Challenges in Diagnosis and Management of Pneumoperitoneum Associated with Pneumatosis Cystoides Intestinalis in Children: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
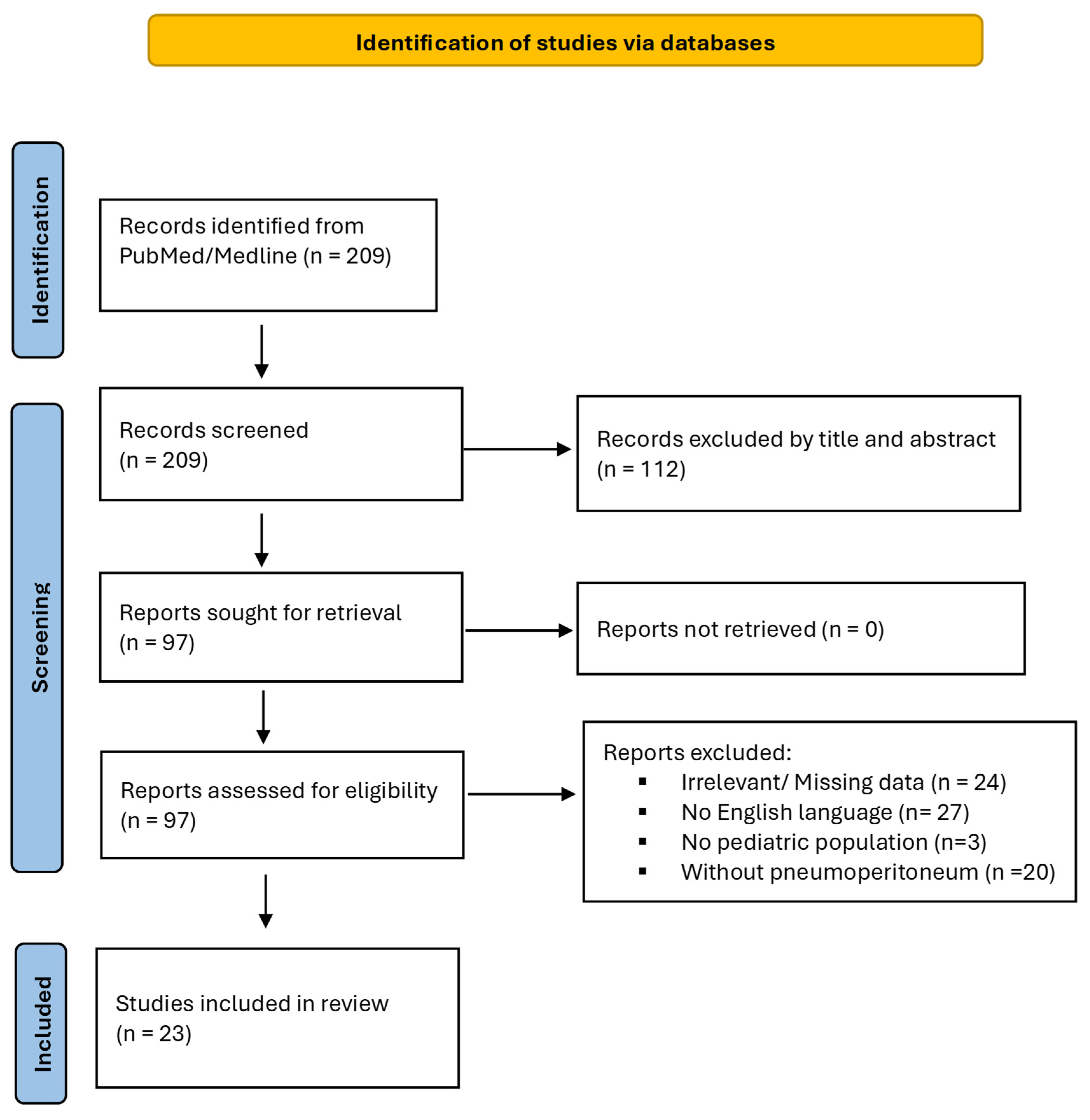
2.2. Study Selection
2.3. Data Extracted
2.4. Quality Assessment
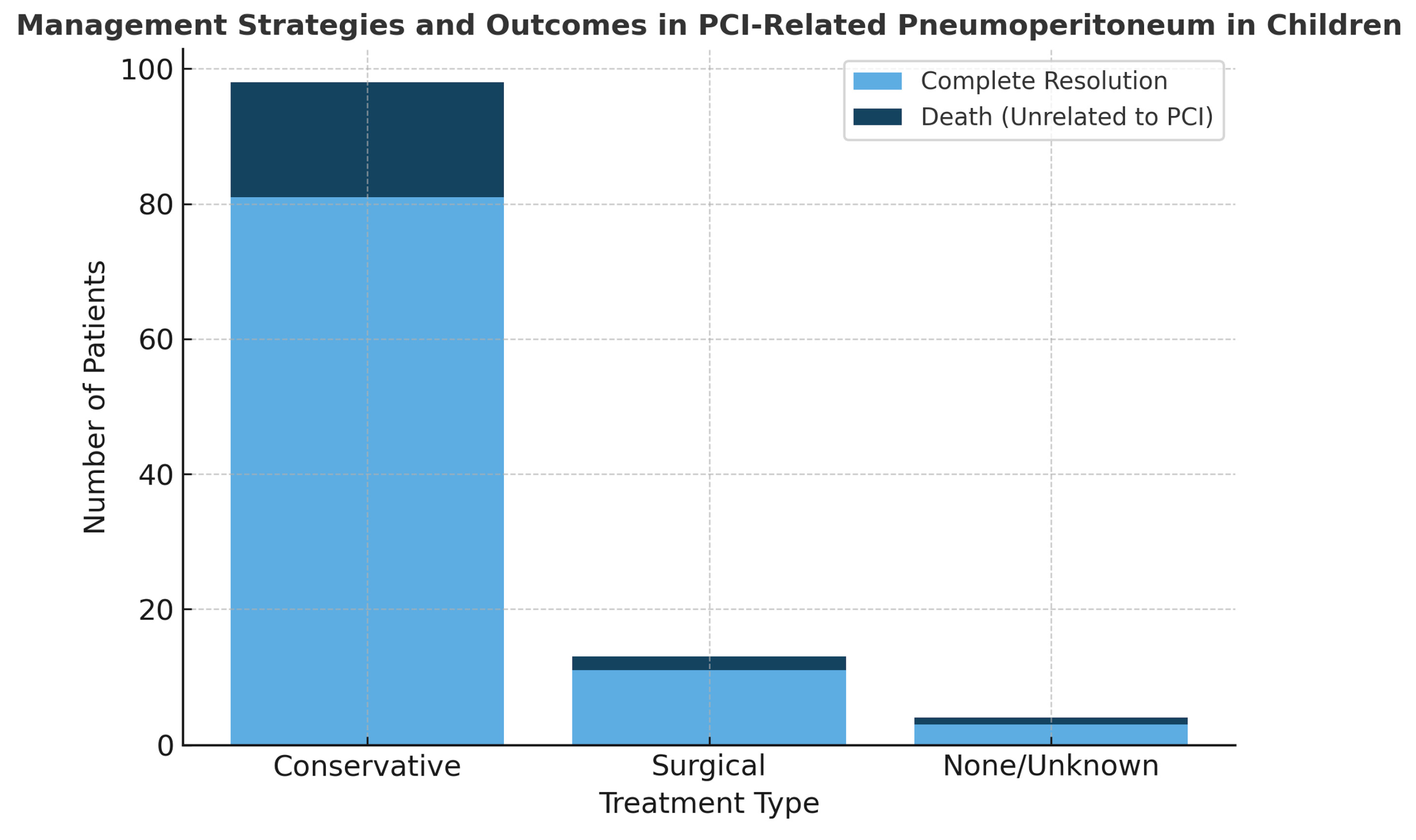
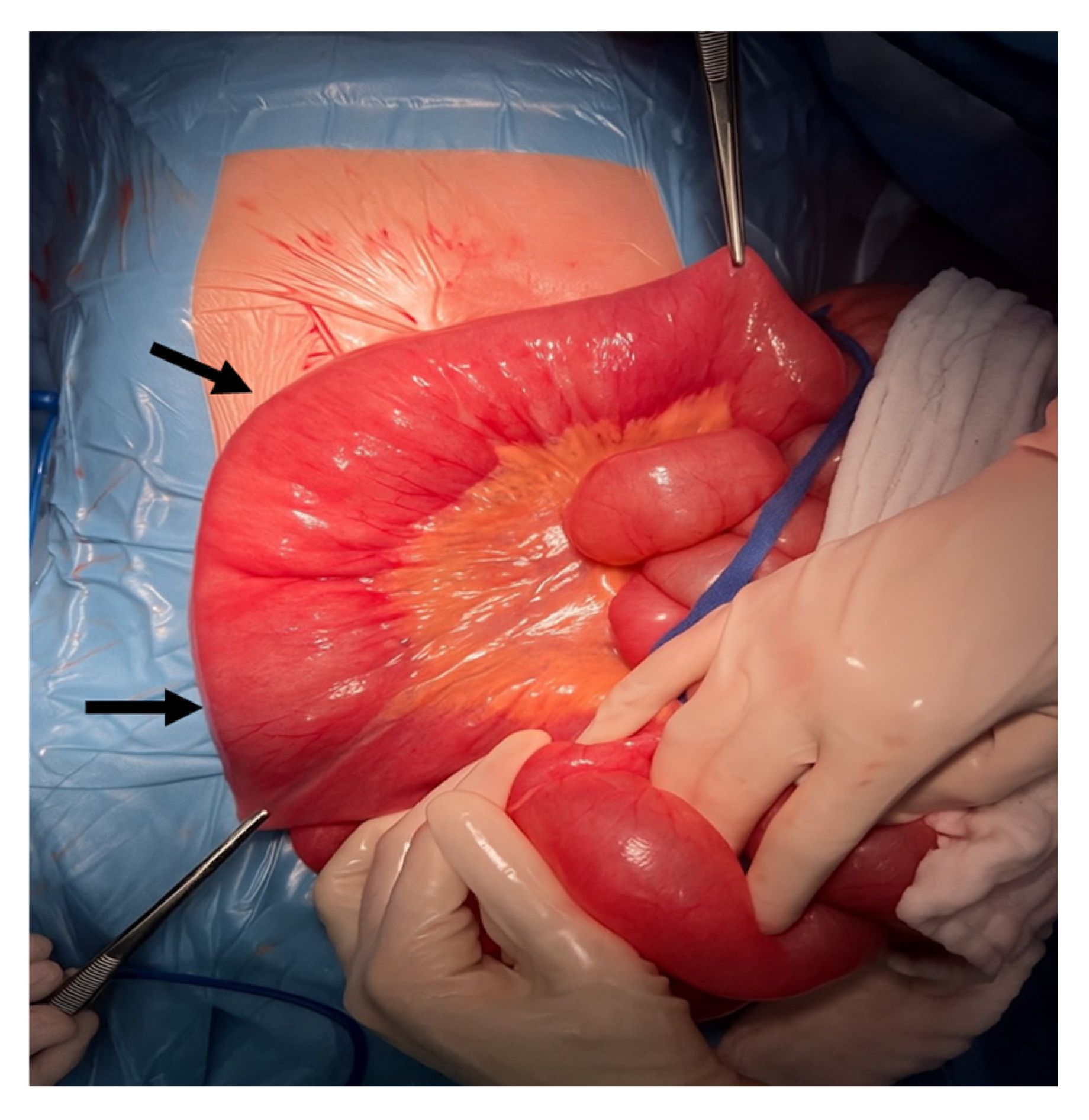
3. Results
Quality Assessment
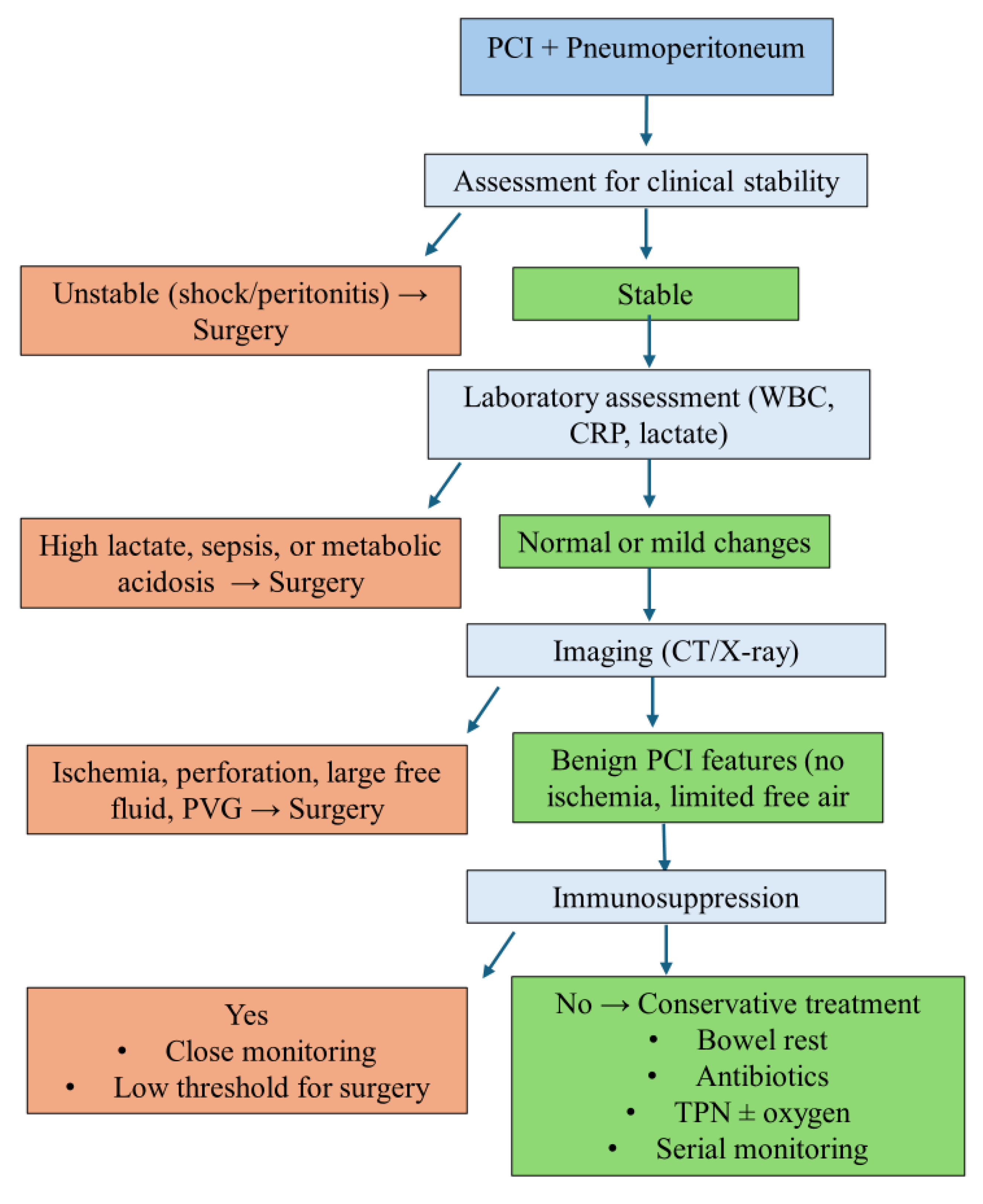
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PCI | Pneumatosis cystoides intestinalis |
| No | Number |
| N/A | Not Applicable |
| M | Male |
| F | Female |
| y.o. | Years Old |
| TB | Tuberculosis |
| ALL | Acute Lymphoblastic Leukemia |
| AML | Acute Myeloid Leukemia |
| TBI | Traumatic Brain Injury |
| BMT | Bone Marrow Transplant |
| GvHD | Graft-versus-Host Disease |
| MTX | Methotrexate |
| ACTH | Adrenocorticotropic Hormone |
| SB | Small Bowel |
| TI | Terminal Ileum |
| AC | Ascending Colon |
| HF | Hepatic Flexure |
| TC | Transverse Colon |
| ICV | Ileocecal Valve |
| U/S | Ultrasound |
| CT | Computed Tomography |
| CXR | Chest X-ray |
| JBI | Joanna Briggs Institute |
References
- Galea, J.; Burnand, K.M.; Dawson, F.L.; Sinha, C.K.; Rex, D.; Okoye, B.O. Pneumoperitoneum in the Setting of Pneumatosis Intestinalis in Children: Is Surgery Always Indicated? Eur. J. Pediatr. Surg. 2017, 27, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.L.; Williams, L.W. Pneumatosis intestinale in children with primary combined immunodeficiency. J. Pediatr. 1998, 132, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Kurbegov, A.C.; Sondheimer, J.M. Pneumatosis intestinalis in non-neonatal pediatric patients. Pediatrics 2001, 108, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Schuster, B.; Mayr, J. Acute pneumatosis cystoides intestinalis with atrophic desmosis of the colon in a child. BMJ Case Rep. 2017, 2017, bcr-2017-219310. [Google Scholar] [CrossRef]
