Isotretinoin Treatment for Acne Vulgaris: A Five-Year Retrospective Analysis of Clinical and Biochemical Adverse Effects
Abstract
1. Introduction
1.1. A Brief Historical Background of Isotretinoin
1.2. A Brief Overview of Isotretinoin’s Mechanisms
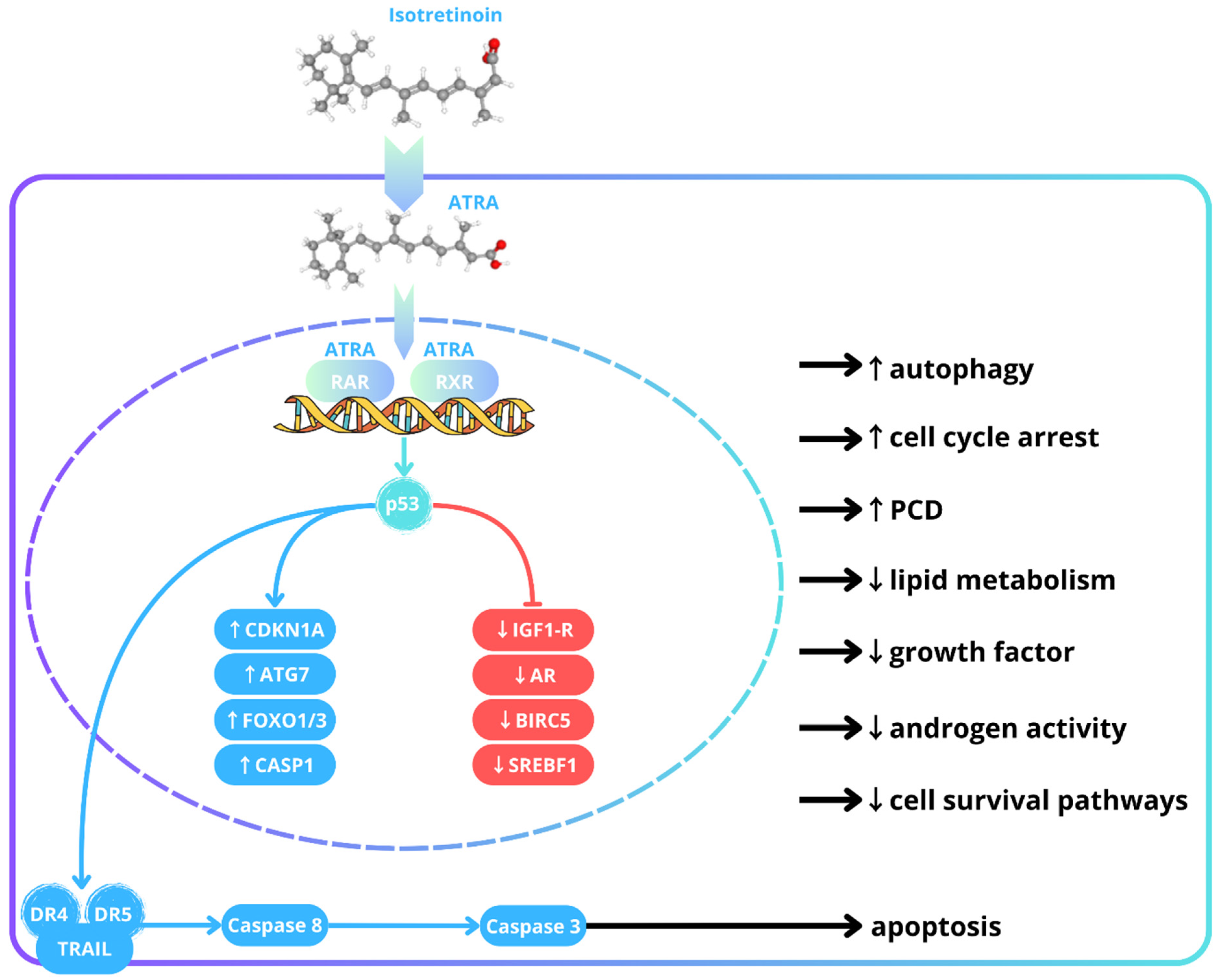
1.3. Dosing and Dose Optimization of Isotretinoin
1.4. Clinical Applications of Isotretinoin
1.5. Adverse Effects of Isotretinoin
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
2.3. Study Group Baseline Characteristics
2.4. Control Group Selection, Matching and Baseline Characteristics
2.5. Treatment Protocol
2.6. Data Analysis and Ethical Considerations
3. Results
3.1. Adverse Effects of Clinical Assessment
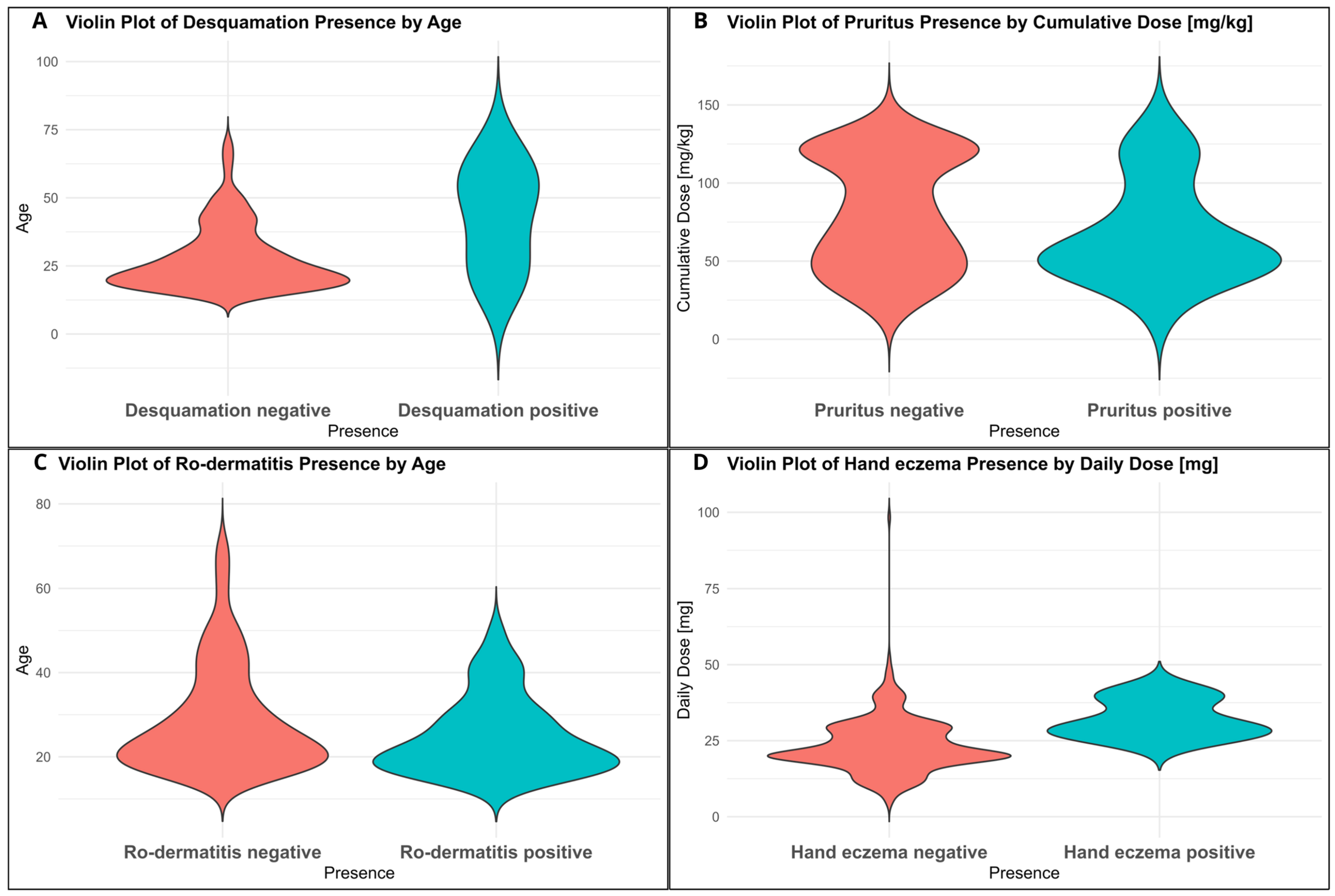
3.2. Correlations Between Adverse Effects and Clinical Variables
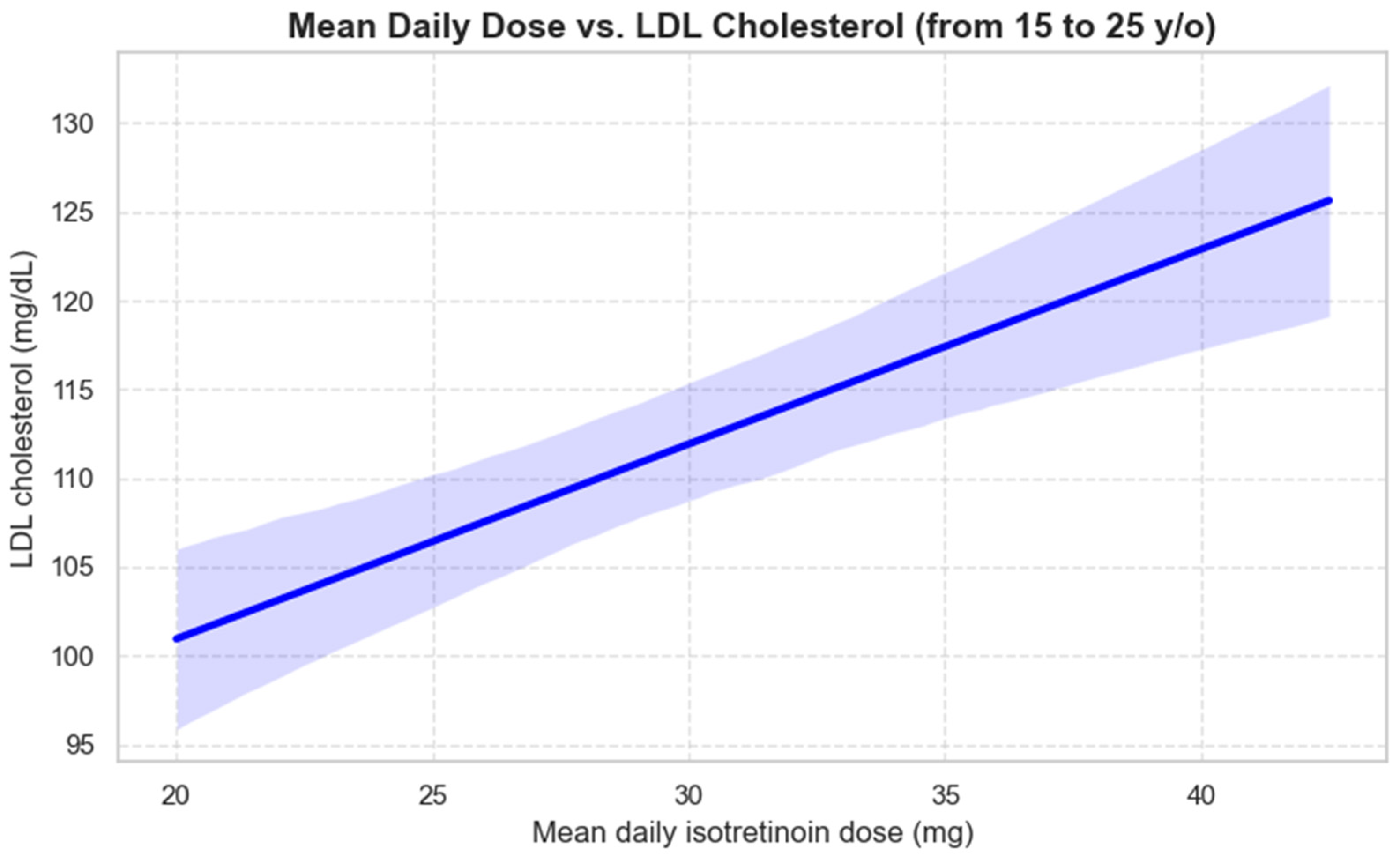
3.3. Impact of Isotretinoin on Lipid Profile
3.4. Isotretinoin on Aminotransferases
3.5. Isotretinoin on Thyroid-Stimulating Hormone
3.6. Impact of Isotretinoin on Prolactin
3.7. Timing of Adverse Effects
4. Discussion
4.1. Optimizing Isotretinoin Dosing: Evidence for Low-Dose Efficacy
4.2. Clinical Adverse Effects
4.3. Correlation Between Adverse Effects, Age and Dose
4.4. Lipid Profile
4.5. Aminotransferases
4.6. Thyroid-Stimulating Hormone
4.7. Prolactin
4.8. Study Limitations
4.9. From the Practical Point of View
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACTH | adrenocorticotropic hormone |
| ALP | alkaline phosphatase |
| ALT | alanine aminotransferase |
| APL | acute promyelocytic leukemia |
