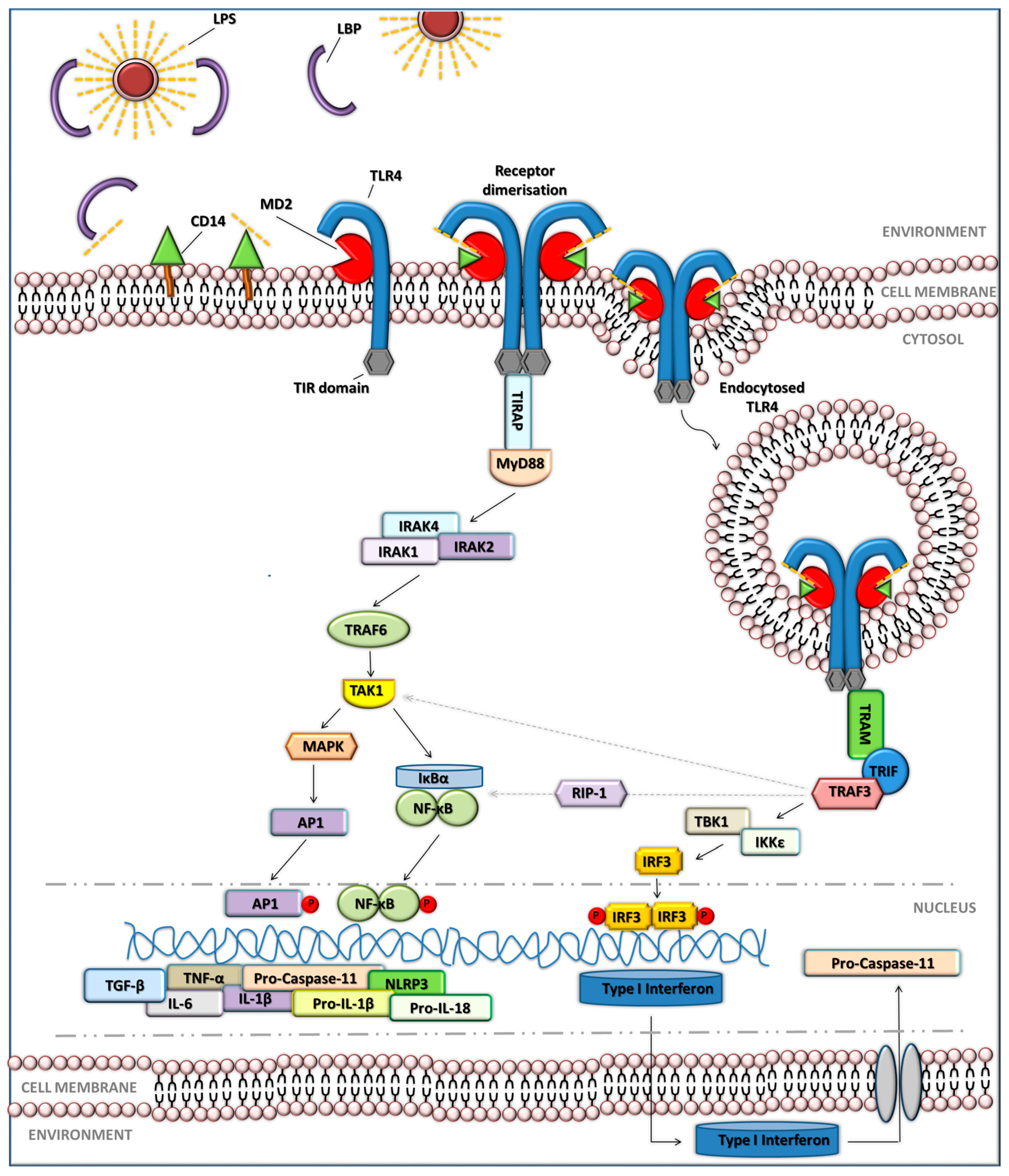
4. How Do Organisms Sense LPS?
5. Inflammasome Signalling
6. Acute Host Response to Endotoxin: Fever as a Hallmark of Endotoxin-Induced Inflammation
7. Beyond the Acute Response: Chronic Inflammation and Endotoxin Tolerance as Distinct Immune Outcomes of Endotoxin Exposure
7.1. Chronic Inflammation
7.2. Endotoxin Tolerance (ET)
8. Two-Faced Nature of ET
8.1. “The Bright Face” of ET
8.2. “The Dark Face” of Endotoxin Tolerance—Increased Disease Susceptibility with a Focus on Cancer
9. Clinical and Therapeutic Applications Related to Endotoxin
9.1. Sepsis and Anti-Endotoxin Strategies
9.2. LPS as a Vaccine Adjuvant
9.3. Endotoxin as a Biomarker and Diagnostic Tool
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASC | Apoptosis-associated speck-like protein containing a CARD |
| CAMPs | Cationic anti-microbial peptides |
| CLP | Caecal ligation and puncture |
| CM | Conditioned medium |
| COX | Cyclooxygenase |
| ERK 1/2 | Extracellular signal-regulated kinases 1/2 |
| ET | Endotoxin tolerance |
| GBPs | Guanylate-binding proteins |
| GLA | Glucopyranosyl lipid A |
| GSDMD | Gasdermin D |
| HBV | Hepatitis B virus |
| HIV | Human immunodeficiency virus |
| hPDLCs | Human periodontal ligament cells |
| HPV | Human papillomavirus |
| IFN | Interferon |
| IL | Interleukin |
| IL-1ra | Interleukin-1 receptor antagonist |
| iNOS | Inducible nitric oxide synthase |
| IR | Ischemia–reperfusion |
| IRAK | Interleukin receptor-associated kinase |
| IRF3 | Interferon regulatory factor 3 |
| JNK | Jun N-terminal kinase |
| LBP | LPS binding protein |
| LOS | Lipooligosaccharide |
| LPS | Lipopolysaccharide |
| LRR | Leucine-rich repeat |
| MAPK | Mitogen-activated protein kinase |
| MD2 | Myeloid differentiation protein 2 |
| MoETs | Monocyte-derived endotoxin-tolerant macrophages |
| MoLPS | Monocyte-derived endotoxin-treated macrophages |
| MPL | Monophosphoryl lipid A |
| MyD88 | Myeloid differentiation primary response gene 88 |
| NAFLD | Nonalcoholic fatty liver disease |
| NET | Neutrophil extracellular trap |
| NF-κB | Nuclear factor-κB |
| NLRP3 | Receptors and pyrin domain-containing protein 3 |
| NOD | Nucleotide-binding oligomerization domain |
| OXPHOS | Oxidative phosphorylation |
| PAMP | Pathogen-associated molecular pattern |
| PEtN | 2-aminoethylphosphate |
| PHAD | Phosphorylated hexaacyl disaccharide |
| PMN | Polymorphonuclear neutrophil |
| PMX-HP | Polymyxin B hemoperfusion cartridge |
| RIP1 | Receptor-interacting protein 1 |
| ROS | Reactive oxygen species |
| RSV | Respiratory syncytial virus |
| SAP | Severe acute pancreatitis |
| SIRS | Systemic inflammatory response syndrome |
| SLA | Second-generation lipid adjuvant |
| SOCS1 | Suppressor of cytokine signalling 1 |
| TAK1 | Transforming growth factor-β-activated kinase 1 |
| TBK1 | TANK binding kinase 1 |
| TGF-β | Transforming growth factor-β |
| TIR | Toll-interleukin-1receptor |
| TIRAP | TIR domain-containing adaptor protein |
| TLR4 | Toll-like receptor 4 |
| TME | Tumour microenvironment |
| TNF-α | Tumor necrosis factor α |
| TRAF3 | TNF receptor-associated factor 3 |
| TRAF6 | Tumour necrosis factor receptor-associated factor 6 |
| TRAM | Toll/IL-1 receptor domain-containing adaptor-inducing IFN-β-related adaptor molecule |
| TRIF | Toll/IL-1 receptor domain-containing adaptor-inducing interferon-β |
| TRIM8 | Tripartite motif containing 8 |
References
- Zhang, L.; Wang, C.C. Inflammatory Response of Macrophages in Infection. Hepatobiliary Pancreat. Dis. Int. 2014, 13, 138–152. [Google Scholar] [CrossRef]
- Page, M.J.; Kell, D.B.; Pretorius, E. The Role of Lipopolysaccharide-Induced Cell Signalling in Chronic Inflammation. Chronic Stress 2022, 6, 24705470221076390. [Google Scholar] [CrossRef]
- Arya, P.; Sharma, V.; Singh, P.; Thapliyal, S.; Sharma, M. Bacterial Endotoxin-Lipopolysaccharide Role in Inflammatory Diseases: An Overview. Iran. J. Basic Med. Sci. 2025, 28, 553–564. [Google Scholar] [CrossRef]
- Määttä, A.M.; Salminen, A.; Pietiäinen, M.; Leskelä, J.; Palviainen, T.; Sattler, W.; Sinisalo, J.; Salomaa, V.; Kaprio, J.; Pussinen, P.J. Endotoxemia Is Associated with an Adverse Metabolic Profile. Innate Immun. 2021, 27, 3–14. [Google Scholar] [CrossRef]
- Denamur, E.; Clermont, O.; Bonacorsi, S.; Gordon, D. The Population Genetics of Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2021, 19, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhao, G.; Chao, X.; Xie, L.; Wang, H. The Characteristic of Virulence, Biofilm and Antibiotic Resistance of Klebsiella Pneumoniae. Int. J. Environ. Res. Public Health 2020, 17, 6278. [Google Scholar] [CrossRef] [PubMed]
- Swain, J.; Askenasy, I.; Rudland Nazeer, R.; Ho, P.M.; Labrini, E.; Mancini, L.; Xu, Q.; Hollendung, F.; Sheldon, I.; Dickson, C.; et al. Pathogenicity and Virulence of Pseudomonas Aeruginosa: Recent Advances and under-Investigated Topics. Virulence 2025, 16, 2503430. [Google Scholar] [CrossRef]
- Di Lorenzo, F.; Duda, K.A.; Lanzetta, R.; Silipo, A.; De Castro, C.; Molinaro, A. A Journey from Structure to Function of Bacterial Lipopolysaccharides. Chem. Rev. 2022, 122, 15767–15821. [Google Scholar] [CrossRef]
- Lorenzo, F.D.; Palmigiano, A.; Paciello, I.; Pallach, M.; Garozzo, D.; Bernardini, M.L.; Cono, V.L.; Yakimov, M.M.; Molinaro, A.; Silipo, A. The Deep-Sea Polyextremophile Halobacteroides Lacunaris TB21 Rough-Type LPS: Structure and Inhibitory Activity towards Toxic LPS. Mar. Drugs 2017, 15, 201. [Google Scholar] [CrossRef] [PubMed]
- Krzyżewska, E.; Rybka, J. Biosynthesis of lipopolysaccharides with different length of the O-specific region as a virulence factor of Gram-negative bacteria. Postep. Hig. Med. Dosw. 2018, 72, 573–586. [Google Scholar] [CrossRef]
- Kaszowska, M. Chemical structure and biosynthesis of lipopolysaccharide--important component of the cell envelope of Gram-negative bacteria. Postep. Hig. Med. Dosw. (Online) 2004, 58, 333–342. [Google Scholar]
- Lodowska, J.; Wolny, D.; Węglarz, L.; Dzierżewicz, Z. Heterogenność strukturalna lipidu A bakterii Gram-ujemnych. Postep. Hig. Med. Dosw. 2007, 61, 106–121. [Google Scholar]
- Samuel, G.; Reeves, P. Biosynthesis of O-Antigens: Genes and Pathways Involved in Nucleotide Sugar Precursor Synthesis and O-Antigen Assembly. Carbohydr. Res. 2003, 338, 2503–2519. [Google Scholar] [CrossRef] [PubMed]