- Feczko, P.J.; Mezwa, D.G.; Farah, M.C.; White, B.D. Clinical significance of pneumatosis of the bowel wall. Radiographics 1992, 12, 1069–1078. [Google Scholar] [CrossRef]
- Ade-Ajayi, N.; Veys, P.; Stanton, M.; Drake, D.P.; Pierro, A. Conservative management of pneumatosis intestinalis and pneumoperitoneum following bone-marrow transplantation. Pediatr. Surg. Int. 2002, 18, 692–695. [Google Scholar] [CrossRef]
- Alp, H.; Orbak, Z.; Sepetcigil, O.; Kantarci, M.; Kartal, I. Abdominal tuberculosis in a child presenting with radiological evidence of pneumatosis intestinalis and portal venous gas. J. Health Popul. Nutr. 2010, 28, 628–632. [Google Scholar] [CrossRef][Green Version]
- Awad, K.; Short, M.; Niyogi, A.; Hosie, G.; Godse, A. Pneumatosis intestinalis in a cohort of children with neurological impairment: A patient group with a management dilemma. J. Paediatr. Child Health 2017, 53, 663–666. [Google Scholar] [CrossRef]
- Aygunes, U.; Karagun, B.S.; Sasmaz, I.; Tutus, K.; Ozden, O.; Antmen, B. Pneumatosis cystoides intestinalis mimicking free intraabdominal air following chemotherapy for relapsed acute myeloblastic leukemia in a transplanted neutropenic child: A case report. Turk. J. Pediatr. 2023, 65, 693–697. [Google Scholar] [CrossRef]
- Bailey, K.A.; Kodikara, H.; Mauguen, A.; Price, A.; LaQuaglia, M.; Boulad, F. Pneumatosis intestinalis in the pediatric oncology population: An 11-year retrospective review at Memorial Sloan Kettering Cancer Center. Pediatr. Blood Cancer 2022, 69, e29539. [Google Scholar] [CrossRef]
- Berard, R.; Chedeville, G.; Saint-Martin, C.; Scuccimarri, R. Benign pneumatosis intestinalis in a patient with juvenile dermatomyositis. J. Rheumatol. 2010, 37, 2442–2444. [Google Scholar] [CrossRef][Green Version]