| AR | androgen receptor |
| AST | aspartate aminotransferase |
| ATG7 | autophagy-related gene 7 |
| ATRA | all-trans retinoic acid |
| BIRC5 | baculoviral inhibitor of apoptosis repeat-containing 5 |
| BMI | body mass index |
| CASP1 | caspase 1 |
| CBC | complete blood count |
| CDKN1A | cyclin-dependent kinase inhibitor 1A |
| CI | confidence interval |
| CML | chronic myelogenous leukemia |
| CRP | C-reactive protein |
| CTCL | cutaneous T-cell lymphoma |
| DR4/DR5 | death receptor 4/5 |
| EMA | European Medicines Agency |
| fT3 | free triiodothyronine |
| fT4 | free thyroxine |
| FDA | Food and Drug Administration |
| FC | fecal calprotectin |
| FOXO1/FOXO3 | forkhead box O1/O3 protein |
| GGTP | gamma-glutamyl transpeptidase |
| GH | growth hormone |
| HDL | high-density lipoprotein |
| IBD | inflammatory bowel disease |
| IGF-1 | insulin-like growth factor 1 |
| IGF1R | insulin-like growth factor 1 receptor |
| IQR | interquartile range |
| ISO | isotretinoin |
| KA | keratoacanthoma |
| LDL | low-density lipoprotein |
| LH | luteinizing hormone |
| MF/SS | mycosis fungoides/Sézary syndrome |
| NHL | non-Hodgkin lymphoma |
| OR | odds ratio |
| PEG | polyethylene glycol |
| PCD | programmed cell death |
| PPP | Pregnancy Prevention Program |
| PRL | prolactin |
| RAR | retinoic acid receptor |
| RARE | retinoic acid response element |
| RR | relative risk |
| RXR | retinoid X receptor |
| SCC | squamous cell carcinoma |
| SD | standard deviation |
| SmPC | summary of product characteristics |