- Silipo, A.; Gargiulo, V.; Sturiale, L.; Marchetti, R.; Prizeman, P.; Grant, W.D.; De Castro, C.; Garozzo, D.; Lanzetta, R.; Parrilli, M.; et al. The Structure of the Carbohydrate Backbone of the Lipooligosaccharide from an Alkaliphilic Halomonas Sp. Carbohydr. Res. 2010, 345, 1971–1975. [Google Scholar] [CrossRef]
- John, C.M.; Phillips, N.J.; Stein, D.C.; Jarvis, G.A. Innate Immune Response to Lipooligosaccharide: Pivotal Regulator of the Pathobiology of Invasive Neisseria Meningitidis Infections. Pathog. Dis. 2017, 75, ftx030. [Google Scholar] [CrossRef] [PubMed]
- Pollard, A.; St Michael, F.; Connor, L.; Nichols, W.; Cox, A. Structural Characterization of Haemophilus parainfluenzae Lipooligosaccharide and Elucidation of Its Role in Adherence Using an Outer Core Mutant. Can. J. Microbiol. 2008, 54, 906–917. [Google Scholar] [CrossRef]
- Di Lorenzo, F.; Palmigiano, A.; Silipo, A.; Desaki, Y.; Garozzo, D.; Lanzetta, R.; Shibuya, N.; Molinaro, A. The Structure of the Lipooligosaccharide from Xanthomonas oryzae Pv. Oryzae: The Causal Agent of the Bacterial Leaf Blight in Rice. Carbohydr. Res. 2016, 427, 38–43. [Google Scholar] [CrossRef]
- Culebro, A.; Machado, M.P.; Carriço, J.A.; Rossi, M. Origin, Evolution, and Distribution of the Molecular Machinery for Biosynthesis of Sialylated Lipooligosaccharide Structures in Campylobacter coli. Sci. Rep. 2018, 8, 3028. [Google Scholar] [CrossRef]
- Mookherjee, N.; Anderson, M.A.; Haagsman, H.P.; Davidson, D.J. Antimicrobial Host Defence Peptides: Functions and Clinical Potential. Nat. Rev. Drug Discov. 2020, 19, 311–332. [Google Scholar] [CrossRef]
- Lorenzo, F.D.; Sturiale, L.; Palmigiano, A.; Lembo-Fazio, L.; Paciello, I.; Coutinho, C.P.; Sá-Correia, I.; Bernardini, M.; Lanzetta, R.; Garozzo, D.; et al. Chemistry and Biology of the Potent Endotoxin from a Burkholderia Dolosa Clinical Isolate from a Cystic Fibrosis Patient. ChemBioChem 2013, 14, 1105–1115. [Google Scholar] [CrossRef]
- McCoy, A.J.; Liu, H.; Falla, T.J.; Gunn, J.S. Identification of Proteus Mirabilis Mutants with Increased Sensitivity to Antimicrobial Peptides. Antimicrob. Agents Chemother. 2001, 45, 2030–2037. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.Y.; Chen, Y.F.; Peng, H.L. Molecular Characterization of the PhoPQ-PmrD-PmrAB Mediated Pathway Regulating Polymyxin B Resistance in Klebsiella Pneumoniae CG43. J. Biomed. Sci. 2010, 17, 60. [Google Scholar] [CrossRef] [PubMed]
- Marceau, M.; Sebbane, F.; Collyn, F.; Simonet, M. Function and Regulation of the Salmonella-like pmrF Antimicrobial Peptide Resistance Operon in Yersinia Pseudotuberculosis. Adv. Exp. Med. Biol. 2003, 529, 253–256. [Google Scholar] [CrossRef]
- Richards, S.M.; Strandberg, K.L.; Conroy, M.; Gunn, J.S. Cationic Antimicrobial Peptides Serve as Activation Signals for the Salmonella Typhimurium PhoPQ and PmrAB Regulons in Vitro and in Vivo. Front. Cell. Infect. Microbiol. 2012, 2, 102. [Google Scholar] [CrossRef]
- Chmiela, M.; Miszczyk, E.; Rudnicka, K. Structural Modifications of Helicobacter Pylori Lipopolysaccharide: An Idea for How to Live in Peace. World J. Gastroenterol. 2014, 20, 9882–9897. [Google Scholar] [CrossRef]
- Ding, P.H.; Wang, C.Y.; Darveau, R.P.; Jin, L. Porphyromonas gingivalis LPS Stimulates the Expression of LPS-Binding Protein in Human Oral Keratinocytes in Vitro. Innate Immun. 2013, 19, 66–75. [Google Scholar] [CrossRef]
- Paciello, I.; Silipo, A.; Lembo-Fazio, L.; Curcurù, L.; Zumsteg, A.; Noël, G.; Ciancarella, V.; Sturiale, L.; Molinaro, A.; Bernardini, M.L. Intracellular Shigella Remodels Its LPS to Dampen the Innate Immune Recognition and Evade Inflammasome Activation. Proc. Natl. Acad. Sci. USA 2013, 110, E4345–E4354. [Google Scholar] [CrossRef]
- Ayoub Moubareck, C.; Hammoudi Halat, D. Insights into Acinetobacter Baumannii: A Review of Microbiological, Virulence, and Resistance Traits in a Threatening Nosocomial Pathogen. Antibiotics 2020, 9, 119. [Google Scholar] [CrossRef]
- Steimle, A.; Autenrieth, I.B.; Frick, J.S. Structure and Function: Lipid A Modifications in Commensals and Pathogens. Int. J. Med. Microbiol. 2016, 306, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Needham, B.D.; Trent, M.S. Fortifying the Barrier: The Impact of Lipid A Remodelling on Bacterial Pathogenesis. Nat. Rev. Microbiol. 2013, 11, 467–481. [Google Scholar] [CrossRef]
- Pither, M.D.; Silipo, A.; Di Lorenzo, F.; Molinaro, A. 5.13—Glycans in Bacterial Infections: Gram-Negative Infections in the Respiratory Tract. In Comprehensive Glycoscience, 2nd ed.; Barchi, J.J., Ed.; Elsevier: Oxford, UK, 2021; pp. 233–249. ISBN 978-0-12-822244-7. [Google Scholar]
- Jennings, M.P.; Srikhanta, Y.N.; Moxon, E.R.; Kramer, M.; Poolman, J.T.; Kuipers, B.; van der Ley, P. The Genetic Basis of the Phase Variation Repertoire of Lipopolysaccharide Immunotypes in Neisseria Meningitidis. Microbiology 1999, 145, 3013–3021. [Google Scholar] [CrossRef]
- Schweda, E.K.H.; Twelkmeyer, B.; Li, J. Profiling Structural Elements of Short-Chain Lipopolysaccharide of Non-Typeable Haemophilus Influenzae. Innate Immun. 2008, 14, 199–211. [Google Scholar] [CrossRef]
- Moran, A.P.; Prendergast, M.M.; Appelmelk, B.J. Molecular Mimicry of Host Structures by Bacterial Lipopolysaccharides and Its Contribution to Disease. FEMS Immunol. Med. Microbiol. 1996, 16, 105–115. [Google Scholar] [CrossRef]
- Varki, A. Since There Are PAMPs and DAMPs, There Must Be SAMPs? Glycan “Self-Associated Molecular Patterns” Dampen Innate Immunity, but Pathogens Can Mimic Them. Glycobiology 2011, 21, 1121–1124. [Google Scholar] [CrossRef]
- Mueller, M.; Lindner, B.; Kusumoto, S.; Fukase, K.; Schromm, A.B.; Seydel, U. Aggregates Are the Biologically Active Units of Endotoxin. J. Biol. Chem. 2004, 279, 26307–26313. [Google Scholar] [CrossRef]
- Brandenburg, K.; Seydel, U.; Schromm, A.B.; Loppnow, H.; Koch, M.H.J.; Rietschel, E.T. Conformation of Lipid A, the Endotoxic Center of Bacterial Lipopolysaccharide. J. Endotoxin Res. 1996, 3, 173–178. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, J.; Liu, X.; Chen, X.; Wang, J. Decoding Nature: Multi-Target Anti-Inflammatory Mechanisms of Natural Products in the TLR4/NF-κB Pathway. Front. Pharmacol. 2025, 15, 1467193. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.A.; Rowe, D.C.; Golenbock, D.T. Endotoxin Recognition and Signal Transduction by the TLR4/MD2-Complex. Microbes Infect. 2004, 6, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 Trafficking and Its Influence on LPS-Induced pro-Inflammatory Signaling. Cell. Mol. Life Sci. 2020, 78, 1233–1261. [Google Scholar] [CrossRef]