- Chan, W.K.; Lee, K.W.; Fan, T.W. Pneumatosis intestinalis in a child with nephrotic syndrome and norovirus gastroenteritis. Pediatr. Nephrol. 2010, 25, 1563–1566. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Marzan, K.A. Benign pneumatosis intestinalis in a pediatric patient with multiple risk factors including granulomatosis with polyangiitis: A case report and review of the literature. Semin. Arthritis Rheum. 2015, 44, 423–427. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, S.; Fabbro, M.A.; Musi, L.; Bozzola, L. Pneumatosis cystoides intestinalis: A rare cause of nonsurgical pneumoperitoneum in an infant. J. Pediatr. Surg. 2000, 35, 1106–1108. [Google Scholar] [CrossRef] [PubMed]
- Galal, O.; Osse, G.; Weigel, W.; Gahr, M. Pneumatosis intestinalis in children with leukaemia; report of three cases. Eur. J. Pediatr. 1981, 137, 91–93. [Google Scholar] [CrossRef]
- Hochwald, O.; Shaoul, R. Pneumatosis intestinalis caused by Clostridium difficile in a neutropenic child. Clin. Gastroenterol. Hepatol. 2007, 5, A24. [Google Scholar] [CrossRef]
- Jaffe, N.; Carlson, D.H.; Vawter, G.F. Pneumatosis cystoides intestinalis in acute leukemia. Cancer 1972, 30, 239–243. [Google Scholar] [CrossRef]
- Kim, C.T.; Kim, H.; Wechsler, B.; Kim, S.W. Pneumatosis intestinalis (PI) following severe traumatic brain injury. Brain Inj. 2005, 19, 1059–1061. [Google Scholar] [CrossRef]
- Kim, S.E.; Lee, S.M.; Kim, J.Y. Blinatumomab-related pneumatosis intestinalis in a pediatric patient with relapsed acute lymphoblastic leukemia: A case report. J. Oncol. Pharm. Pract. 2021, 27, 2045–2048. [Google Scholar] [CrossRef]
- McCarville, M.B.; Whittle, S.B.; Goodin, G.S.; Li, C.S.; Smeltzer, M.P.; Hale, G.A.; Kaufman, R.A. Clinical and CT features of benign pneumatosis intestinalis in pediatric hematopoietic stem cell transplant and oncology patients. Pediatr. Radiol. 2008, 38, 1074–1083. [Google Scholar] [CrossRef][Green Version]