| SREBF1 | sterol regulatory element-binding transcription factor 1 |
| TC | total cholesterol |
| TG/TGs | triglyceride(s) |
| TRAIL | TNF-related apoptosis-inducing ligand |
| TSH | thyroid-stimulating hormone |
| ρ | Spearman correlation coefficient |
References
- Reynolds, R.V.; Yeung, H.; Cheng, C.E.; Cook-Bolden, F.; Desai, S.R.; Druby, K.M.; Freeman, E.E.; Keri, J.E.; Stein Gold, L.F.; Tan, J.K.L.; et al. Guidelines of Care for the Management of Acne Vulgaris. J. Am. Acad. Dermatol. 2024, 90, 1006.e1–1006.e30. [Google Scholar] [CrossRef] [PubMed]
- Bagatin, E.; Costa, C.S.; Rocha, M.A.D.D.; Picosse, F.R.; Kamamoto, C.S.L.; Pirmez, R.; Ianhez, M.; Miot, H.A. Consensus on the Use of Oral Isotretinoin in Dermatology—Brazilian Society of Dermatology. An. Bras. Dermatol. 2020, 95, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B. Apoptosis May Explain the Pharmacological Mode of Action and Adverse Effects of Isotretinoin, Including Teratogenicity. Acta Derm.-Venereol. 2017, 97, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C. Acne Transcriptomics: Fundamentals of Acne Pathogenesis and Isotretinoin Treatment. Cells 2023, 12, 2600. [Google Scholar] [CrossRef]
- Agamia, N.F.; El Mulla, K.F.; Alsayed, N.M.; Ghazala, R.M.; El Maksoud, R.E.A.; Abdelmeniem, I.M.; Talaat, I.M.; Zaki, I.I.; Sabah, R.M.; Melnik, B.C. Isotretinoin Treatment Upregulates the Expression of P53 in the Skin and Sebaceous Glands of Patients with Acne Vulgaris. Arch. Dermatol. Res. 2023, 315, 1355–1365. [Google Scholar] [CrossRef]