- West, A.P.; Koblansky, A.A.; Ghosh, S. Recognition and Signaling by Toll-like Receptors. Annu. Rev. Cell Dev. Biol. 2006, 22, 409–437. [Google Scholar] [CrossRef] [PubMed]
- Płóciennikowska, A.; Hromada-Judycka, A.; Borzęcka, K.; Kwiatkowska, K. Co-Operation of TLR4 and Raft Proteins in LPS-Induced pro-Inflammatory Signaling. Cell. Mol. Life Sci. 2015, 72, 557–581. [Google Scholar] [CrossRef]
- Jiang, Z.; Georgel, P.; Du, X.; Shamel, L.; Sovath, S.; Mudd, S.; Huber, M.; Kalis, C.; Keck, S.; Galanos, C.; et al. CD14 Is Required for MyD88-Independent LPS Signaling. Nat. Immunol. 2005, 6, 565–570. [Google Scholar] [CrossRef]
- Oshiumi, H.; Matsumoto, M.; Funami, K.; Akazawa, T.; Seya, T. TICAM-1, an Adaptor Molecule That Participates in Toll-like Receptor 3-Mediated Interferon-Beta Induction. Nat. Immunol. 2003, 4, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Sato, S.; Mori, K.; Hoshino, K.; Takeuchi, O.; Takeda, K.; Akira, S. Cutting Edge: A Novel Toll/IL-1 Receptor Domain-Containing Adapter That Preferentially Activates the IFN-Beta Promoter in the Toll-like Receptor Signaling. J. Immunol. 2002, 169, 6668–6672. [Google Scholar] [CrossRef]
- Takeda, K.; Akira, S. TLR Signaling Pathways. Semin. Immunol. 2004, 16, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Youn, H.S.; Lee, J.Y.; Fitzgerald, K.A.; Young, H.A.; Akira, S.; Hwang, D.H. Specific Inhibition of MyD88-Independent Signaling Pathways of TLR3 and TLR4 by Resveratrol: Molecular Targets Are TBK1 and RIP1 in TRIF Complex. J. Immunol. 2005, 175, 3339–3346. [Google Scholar] [CrossRef]
- Häcker, H.; Tseng, P.H.; Karin, M. Expanding TRAF Function: TRAF3 as a Tri-Faced Immune Regulator. Nat. Rev. Immunol. 2011, 11, 457–468. [Google Scholar] [CrossRef]
- Kawai, T.; Takeuchi, O.; Fujita, T.; Inoue, J.; Mühlradt, P.F.; Sato, S.; Hoshino, K.; Akira, S. Lipopolysaccharide Stimulates the MyD88-Independent Pathway and Results in Activation of IFN-Regulatory Factor 3 and the Expression of a Subset of Lipopolysaccharide-Inducible Genes. J. Immunol. 2001, 167, 5887–5894. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.H.; Kim, I.K.; Lee, H.I.; Joo, H.; Lim, J.U.; Lee, J.; Lee, S.H.; Moon, H.S. Chronic Intermittent Hypoxia Induces Liver Fibrosis in Mice with Diet-Induced Obesity via TLR4/MyD88/MAPK/NF-kB Signaling Pathways. Biochem. Biophys. Res. Commun. 2017, 490, 349–355. [Google Scholar] [CrossRef]
- Wang, Y.; Tu, Q.; Yan, W.; Xiao, D.; Zeng, Z.; Ouyang, Y.; Huang, L.; Cai, J.; Zeng, X.; Chen, Y.J.; et al. CXC195 Suppresses Proliferation and Inflammatory Response in LPS-Induced Human Hepatocellular Carcinoma Cells via Regulating TLR4-MyD88-TAK1-Mediated NF-κB and MAPK Pathway. Biochem. Biophys. Res. Commun. 2015, 456, 373–379. [Google Scholar] [CrossRef]
- Xing, Y.; Yao, X.; Li, H.; Xue, G.; Guo, Q.; Yang, G.; An, L.; Zhang, Y.; Meng, G. Cutting Edge: TRAF6 Mediates TLR/IL-1R Signaling-Induced Nontranscriptional Priming of the NLRP3 Inflammasome. J. Immunol. 2017, 199, 1561–1566. [Google Scholar] [CrossRef]
- Platnich, J.M.; Muruve, D.A. NOD-like Receptors and Inflammasomes: A Review of Their Canonical and Non-Canonical Signaling Pathways. Arch. Biochem. Biophys. 2019, 670, 4–14. [Google Scholar] [CrossRef]
- Bauernfeind, F.G.; Horvath, G.; Stutz, A.; Alnemri, E.S.; MacDonald, K.; Speert, D.; Fernandes-Alnemri, T.; Wu, J.; Monks, B.G.; Fitzgerald, K.A.; et al. Cutting Edge: NF-kappaB Activating Pattern Recognition and Cytokine Receptors License NLRP3 Inflammasome Activation by Regulating NLRP3 Expression. J. Immunol. 2009, 183, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, S.O.; Russo, A.J.; Kumari, P.; Vanaja, S.K.; Rathinam, V.A. A TLR4-Independent Critical Role for CD14 in Intracellular LPS Sensing. Cell Rep. 2022, 39, 110755. [Google Scholar] [CrossRef]
- Diamond, C.E.; Khameneh, H.J.; Brough, D.; Mortellaro, A. Novel Perspectives on Non-Canonical Inflammasome Activation. ImmunoTargets Ther. 2015, 4, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Hagar, J.A.; Powell, D.A.; Aachoui, Y.; Ernst, R.K.; Miao, E.A. Cytoplasmic LPS Activates Caspase-11: Implications in TLR4-Independent Endotoxic Shock. Science 2013, 341, 1250–1253. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, Y.; Gao, W.; Ding, J.; Li, P.; Hu, L.; Shao, F. Inflammatory Caspases Are Innate Immune Receptors for Intracellular LPS. Nature 2014, 514, 187–192. [Google Scholar] [CrossRef]
- He, W.; Wan, H.; Hu, L.; Chen, P.; Wang, X.; Huang, Z.; Yang, Z.H.; Zhong, C.Q.; Han, J. Gasdermin D Is an Executor of Pyroptosis and Required for Interleukin-1β Secretion. Cell Res. 2015, 25, 1285–1298. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by Inflammatory Caspases Determines Pyroptotic Cell Death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Elizagaray, M.L.; Gomes, M.T.R.; Guimaraes, E.S.; Rumbo, M.; Hozbor, D.F.; Oliveira, S.C.; Moreno, G. Canonical and Non-Canonical Inflammasome Activation by Outer Membrane Vesicles Derived From Bordetella Pertussis. Front. Immunol. 2020, 11, 1879. [Google Scholar] [CrossRef]
- Wrotek, S.; LeGrand, E.K.; Dzialuk, A.; Alcock, J. Let Fever Do Its Job: The Meaning of Fever in the Pandemic Era. Evol. Med. Public. Health 2021, 9, 26–35. [Google Scholar] [CrossRef] [PubMed]
- González Plaza, J.J.; Hulak, N.; Zhumadilov, Z.; Akilzhanova, A. Fever as an Important Resource for Infectious Diseases Research. Intractable Rare Dis. Res. 2016, 5, 97–102. [Google Scholar] [CrossRef]
- Roth, J.; Blatteis, C.M. Mechanisms of Fever Production and Lysis: Lessons from Experimental LPS Fever. Compr. Physiol. 2014, 4, 1563–1604. [Google Scholar] [CrossRef] [PubMed]
- Wrotek, S.; Sobocińska, J.; Kozłowski, H.M.; Pawlikowska, M.; Jędrzejewski, T.; Dzialuk, A. New Insights into the Role of Glutathione in the Mechanism of Fever. Int. J. Mol. Sci. 2020, 21, 1393. [Google Scholar] [CrossRef] [PubMed]