- Naiditch, J.A.; Duerst, R.; Pillai, S.; Chin, A. Nonoperative management of pneumatosis intestinalis and pneumoperitoneum in a patient with acute lymphoblastic leukemia: Case report and review of the literature. Eur. J. Pediatr. Surg. 2010, 20, 426–429. [Google Scholar] [CrossRef]
- Ryan, J.L.; Dandridge, L.M.; Andrews, W.S.; Daniel, J.F.; Fischer, R.T.; Rivard, D.C.; Wieser, A.B.; Kane, B.J.; Hendrickson, R.J. Conservative Management of Pneumatosis Intestinalis and Portal Venous Gas After Pediatric Liver Transplantation. Transplant. Proc. 2020, 52, 938–942. [Google Scholar] [CrossRef]
- Wallace, G.; Rosen, N.; Towbin, A.J.; Jodele, S.; Myers, K.C.; Davies, S.M.; Flannery, A.; Gurria, J.P. Pneumatosis intestinalis after hematopoietic stem cell transplantation: When not doing anything is good enough. J. Pediatr. Surg. 2021, 56, 2073–2077. [Google Scholar] [CrossRef] [PubMed]
- Yeager, A.M.; Kanof, M.E.; Kramer, S.S.; Jones, B.; Saral, R.; Lake, A.M.; Santos, G.W. Pneumatosis intestinalis in children after allogeneic bone marrow transplantation. Pediatr. Radiol. 1987, 17, 18–22. [Google Scholar] [CrossRef] [PubMed]
- McDonald, T.; Siddle, K.; Starzyk, B.; McDonald, L.; Riley, G. Pneumatosis cystoides intestinalis in the paediatric population: A radiological diagnostic dilemma. Clin. Radiol. 2022, 77, e14. [Google Scholar] [CrossRef]
- Ling, F.; Guo, D.; Zhu, L. Pneumatosis cystoides intestinalis: A case report and literature review. BMC Gastroenterol. 2019, 19, 176. [Google Scholar] [CrossRef]
- Gui, X.; Zhou, Y.; Eidus, L.; Falck, V.; Gao, Z.H.; Qin, L. Is pneumatosis cystoides intestinalis gas-distended and ruptured lymphatics? Reappraisal by immunohistochemistry. Arch. Pathol. Lab. Med. 2014, 138, 1059–1066. [Google Scholar] [CrossRef]
- Suryawan, I.W.; Artana, T.G.M.; Sutarga, I.M.; Sumardika, I.W. Imaging of multi-etiologies of pneumatosis intestinalis: Role of ultrasound and GRE MRI sequences. Medicina 2024, 60, 671. [Google Scholar] [CrossRef]