- Melnik, B.C. Overexpression of P53 Explains Isotretinoin’s Teratogenicity. Exp. Dermatol. 2018, 27, 91–93. [Google Scholar] [CrossRef]
- Rabello-Fonseca, R.; Azulay, D.; Luiz, R.; Mandarim-de-Lacerda, C.; Cuzzi, T.; Manela-Azulay, M. Oral Isotretinoin in Photoaging: Clinical and Histopathological Evidence of Efficacy of an Off-label Indication. Acad. Dermatol. Venereol. 2009, 23, 115–123. [Google Scholar] [CrossRef]
- Akyol, M.; Özçelik, S. Non-Acne Dermatologic Indications for Systemic Isotretinoin. Am. J. Clin. Dermatol. 2005, 6, 175–184. [Google Scholar] [CrossRef]
- Rademaker, M. Adverse Effects of Isotretinoin: A Retrospective Review of 1743 Patients Started on Isotretinoin. Australas. J. Dermatol. 2010, 51, 248–253. [Google Scholar] [CrossRef]
- Lai, J.; Barbieri, J.S. Acne Relapse and Isotretinoin Retrial in Patients with Acne. JAMA Dermatol. 2025, 161, 367–374. [Google Scholar] [CrossRef]
- Cortes, J.; Kantarjian, H.; O’Brien, S.; Beran, M.; Estey, E.; Keating, M.; Talpaz, M. A Pilot Study of All-Trans Retinoic Acid in Patients with Philadelphia Chromosome-Positive Chronic Myelogenous Leukemia. Leukemia 1997, 11, 929–932. [Google Scholar] [CrossRef]
- Zhang, Y.; Ning, X.; Zhang, D.; Wang, W.; Ren, J. Efficacy and Safety of Oral Isotretinoin in the Treatment of Moderate to Severe Seborrheic Dermatitis: A Retrospective Study. Int. J. Dermatol. 2023, 62, 759–763. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Luo, Q. Off-Label Uses of Retinoids in Dermatology. Our Dermatol. Online 2012, 3 (Suppl. S1), 259–278. [Google Scholar] [CrossRef]