- Kozak, W.; Jedrzejewski, T.; Pawlikowska, M.; Piotrowski, J.; Wrotek, S. Toward Antitumor Immunity and Febrile Infections: Gamma/Delta (Γδ) T Cells Hypothesis. Q. Rev. Biol. 2018, 93, 187–205. [Google Scholar] [CrossRef]
- Glaros, T.G.; Chang, S.; Gilliam, E.A.; Maitra, U.; Deng, H.; Li, L. Causes and Consequences of Low Grade Endotoxemia and Inflammatory Diseases. FBS 2013, 5, 754–765. [Google Scholar] [CrossRef]
- Park, J.E.; Park, H.Y.; Kim, Y.S.; Park, M. The Role of Diet, Additives, and Antibiotics in Metabolic Endotoxemia and Chronic Diseases. Metabolites 2024, 14, 704. [Google Scholar] [CrossRef]
- Mohammad, S.; Thiemermann, C. Role of Metabolic Endotoxemia in Systemic Inflammation and Potential Interventions. Front. Immunol. 2020, 11, 594150. [Google Scholar] [CrossRef]
- Kusters, J.G.; van Vliet, A.H.M.; Kuipers, E.J. Pathogenesis of Helicobacter Pylori Infection. Clin. Microbiol. Rev. 2006, 19, 449–490. [Google Scholar] [CrossRef]
- Hajishengallis, G. The Inflammophilic Character of the Periodontitis-Associated Microbiota. Mol. Oral. Microbiol. 2014, 29, 248–257. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Fei, N.; Zhao, L. An Opportunistic Pathogen Isolated from the Gut of an Obese Human Causes Obesity in Germfree Mice. ISME J. 2013, 7, 880–884. [Google Scholar] [CrossRef]
- d’Hennezel, E.; Abubucker, S.; Murphy, L.O.; Cullen, T.W. Total Lipopolysaccharide from the Human Gut Microbiome Silences Toll-Like Receptor Signaling. mSystems 2017, 2, e00046-17. [Google Scholar] [CrossRef]
- Miller, S.; Ernst, R.; Bader, M. LPS, TLR4 and Infectious Disease Diversity. Nat. Rev. Microbiol. 2005, 3, 36–46. Available online: https://www.nature.com/articles/nrmicro1068 (accessed on 9 September 2025). [CrossRef]
- Caesar, R.; Reigstad, C.S.; Bäckhed, H.K.; Reinhardt, C.; Ketonen, M.; Lundén, G.Ö.; Cani, P.D.; Bäckhed, F. Gut-Derived Lipopolysaccharide Augments Adipose Macrophage Accumulation but Is Not Essential for Impaired Glucose or Insulin Tolerance in Mice. Gut 2012, 61, 1701–1707. [Google Scholar] [CrossRef]
- Miquel, S.; Martín, R.; Rossi, O.; Bermúdez-Humarán, L.; Chatel, J.; Sokol, H.; Thomas, M.; Wells, J.; Langella, P. Faecalibacterium prausnitzii and Human Intestinal Health. Curr. Opin. Microbiol. 2013, 16, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Levy, M.; Grosheva, I.; Zheng, D.; Soffer, E.; Blacher, E.; Braverman, S.; Tengeler, A.C.; Barak, O.; Elazar, M.; et al. Hyperglycemia Drives Intestinal Barrier Dysfunction and Risk for Enteric Infection. Science 2018, 359, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Serino, M.; Luche, E.; Gres, S.; Baylac, A.; Bergé, M.; Cenac, C.; Waget, A.; Klopp, P.; Iacovoni, J.; Klopp, C.; et al. Metabolic Adaptation to a High-Fat Diet Is Associated with a Change in the Gut Microbiota. Gut 2012, 61, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Vatanen, T.; Kostic, A.D.; d’Hennezel, E.; Siljander, H.; Franzosa, E.A.; Yassour, M.; Kolde, R.; Vlamakis, H.; Arthur, T.D.; Hämäläinen, A.M.; et al. Variation in Microbiome LPS Immunogenicity Contributes to Autoimmunity in Humans. Cell 2016, 165, 842–853. [Google Scholar] [CrossRef]
- Guo, H.; Callaway, J.B.; Ting, J.P.Y. Inflammasomes: Mechanism of Action, Role in Disease, and Therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef]
- Boutagy, N.E.; McMillan, R.P.; Frisard, M.I.; Hulver, M.W. Metabolic Endotoxemia with Obesity: Is It Real and Is It Relevant? Biochimie 2016, 124, 11–20. [Google Scholar] [CrossRef]
- Larsson, L.; Garaicoa-Pazmino, C.; Asa’ad, F.; Castilho, R.M. Understanding the Role of Endotoxin Tolerance in Chronic Inflammatory Conditions and Periodontal Disease. J. Clin. Periodontol. 2022, 49, 270–279. [Google Scholar] [CrossRef]
- Beeson, P.B. Development of Tolerance to Typhoid Bacterial Pyrogen and Its Abolition by Reticulo-Endothelial Blockade. Proc. Soc. Exp. Biol. Med. 1946, 61, 248–250. [Google Scholar] [CrossRef] [PubMed]
- Greisman, S.E.; Hornick, R.B. The Nature of Endotoxin Tolerance. Trans. Am. Clin. Climatol. Assoc. 1975, 86, 43–50. [Google Scholar]
- Kox, M.; de Kleijn, S.; Pompe, J.C.; Ramakers, B.P.; Netea, M.G.; van der Hoeven, J.G.; Hoedemaekers, C.W.; Pickkers, P. Differential Ex Vivo and in Vivo Endotoxin Tolerance Kinetics Following Human Endotoxemia. Crit. Care Med. 2011, 39, 1866–1870. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Cao, S.; Zhou, Y.; Xiong, Y. Recent Advances in Endotoxin Tolerance. J. Cell. Biochem. 2019, 120, 56–70. [Google Scholar] [CrossRef]
- West, M.A.; Heagy, W. Endotoxin Tolerance: A Review. Crit. Care Med. 2002, 30, S64–S73. [Google Scholar] [CrossRef]
- Cohen, P. The Search for Physiological Substrates of MAP and SAP Kinases in Mammalian Cells. Trends Cell Biol. 1997, 7, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Kopanakis, K.; Tzepi, I.M.; Pistiki, A.; Carrer, D.P.; Netea, M.G.; Georgitsi, M.; Lymperi, M.; Droggiti, D.-I.; Liakakos, T.; Machairas, A.; et al. Pre-Treatment with Low-Dose Endotoxin Prolongs Survival from Experimental Lethal Endotoxic Shock: Benefit for Lethal Peritonitis by Escherichia coli. Cytokine 2013, 62, 382–388. [Google Scholar] [CrossRef]
- Loosbroock, C.; Hunter, K.W. Inhibiting TNF-α Signaling Does Not Attenuate Induction of Endotoxin Tolerance. J. Inflamm. Res. 2014, 7, 159–167. [Google Scholar] [CrossRef][Green Version]
- Nakagawa, R.; Naka, T.; Tsutsui, H.; Fujimoto, M.; Kimura, A.; Abe, T.; Seki, E.; Sato, S.; Takeuchi, O.; Takeda, K.; et al. SOCS-1 Participates in Negative Regulation of LPS Responses. Immunity 2002, 17, 677–687. [Google Scholar] [CrossRef]
- Lee, M.J.; Bae, J.; Lee, J.H.; Park, Y.J.; Lee, H.A.R.; Mun, S.; Kim, Y.S.; Yune, C.J.; Chung, T.N.; Kim, K. Serial Change of Endotoxin Tolerance in a Polymicrobial Sepsis Model. Int. J. Mol. Sci. 2022, 23, 6581. [Google Scholar] [CrossRef]
- Zhang, W.; Shen, X.; Xie, L.; Chu, M.; Ma, Y. MicroRNA-181b Regulates Endotoxin Tolerance by Targeting IL-6 in Macrophage RAW264.7 Cells. J. Inflamm. 2015, 12, 18. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, L.; Liang, X.; Zhu, G. MicroRNA-155 Promotes Atherosclerosis Inflammation via Targeting SOCS1. Cell. Physiol. Biochem. 2015, 36, 1371–1381. [Google Scholar] [CrossRef]