- Panda, A.; Kayali, M.; Aswani, Y. Emphysematous conditions of the abdomen and pelvis: Imaging mimics and pitfalls. Abdom. Radiol. 2025. [Google Scholar] [CrossRef]
- Olson, D.E.; Kim, Y.W.; Ying, J.; Donnelly, L.F. CT predictors for differentiating benign and clinically worrisome pneumatosis intestinalis in children beyond the neonatal period. Radiology 2009, 253, 513–519. [Google Scholar] [CrossRef]
- Than, V.S.; Nguyen, M.D.; Gallon, A.; Pham, M.T.; Nguyen, D.H.; Boyer, L.; Le, T.D. Pneumatosis intestinalis with pneumoperitoneum: Not always a surgical emergency. Radiol Case Rep. 2020, 15, 2459–2463. [Google Scholar] [CrossRef]
- Perrone, A.; D’Angelo, V.; Pilla, R.; Ialongo, P.; Serino, I.; Federico, A. The Challenge of Pneumatosis Intestinalis: A Contemporary Systematic Review. J. Pers. Med. 2024, 14, 167. [Google Scholar] [CrossRef]
- Tropeano, G.; Di Grezia, M.; Puccioni, C.; Lanzillotta, M.; Pellegrino, L.; Pontoriero, A.; Grande, R.; Perri, F.; Marrelli, D.; Pinto, A. The spectrum of pneumatosis intestinalis in the adult: A surgical dilemma. World J. Gastrointest. Surg. 2023, 15, 553–565. [Google Scholar] [CrossRef]
| Study (Year) | Patient No. | Gender | Age (y.o.) | Underlying Disease (No Patients) | Etiology | Symptoms (No Patients) | PCI Location (No Patients) | Diagnosis (No Patients) | Treatment (No Patients) | Outcome (No Patients) | Follow-Up (No Patients) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alp et al. (2010) [7] | 1 | N/A | 8 | Abdominal TB | Abdominal TB | Distension, tenderness | SB, gastritis, duodenitis | AXR, U/S, CT, endoscopy | Conservative | Died (unknown cause) | 3 weeks: little improvement |
| Schuster et al. (2017) [4] | 1 | M | 14 | Fanconi anemia | Corticosteroids for GvHD, incomplete atrophic desmosis of colon | Bloody diarrhea, pain, anemia | TI, colon (sparing sigmoid + rectum) | CT | Surgical | Ostomy reversal after 4 months | 1 year: resolved |