- Son, C.J.; Carnino, J.M.; Lee, H.; Jin, Y. Emerging Roles of Circular RNA in Macrophage Activation and Inflammatory Lung Responses. Cells 2024, 13, 1407. [Google Scholar] [CrossRef]
- Ng, W.L.; Marinov, G.K.; Chin, Y.M.; Lim, Y.Y.; Ea, C.K. Transcriptomic Analysis of the Role of RasGEF1B Circular RNA in the TLR4/LPS Pathway. Sci. Rep. 2017, 7, 12227. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Liang, Q.; Qin, C.; Li, Y. CircANKRD36 Knockdown Suppressed Cell Viability and Migration of LPS-Stimulated RAW264.7 Cells by Sponging MiR-330. Inflammation 2021, 44, 2044–2053. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Hong, R.; Jin, Y. Altered Circular RNA Expressions in Extracellular Vesicles from Bronchoalveolar Lavage Fluids in Mice after Bacterial Infections. Front. Immunol. 2024, 15, 1354676. [Google Scholar] [CrossRef]
- Michel, O.; LeVan, T.D.; Stern, D.; Dentener, M.; Thorn, J.; Gnat, D.; Beijer, M.L.; Cochaux, P.; Holt, P.G.; Martinez, F.D.; et al. Systemic Responsiveness to Lipopolysaccharide and Polymorphisms in the Toll-like Receptor 4 Gene in Human Beings. J. Allergy Clin. Immunol. 2003, 112, 923–929. [Google Scholar] [CrossRef]
- Schmitt, C.; Humeny, A.; Becker, C.M.; Brune, K.; Pahl, A. Polymorphisms of TLR4: Rapid Genotyping and Reduced Response to Lipopolysaccharide of TLR4 Mutant Alleles. Clin. Chem. 2002, 48, 1661–1667. [Google Scholar] [CrossRef] [PubMed]
- Arbour, N.C.; Lorenz, E.; Schutte, B.C.; Zabner, J.; Kline, J.N.; Jones, M.; Frees, K.; Watt, J.L.; Schwartz, D.A. TLR4 Mutations Are Associated with Endotoxin Hyporesponsiveness in Humans. Nat. Genet. 2000, 25, 187–191. [Google Scholar] [CrossRef]
- Norata, G.D.; Garlaschelli, K.; Ongari, M.; Raselli, S.; Grigore, L.; Benvenuto, F.; Maggi, F.M.; Catapano, A.L. Effect of the Toll-like Receptor 4 (TLR-4) Variants on Intima-Media Thickness and Monocyte-Derived Macrophage Response to LPS. J. Intern. Med. 2005, 258, 21–27. [Google Scholar] [CrossRef]
- Richard, K.; Piepenbrink, K.H.; Shirey, K.A.; Gopalakrishnan, A.; Nallar, S.; Prantner, D.J.; Perkins, D.J.; Lai, W.; Vlk, A.; Toshchakov, V.Y.; et al. A Mouse Model of Human TLR4 D299G/T399I SNPs Reveals Mechanisms of Altered LPS and Pathogen Responses. J. Exp. Med. 2021, 218, e20200675. [Google Scholar] [CrossRef]
- Lundberg, A.; Wikberg, L.A.; Ilonen, J.; Vaarala, O.; Böttcher, M.F. Lipopolysaccharide-Induced Immune Responses in Relation to the TLR4(Asp299Gly) Gene Polymorphism. Clin. Vaccine Immunol. 2008, 15, 1878–1883. [Google Scholar] [CrossRef]
- Roy, K.; Kozłowski, H.M.; Jędrzejewski, T.; Sobocińska, J.; Maciejewski, B.; Dzialuk, A.; Wrotek, S. Endotoxin Tolerance Creates Favourable Conditions for Cancer Development. Cancers 2023, 15, 5113. [Google Scholar] [CrossRef] [PubMed]
- Pena, O.M.; Pistolic, J.; Raj, D.; Fjell, C.D.; Hancock, R.E.W. Endotoxin Tolerance Represents a Distinctive State of Alternative Polarization (M2) in Human Mononuclear Cells. J. Immunol. 2011, 186, 7243–7254. [Google Scholar] [CrossRef] [PubMed]
- Hosseinalizadeh, H.; Mohamadzadeh, O.; Kahrizi, M.S.; Razaghi Bahabadi, Z.; Klionsky, D.J.; Mirzei, H. TRIM8: A Double-Edged Sword in Glioblastoma with the Power to Heal or Hurt. Cell. Mol. Biol. Lett. 2023, 28, 6. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Wang, W.; Li, Y.; Yang, D.; Li, X.; Shen, C.; Liu, Y.; Ke, X.; Guo, S.; Guo, Z. HSP90AA1-Mediated Autophagy Promotes Drug Resistance in Osteosarcoma. J. Exp. Clin. Cancer Res. 2018, 37, 201. [Google Scholar] [CrossRef]
- Xu, Z.; Han, X.; Ou, D.; Liu, T.; Li, Z.; Jiang, G.; Liu, J.; Zhang, J. Targeting PI3K/AKT/mTOR-Mediated Autophagy for Tumor Therapy. Appl. Microbiol. Biotechnol. 2020, 104, 575–587. [Google Scholar] [CrossRef]
- Fernández, E.D.; Flohé, S.; Siemers, F.; Nau, M.; Ackermann, M.; Ruwe, M.; Schade, F.U. Endotoxin Tolerance Protects against Local Hepatic Ischemia/Reperfusion Injury in the Rat. J. Endotoxin Res. 2000, 6, 321–328. [Google Scholar] [CrossRef]
- Godet, C.; Goujon, J.M.; Petit, I.; Lecron, J.C.; Hauet, T.; Mauco, G.; Carretier, M.; Robert, R. Endotoxin Tolerance Enhances Interleukin-10 Renal Expression and Decreases Ischemia-Reperfusion Renal Injury in Rats. Shock 2006, 25, 384–388. [Google Scholar] [CrossRef]
- Rosenzweig, H.L.; Lessov, N.S.; Henshall, D.C.; Minami, M.; Simon, R.P.; Stenzel-Poore, M.P. Endotoxin Preconditioning Prevents Cellular Inflammatory Response during Ischemic Neuroprotection in Mice. Stroke 2004, 35, 2576–2581. [Google Scholar] [CrossRef]
- Yao, L.; Li, P.; Chen, Q.; Hu, A.; Wu, Y.; Li, B. Protective Effects of Endotoxin Tolerance on Peripheral Lipopolysaccharide-Induced Neuroinflammation and Dopaminergic Neuronal Injury. Immunopharmacol. Immunotoxicol. 2022, 44, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Chousterman, B.G.; Swirski, F.K.; Weber, G.F. Cytokine Storm and Sepsis Disease Pathogenesis. Semin. Immunopathol. 2017, 39, 517–528. [Google Scholar] [CrossRef]
- Wheeler, D.S.; Lahni, P.M.; Denenberg, A.G.; Poynter, S.E.; Wong, H.R.; Cook, J.A.; Zingarelli, B. Induction of Endotoxin Tolerance Enhances Bacterial Clearance and Survival in Murine Polymicrobial Sepsis. Shock 2008, 30, 267–273. [Google Scholar] [CrossRef]
- Melo, E.S.; Goloubkova, T.; Barbeiro, D.F.; Gorjão, R.; Vasconcelos, D.; Szabo, C.; Curi, R.; de Lima Salgado, T.M.; Velasco, I.T.; Soriano, F.G. Endotoxin Tolerance: Selective Alterations in Gene Expression and Protection against Lymphocyte Death. Immunobiology 2010, 215, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.M.C.; Ariga, S.S.K.; Barbeiro, D.F.; Barbeiro, H.V.; Pimentel, R.N.; Petroni, R.C.; Soriano, F.G. Endotoxin Tolerance Modulates TREG and TH17 Lymphocytes Protecting Septic Mice. Oncotarget 2019, 10, 3451–3461. [Google Scholar] [CrossRef]
- Draisma, A.; Pickkers, P.; Bouw, M.P.W.J.M.; van der Hoeven, J.G. Development of Endotoxin Tolerance in Humans in Vivo. Crit. Care Med. 2009, 37, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Rearte, B.; Maglioco, A.; Balboa, L.; Bruzzo, J.; Landoni, V.I.; Laborde, E.A.; Chiarella, P.; Ruggiero, R.A.; Fernández, G.C.; Isturiz, M.A. Mifepristone (RU486) Restores Humoral and T Cell-Mediated Immune Response in Endotoxin Immunosuppressed Mice. Clin. Exp. Immunol. 2010, 162, 568–577. [Google Scholar] [CrossRef]
- Landoni, V.I.; Chiarella, P.; Martire-Greco, D.; Schierloh, P.; van-Rooijen, N.; Rearte, B.; Palermo, M.S.; Isturiz, M.A.; Fernández, G.C. Tolerance to Lipopolysaccharide Promotes an Enhanced Neutrophil Extracellular Traps Formation Leading to a More Efficient Bacterial Clearance in Mice. Clin. Exp. Immunol. 2012, 168, 153–163. [Google Scholar] [CrossRef]
- López-Collazo, E.; Del Fresno, C. Endotoxin Tolerance and Trained Immunity: Breaking down Immunological Memory Barriers. Front. Immunol. 2024, 15, 1393283. [Google Scholar] [CrossRef]
- Vasilescu, C.; Buttenschoen, K.; Olteanu, M.; Flondor, P. Severe Acute Pancreatitis between Systematic Inflammatory Response Syndrome and Sepsis: Insights from a Mathematical Model of Endotoxin Tolerance. Am. J. Surg. 2007, 194, S33–S38. [Google Scholar] [CrossRef]
- Pena, O.M.; Hancock, D.G.; Lyle, N.H.; Linder, A.; Russell, J.A.; Xia, J.; Fjell, C.D.; Boyd, J.H.; Hancock, R.E.W. An Endotoxin Tolerance Signature Predicts Sepsis and Organ Dysfunction at Initial Clinical Presentation. EBioMedicine 2014, 1, 64–71. [Google Scholar] [CrossRef]
- Leligdowicz, A.; Kamm, J.; Kalantar, K.; Jauregui, A.; Vessel, K.; Caldera, S.; Serpa, P.H.; Abbott, J.; Fang, X.; Tian, X.; et al. Functional Transcriptomic Studies of Immune Responses and Endotoxin Tolerance in Early Human Sepsis. Shock 2022, 57, 180–190. [Google Scholar] [CrossRef]
- Leligdowicz, A.; Langelier, C.; Kamm, J.A.; Jauregui, A.; Vessel, K.; Caldera, S.; Hayakawa, P.; Fang, X.; Abbott, J.A.; Tian, X.; et al. Physiologically Derived Transcriptional Signature of Endotoxin Tolerance Identifies Immune Suppression in Early Sepsis. In A103. SEPSIS: TRANSLATIONAL STUDIES; American Thoracic Society International Conference Abstracts; American Thoracic Society: New York, NY, USA, 2020; p. A2586. [Google Scholar]
- Touyz, L.Z.G. Periodontitis Contributes to Initiation, Progress and Aggravation of Septic Shock; a Feasible Hypothesis. Med. Hypotheses 2013, 81, 650–652. [Google Scholar] [CrossRef] [PubMed]
- Muthukuru, M.; Jotwani, R.; Cutler, C.W. Oral Mucosal Endotoxin Tolerance Induction in Chronic Periodontitis. Infect. Immun. 2005, 73, 687–694. [Google Scholar] [CrossRef] [PubMed]
- van der Poll, T.; van de Veerdonk, F.L.; Scicluna, B.P.; Netea, M.G. The Immunopathology of Sepsis and Potential Therapeutic Targets. Nat. Rev. Immunol. 2017, 17, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Sepsis-Induced Immunosuppression: From Cellular Dysfunctions to Immunotherapy. Nat. Rev. Immunol. 2013, 13, 862–874. [Google Scholar] [CrossRef]
- Shalova, I.N.; Lim, J.Y.; Chittezhath, M.; Zinkernagel, A.S.; Beasley, F.; Hernández-Jiménez, E.; Toledano, V.; Cubillos-Zapata, C.; Rapisarda, A.; Chen, J.; et al. Human Monocytes Undergo Functional Re-Programming during Sepsis Mediated by Hypoxia-Inducible Factor-1α. Immunity 2015, 42, 484–498. [Google Scholar] [CrossRef]
- Foster, S.L.; Hargreaves, D.C.; Medzhitov, R. Gene-Specific Control of Inflammation by TLR-Induced Chromatin Modifications. Nature 2007, 447, 972–978. [Google Scholar] [CrossRef]
- Biswas, S.K.; Lopez-Collazo, E. Endotoxin Tolerance: New Mechanisms, Molecules and Clinical Significance. Trends Immunol. 2009, 30, 475–487. [Google Scholar] [CrossRef]
- Medzhitov, R.; Schneider, D.S.; Soares, M.P. Disease Tolerance as a Defense Strategy. Science 2012, 335, 936–941. [Google Scholar] [CrossRef]
- Cavaillon, J.M.; Adib-Conquy, M. Bench-to-Bedside Review: Endotoxin Tolerance as a Model of Leukocyte Reprogramming in Sepsis. Crit. Care 2006, 10, 233. [Google Scholar] [CrossRef][Green Version]
- Seeley, J.J.; Ghosh, S. Molecular Mechanisms of Innate Memory and Tolerance to LPS. J. Leukoc. Biol. 2017, 101, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Wrotek, S.; Kamecki, K.; Kwiatkowski, S.; Kozak, W. Cancer Patients Report a History of Fewer Fevers during Infections than Healthy Controls. J. Pre-Clin. Clin. Res. 2009, 3, 31–35. [Google Scholar]
- Maurer, S.; Kölmel, K.F. Spontaneous Regression of Advanced Malignant Melanoma. Oncol. Res. Treat. 1998, 21, 14–18. [Google Scholar] [CrossRef]
- Wrotek, S.; Brycht, Ł.; Wrotek, W.; Kozak, W. Fever as a Factor Contributing to Long-Term Survival in a Patient with Metastatic Melanoma: A Case Report. Complement. Ther. Med. 2018, 38, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, D.S.; Tevis, K.M.; Blessing, W.A.; Colson, Y.L.; Zaman, M.H.; Grinstaff, M.W. Breast Cancer Spheroids Reveal a Differential Cancer Stem Cell Response to Chemotherapeutic Treatment. Sci. Rep. 2017, 7, 10382. [Google Scholar] [CrossRef] [PubMed]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H.K. The Third Dimension Bridges the Gap between Cell Culture and Live Tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef]
- Fan, H.; Cook, J.A. Molecular Mechanisms of Endotoxin Tolerance. J. Endotoxin Res. 2004, 10, 71–84. [Google Scholar] [CrossRef]
- Li, Q.; von Ehrlich-Treuenstätt, V.; Schardey, J.; Wirth, U.; Zimmermann, P.; Andrassy, J.; Bazhin, A.V.; Werner, J.; Kühn, F. Gut Barrier Dysfunction and Bacterial Lipopolysaccharides in Colorectal Cancer. J. Gastrointest. Surg. 2023, 27, 1466–1472. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Peng, R.Q.; Wu, X.J.; Xia, Q.; Hou, J.H.; Ding, Y.; Zhou, Q.M.; Zhang, X.; Pang, Z.Z.; Wan, D.S.; et al. The Density of Macrophages in the Invasive Front Is Inversely Correlated to Liver Metastasis in Colon Cancer. J. Transl. Med. 2010, 8, 13. [Google Scholar] [CrossRef]
- Gulubova, M.; Ananiev, J.; Yovchev, Y.; Julianov, A.; Karashmalakov, A.; Vlaykova, T. The Density of Macrophages in Colorectal Cancer Is Inversely Correlated to TGF-Β1 Expression and Patients’ Survival. J. Mol. Histol. 2013, 44, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhang, Z.; Lei, Z.; Lei, P. CD14: Biology and Role in the Pathogenesis of Disease. Cytokine Growth Factor Rev. 2019, 48, 24–31. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, K.H.; Park, H.S.; Choi, S.J. LPS Sensing Mechanism of Human Astrocytes: Evidence of Functional TLR4 Expression and Requirement of Soluble CD14. J. Bacteriol. Virol. 2017, 47, 189. [Google Scholar] [CrossRef]
- Lin, C.Y.; Kao, S.H.; Hung, L.C.; Chien, H.J.; Wang, W.H.; Chang, Y.W.; Chen, Y.H. Lipopolysaccharide-Induced Nitric Oxide and Prostaglandin E2 Production Is Inhibited by Tellimagrandin II in Mouse and Human Macrophages. Life 2021, 11, 411. [Google Scholar] [CrossRef]