| Berard et al. (2010) [11] | 1 | F | 8 | Juvenile dermatomyositis | Corticosteroids, MTX | Asymptomatic | Colon | CXR, CT | Conservative | Resolved | 6 weeks: resolved |
| Chang et al. (2015) [13] | 1 | F | 13 | Granulomatosis with polyangiitis | Corticosteroids, MTX | Asymptomatic | SB, colon | AXR, CT | Conservative | Resolved | 17 months: resolved |
| Kim et al. (2021) [19] | 1 | F | 1 | B-ALL | Blinatumomab | Constipation | AC, HF | CT | Conservative | Resolved | 1 year: resolved |
| McCarville et al. (2008) [20] | 16 | 11M/3F | 1–13 | AML (5) ALL (5) Sickle cell anemia (2) Neuroblastoma (1) Myeloblastoma (1) | Corticosteroids | Emesis (2) Pain/diarrhea (9) Asymptomatic (3) | Colon | AXR, CT | Conservative | Resolved | N/A |
| Ryan et al. (2020) [22] | 8 | 4M/4F | 1–7 | Hepatoblastoma (3) Biliary atresia (4) Alpha-1 antitrypsin deficiency (1) | Immunosuppressive therapy post-transplant, corticosteroids | Diarrhea (5) Bloody stools (1) Asymptomatic (2) | Cecum, AC, TC | US, CT | Conservative | Resolved | N/A |
| Naiditch et al. (2010) [21] | 1 | M | 3 | ALL | Immunosuppressive therapy for GvHD, corticosteroids, antibiotics | Pain, bilious emesis, fever | SB | CXR, CT | Conservative | Resolved | N/A |
| Jaffe et al. (1972) [17] | 6 | 4M/2F | 1–5 | ALL | Chemotherapy, corticosteroids, ACTH, blood transfusions, antibiotics | Tenderness/pain (3) Left pneumothorax, pleural effusion, lobar pneumonia (1) Distention (1) Asymptomatic (1) | Colon (1) Colon + rectum (1) Cecum (1) AC (1) Ileus (1) ICV, cecum, AC (1) | AXR (3) Autopsy (3) | Conservative (4) N/A (2) | Resolved (1) Died (5) | Died due to sepsis 5 years later (1) Autopsy showed PCI (5); cause of death was leukemia and sepsis (4) and electrolyte imbalance (1) |
| Aygunes et al. (2023) [9] | 1 | F | 4 | AML | Chemotherapy | Fever, pain | Colon | AXR, CT | Conservative | Resolved (21 days) | Inpatient for transplant |