- Wautier, J.L.; Wautier, M.P. Pro- and Anti-Inflammatory Prostaglandins and Cytokines in Humans: A Mini Review. Int. J. Mol. Sci. 2023, 24, 9647. [Google Scholar] [CrossRef]
- Martin, M.; Katz, J.; Vogel, S.N.; Michalek, S.M. Differential Induction of Endotoxin Tolerance by Lipopolysaccharides Derived from Porphyromonas gingivalis and Escherichia coli. J. Immunol. 2001, 167, 5278–5285. [Google Scholar] [CrossRef]
- Anavi, S.; Tirosh, O. iNOS as a Metabolic Enzyme under Stress Conditions. Free Radic. Biol. Med. 2020, 146, 16–35. [Google Scholar] [CrossRef]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible Nitric Oxide Synthase: Regulation, Structure, and Inhibition. Med. Res. Rev. 2020, 40, 158–189. [Google Scholar] [CrossRef] [PubMed]
- Kotsafti, A.; Scarpa, M.; Castagliuolo, I.; Scarpa, M. Reactive Oxygen Species and Antitumor Immunity-From Surveillance to Evasion. Cancers 2020, 12, 1748. [Google Scholar] [CrossRef]
- Cheung, E.C.; Vousden, K.H. The Role of ROS in Tumour Development and Progression. Nat. Rev. Cancer 2022, 22, 280–297. [Google Scholar] [CrossRef] [PubMed]
- Roy, K.; Jędrzejewski, T.; Sobocińska, J.; Spisz, P.; Maciejewski, B.; Hövelmeyer, N.; Passeri, B.; Wrotek, S. Divergent Impact of Endotoxin Priming and Endotoxin Tolerance on Macrophage Responses to Cancer Cells. Cell Immunol. 2025, 411–412, 104934. [Google Scholar] [CrossRef]
- Zheng, J. Energy Metabolism of Cancer: Glycolysis versus Oxidative Phosphorylation (Review). Oncol. Lett. 2012, 4, 1151–1157. [Google Scholar] [CrossRef]
- Alberghina, L.; Gaglio, D.; Gelfi, C.; Moresco, R.M.; Mauri, G.; Bertolazzi, P.; Messa, C.; Gilardi, M.C.; Chiaradonna, F.; Vanoni, M. Cancer Cell Growth and Survival as a System-Level Property Sustained by Enhanced Glycolysis and Mitochondrial Metabolic Remodeling. Front. Physiol. 2012, 3, 362. [Google Scholar] [CrossRef]
- Van den Bossche, J.; Baardman, J.; Otto, N.A.; van der Velden, S.; Neele, A.E.; van den Berg, S.M.; Luque-Martin, R.; Chen, H.J.; Boshuizen, M.C.S.; Ahmed, M.; et al. Mitochondrial Dysfunction Prevents Repolarization of Inflammatory Macrophages. Cell Rep. 2016, 17, 684–696. [Google Scholar] [CrossRef]
- Zakuan, Z.D.; Suresh, K. Rational Use of Intravenous Polymyxin B and Colistin: A Review. Med. J. Malays. 2018, 73, 351–359. [Google Scholar]
- Padhy, I.; Dwibedy, S.K.; Mohapatra, S.S. A Molecular Overview of the Polymyxin-LPS Interaction in the Context of Its Mode of Action and Resistance Development. Microbiol. Res. 2024, 283, 127679. [Google Scholar] [CrossRef]
- Aysert-Yildiz, P.; Özgen-Top, Ö.; Şentürk, A.F.; Kanik, S.; Özger, H.S.; Dizbay, M. Polymyxin B vs. Colistin: The Comparison of Neurotoxic and Nephrotoxic Effects of the Two Polymyxins. BMC Infect. Dis. 2024, 24, 862. [Google Scholar] [CrossRef] [PubMed]
- Yessayan, L.; Pino, C.J.; Humes, H.D. Extracorporeal Therapies in Sepsis: A Comprehensive Review of the Selective Cytopheretic Device, Polymyxin B and Seraph Cartridges. Ren. Fail. 2025, 47, 2459349. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, C.; Mao, Z.; Qi, S.; Song, R.; Zhou, F. Effectiveness of Polymyxin B-Immobilized Hemoperfusion against Sepsis and Septic Shock: A Systematic Review and Meta-Analysis. J. Crit. Care 2021, 63, 187–195. [Google Scholar] [CrossRef]
- Chen, J.J.; Lai, P.C.; Lee, T.H.; Huang, Y.T. Blood Purification for Adult Patients With Severe Infection or Sepsis/Septic Shock: A Network Meta-Analysis of Randomized Controlled Trials. Crit. Care Med. 2023, 51, 1777–1789. [Google Scholar] [CrossRef]
- Rice, T.W.; Wheeler, A.P.; Bernard, G.R.; Vincent, J.L.; Angus, D.C.; Aikawa, N.; Demeyer, I.; Sainati, S.; Amlot, N.; Cao, C.; et al. A Randomized, Double-Blind, Placebo-Controlled Trial of TAK-242 for the Treatment of Severe Sepsis. Crit. Care Med. 2010, 38, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Opal, S.M.; Laterre, P.F.; Francois, B.; LaRosa, S.P.; Angus, D.C.; Mira, J.P.; Wittebole, X.; Dugernier, T.; Perrotin, D.; Tidswell, M.; et al. Effect of Eritoran, an Antagonist of MD2-TLR4, on Mortality in Patients with Severe Sepsis: The ACCESS Randomized Trial. JAMA 2013, 309, 1154–1162. [Google Scholar] [CrossRef]
- Heine, H.; Zamyatina, A. Therapeutic Targeting of TLR4 for Inflammation, Infection, and Cancer: A Perspective for Disaccharide Lipid A Mimetics. Pharmaceuticals 2022, 16, 23. [Google Scholar] [CrossRef]
- Del Giudice, G.; Rappuoli, R.; Didierlaurent, A.M. Correlates of Adjuvanticity: A Review on Adjuvants in Licensed Vaccines. Semin. Immunol. 2018, 39, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.X.; Tseng, J.C.; Yu, G.Y.; Luo, Y.; Huang, C.Y.F.; Hong, Y.R.; Chuang, T.H. Recent Advances in the Development of Toll-like Receptor Agonist-Based Vaccine Adjuvants for Infectious Diseases. Pharmaceutics 2022, 14, 423. [Google Scholar] [CrossRef]
- Carter, D.; De La Rosa, G.; Garçon, N.; Moon, H.M.; Nam, H.J.; Skibinski, D.A.G. The Success of Toll-like Receptor 4 Based Vaccine Adjuvants. Vaccine 2025, 61, 127413. [Google Scholar] [CrossRef]
- Wang, Z.B.; Xu, J. Better Adjuvants for Better Vaccines: Progress in Adjuvant Delivery Systems, Modifications, and Adjuvant-Antigen Codelivery. Vaccines 2020, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.G.; Carter, D.; Casper, C.; Duthie, M.S.; Fox, C.B. Correlates of GLA Family Adjuvants’ Activities. Semin. Immunol. 2018, 39, 22–29. [Google Scholar] [CrossRef]
- Kelleher, K.; Subramaniam, N.; Drysdale, S.B. The Recent Landscape of RSV Vaccine Research. Ther. Adv. Vaccines Immunother. 2025, 13, 25151355241310601. [Google Scholar] [CrossRef]
- Su, W.; Ding, X. Methods of Endotoxin Detection. SLAS Technol. 2015, 20, 354–364. [Google Scholar] [CrossRef]
- Schneier, M.; Razdan, S.; Miller, A.M.; Briceno, M.E.; Barua, S. Current Technologies to Endotoxin Detection and Removal for Biopharmaceutical Purification. Biotechnol. Bioeng. 2020, 117, 2588–2609. [Google Scholar] [CrossRef] [PubMed]
- Dullah, E.C.; Ongkudon, C.M. Current Trends in Endotoxin Detection and Analysis of Endotoxin-Protein Interactions. Crit. Rev. Biotechnol. 2017, 37, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Jackie, J.; Lau, W.K.; Feng, H.T.; Li, S.F.Y. Detection of Endotoxins: From Inferring the Responses of Biological Hosts to the Direct Chemical Analysis of Lipopolysaccharides. Crit. Rev. Anal. Chem. 2019, 49, 126–137. [Google Scholar] [CrossRef]
- Foster, D.M.; Kellum, J.A. Endotoxic Septic Shock: Diagnosis and Treatment. Int. J. Mol. Sci. 2023, 24, 16185. [Google Scholar] [CrossRef]
- He, R.R.; Yue, G.L.; Dong, M.L.; Wang, J.Q.; Cheng, C. Sepsis Biomarkers: Advancements and Clinical Applications-A Narrative Review. Int. J. Mol. Sci. 2024, 25, 9010. [Google Scholar] [CrossRef]
- Kataria, Y.; Remick, D. Sepsis Biomarkers. Methods Mol. Biol. 2021, 2321, 177–189. [Google Scholar] [CrossRef]
- Fux, A.C.; Casonato Melo, C.; Michelini, S.; Swartzwelter, B.J.; Neusch, A.; Italiani, P.; Himly, M. Heterogeneity of Lipopolysaccharide as Source of Variability in Bioassays and LPS-Binding Proteins as Remedy. Int. J. Mol. Sci. 2023, 24, 8395. [Google Scholar] [CrossRef] [PubMed]
- Burgmaier, L.; Lifka, J.; Avci-Adali, M.; Reich, J. Endotoxin Masking in Human Plasma: The Role of Protein Interactions and Lipopolysaccharide Structure. Eur. J. Pharm. Biopharm. 2025, 214, 114786. [Google Scholar] [CrossRef]
- Soppert, J.; Brandt, E.F.; Heussen, N.M.; Barzakova, E.; Blank, L.M.; Kuepfer, L.; Hornef, M.W.; Trebicka, J.; Jankowski, J.; Berres, M.L.; et al. Blood Endotoxin Levels as Biomarker of Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2023, 21, 2746–2758. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, P.C.; Dorneles, G.P.; Santana Filho, P.C.; da Silva, I.M.; Schipper, L.L.; Postiga, I.A.L.; Neves, C.A.M.; Rodrigues Junior, L.C.; Peres, A.; de Souto, J.T.; et al. Increased LPS Levels Coexist with Systemic Inflammation and Result in Monocyte Activation in Severe COVID-19 Patients. Int. Immunopharmacol. 2021, 100, 108125. [Google Scholar] [CrossRef]
- Larsen, P.P.; Féart, C.; Pais de Barros, J.P.; Merle, B.M.J.; Gayraud, L.; Delyfer, M.N.; Korobelnik, J.F.; Delcourt, C. Association of Age-Related Macular Degeneration with a Blood Biomarker of Lipopolysaccharide, a Gut Bacterial Proinflammatory Toxin. Investig. Ophthalmol. Vis. Sci. 2023, 64, 47. [Google Scholar] [CrossRef]
- Ribeiro, C.B.; Castro, F. de O.F. de; Dorneles, G.P.; de Sousa Barros, J.B.; Silva, J.M.; Tavares, C.; Carvalho, H.R.; Carlos da Cunha, L.; Nagib, P.; Hoffmann, C.; et al. The Concomitant Use of Cannabis and Cocaine Coexists with Increased LPS Levels and Systemic Inflammation in Male Drug Users. Cytokine 2021, 141, 155472. [Google Scholar] [CrossRef]
- Gallucci, G.; Santucci, N.; Díaz, A.; Bongiovanni, B.; Bértola, D.; Gardeñez, W.; Rassetto, M.; Bay, M.L.; Bottasso, O.; D’Attilio, L. Increased Levels of Circulating LPS during Tuberculosis Prevails in Patients with Advanced Pulmonary Involvement. PLoS ONE 2021, 16, e0257214. [Google Scholar] [CrossRef]
- Jayashree, B.; Bibin, Y.S.; Prabhu, D.; Shanthirani, C.S.; Gokulakrishnan, K.; Lakshmi, B.S.; Mohan, V.; Balasubramanyam, M. Increased Circulatory Levels of Lipopolysaccharide (LPS) and Zonulin Signify Novel Biomarkers of Proinflammation in Patients with Type 2 Diabetes. Mol. Cell. Biochem. 2014, 388, 203–210. [Google Scholar] [CrossRef] [PubMed]
- de Waal, G.M.; de Villiers, W.J.S.; Forgan, T.; Roberts, T.; Pretorius, E. Colorectal Cancer Is Associated with Increased Circulating Lipopolysaccharide, Inflammation and Hypercoagulability. Sci. Rep. 2020, 10, 8777. [Google Scholar] [CrossRef] [PubMed]


| Stimulation | Chronic Inflammation | Endotoxin Tolerance |
|---|---|---|
| The alarm goes off (LPS) | The system keeps running and will not shut down | The system has “overheated” and stopped responding |
| Immunological outcome | Ongoing battle, tissue destruction | Immune silence due to hyporesponsivenessbut at the cost of vulnerability to other threats such as new infections or secondary pathogens |
| Adaptive function | Low—destructive and uncontrolled response | High—protective mechanism against immunopathology |
| Mechanism responsible for lack of fever | Low-level immune activation that does not reach the threshold needed to trigger a robust febrile response | Functional reprogramming of innate immune cells leading to sustained suppression of fever, even after repeated LPS exposure, unless tolerance is overcome |
| Type of LPS Analogues | Advantage of LPS Modification | Adjuvant | Composition | Conditions | Phase of Development |
|---|---|---|---|---|---|
| MPL | Reduced toxicity in comparison to LPS | AS01 | MPL with QS21 in liposome form | Malaria Herpes Zoster RSV | Approved Approved Approved |
| AS02 | MPL with QS21 in squalene oil-in-water emulsion | HIV Tuberculosis Hepatitis B | I II III | ||
| AS04 | MPL adsorbed onto aluminium | HBV HPV | Approved Approved | ||
| GLA | Increased immunological response compared to MPL. Synthetic substance. | GLA-AF | GLA in an aqueous form | HIV Influenza Schistosomiasis Hookworm | I I I I |
| GLA-LSQ | GLA with QS21 in liposome form | Malaria Plasmodium Falciparum | I I | ||
| GLA-SE | GLA in squalene oil-in-water emulsion | Schistosomiasis Leprosy Tuberculosis Influenza Malaria HIV Leishmaniasis RSV | II I II II I I I II | ||
| SLA | Increased immunological response compared to GLA. Synthetic substance. | SLA-SE | SLA in squalene oil-in-water emulsion | Leishmaniasis Herpes Zoster | I II |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roszak, K.; Roy, K.; Sobocińska, J.; Spisz, P.; Jędrzejewski, T.; Wrotek, S. Endotoxin’s Impact on Organism: From Immune Activation to Tolerance and Beyond. J. Clin. Med. 2025, 14, 6478. https://doi.org/10.3390/jcm14186478
Roszak K, Roy K, Sobocińska J, Spisz P, Jędrzejewski T, Wrotek S. Endotoxin’s Impact on Organism: From Immune Activation to Tolerance and Beyond. Journal of Clinical Medicine. 2025; 14(18):6478. https://doi.org/10.3390/jcm14186478
Chicago/Turabian StyleRoszak, Kacper, Konkonika Roy, Justyna Sobocińska, Paulina Spisz, Tomasz Jędrzejewski, and Sylwia Wrotek. 2025. "Endotoxin’s Impact on Organism: From Immune Activation to Tolerance and Beyond" Journal of Clinical Medicine 14, no. 18: 6478. https://doi.org/10.3390/jcm14186478
APA StyleRoszak, K., Roy, K., Sobocińska, J., Spisz, P., Jędrzejewski, T., & Wrotek, S. (2025). Endotoxin’s Impact on Organism: From Immune Activation to Tolerance and Beyond. Journal of Clinical Medicine, 14(18), 6478. https://doi.org/10.3390/jcm14186478