| D’Agostino et al. (2000) [14] | 1 | M | 3 | Down syndrome | N/A | Distension, emesis | Jejunum, ileum, colon | AXR | Surgical | Resolved | 1 year: resolved |
| Tang et al. (1998) [2] | 5 | N/A | 4 mo–11 y.o. | Combined immunodeficiency | GvHD, corticosteroids | Diarrhea ± blood, distension, pain, fever (4) Asymptomatic (1) | N/A | AXR, US | Conservative | Resolved (4) Died (1) | 2–3.5 weeks: resolved |
| Kim et al. (2005) [18] | 1 | F | 9 | TBI | N/A | Distension, bloody diarrhea | N/A | AXR | Conservative | Resolved | 10 days: resolved |
| Hochwald et al. (2007) [16] | 1 | F | 1.5 | Rhabdomyosarcoma | N/A | Diarrhea, pain, distention | Colon | CT | Conservative | Resolved | N/A |
| Chan et al. (2010) [12] | 1 | M | 2 | Nephrotic syndrome | Corticosteroids | Diarrhea, distension, | Colon, rectum | CXR, CT | Conservative | Resolved | N/A |
| Awad et al. (2017) [8] | 5 | 2M/3F | 5–9 | Cerebral palsy | No corticosteroids or immunosuppression | Pain, distention | Colon | AXR | Surgical (4) Conservative (1) | Resolved | Recurrence (4): conservative management |
| Yeager et al. (1987) [24] | 1 | M | 5 | Aplastic anemia/BMT | Corticosteroids | Tenderness | Colon | AXR | Conservative | Resolved | 220 days: resolved |
| Galal et al. (1981) [15] | 2 | 2F | 4–6 | ALL | Corticosteroids, chemotherapy | Maxillary sinusitis (1) Cough, thoracic tenderness, distention (1) | AC, TC | AXR, CXR | Conservative | Resolved | N/A |
| Ade-Ajayi et al. (2002) [6] | 3 | 1M/2F | 11 | ALL (1) AML (2) | Corticosteroids | Pain, diarrhea, distension | N/A | AXR | Conservative | Resolved | Died due to leukemia |
| Wallace et al. (2021) [23] | 15 | N/A | N/A | GvHD | Corticosteroids | Distention (11) Asymptomatic (4) | N/A | AXR | Conservative | Resolved | 100 days: died (8) due to other reasons |
| Kurbegov et al. (2001) [3] | 8 | N/A | N/A | Tissue transplant, CHD, GI dysmotility | GvHD, Colitis, Ischemia | Distension, pain. diarrhea, fever, emesis | N/A | AXR | Conservative (5) Surgical (2) None (1) | Resolved | N/A |
| Bailey et al. (2022) [10] | 8 | N/A | N/A | Oncology patients | Corticosteroids | Pain, distention | N/A | AXR | Conservative (6) Surgical (2) | Resolved | N/A |
| Galea et al. (2016) [1] | 7 | 4M/3F | 5–14 | Developmental delay, ALL, encephalitis, eosinophilic gastroenteritis | N/A | Chest pain, seizure, gastroenteritis | Cecum, AC | CT | Conservative (6) Surgical (1) | Resolved | Died (1) due to encephalitis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siouli, C.; Dimopoulou, K.; Dimopoulou, D.; Krikri, A.; Kelaidi, N.; Zavras, N.; Dimopoulou, A. Challenges in Diagnosis and Management of Pneumoperitoneum Associated with Pneumatosis Cystoides Intestinalis in Children: A Systematic Review. J. Clin. Med. 2025, 14, 6479. https://doi.org/10.3390/jcm14186479
Siouli C, Dimopoulou K, Dimopoulou D, Krikri A, Kelaidi N, Zavras N, Dimopoulou A. Challenges in Diagnosis and Management of Pneumoperitoneum Associated with Pneumatosis Cystoides Intestinalis in Children: A Systematic Review. Journal of Clinical Medicine. 2025; 14(18):6479. https://doi.org/10.3390/jcm14186479
Chicago/Turabian StyleSiouli, Christina, Konstantina Dimopoulou, Dimitra Dimopoulou, Aggeliki Krikri, Natalia Kelaidi, Nikolaos Zavras, and Anastasia Dimopoulou. 2025. "Challenges in Diagnosis and Management of Pneumoperitoneum Associated with Pneumatosis Cystoides Intestinalis in Children: A Systematic Review" Journal of Clinical Medicine 14, no. 18: 6479. https://doi.org/10.3390/jcm14186479
APA StyleSiouli, C., Dimopoulou, K., Dimopoulou, D., Krikri, A., Kelaidi, N., Zavras, N., & Dimopoulou, A. (2025). Challenges in Diagnosis and Management of Pneumoperitoneum Associated with Pneumatosis Cystoides Intestinalis in Children: A Systematic Review. Journal of Clinical Medicine, 14(18), 6479. https://doi.org/10.3390/jcm14186479