- Muthu, S.K.; Narang, T.; Saikia, U.N.; Kanwar, A.J.; Parsad, D.; Dogra, S. Low-Dose Oral Isotretinoin Therapy in Lichen Planus Pigmentosus: An Open-Label Non-Randomized Prospective Pilot Study. Int. J. Dermatol. 2016, 55, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Badaoui, A.; Reygagne, P.; Cavelier-Balloy, B.; Pinquier, L.; Deschamps, L.; Crickx, B.; Descamps, V. Dissecting Cellulitis of the Scalp: A Retrospective Study of 51 Patients and Review of Literature. Br. J. Dermatol. 2016, 174, 421–423. [Google Scholar] [CrossRef] [PubMed]
- Baden, H.P.; Buxman, M.M.; Weinstein, G.D.; Yoder, F.W. Treatment of Ichthyosis with Isotretinoin. J. Am. Acad. Dermatol. 1982, 6, 716–720. [Google Scholar] [CrossRef]
- Zaenglein, A.L.; Levy, M.L.; Stefanko, N.S.; Benjamin, L.T.; Bruckner, A.L.; Choate, K.; Craiglow, B.G.; DiGiovanna, J.J.; Eichenfield, L.F.; Elias, P.; et al. Consensus Recommendations for the Use of Retinoids in Ichthyosis and Other Disorders of Cornification in Children and Adolescents. Pediatr. Dermatol. 2021, 38, 164–180. [Google Scholar] [CrossRef]
- Goldsmith, L.A.; Elise Weinrich, A.; Shupack, J. Pityriasis Rubra Pilaris Response to 13-Cis-Retinoic Acid (Isotretinoin). J. Am. Acad. Dermatol. 1982, 6, 710–715. [Google Scholar] [CrossRef]
- Mortazavi, H.; Khezri, S.; Hosseini, H.; Khezri, F.; Vasigh, M. A Single Blind Randomized Clinical Study: The Efficacy of Isotretinoin plus Narrow Band Ultraviolet B in the Treatment of Psoriasis Vulgaris. Photodermatol. Photoimmunol. Photomed. 2011, 27, 159–161. [Google Scholar] [CrossRef]
- Aksoy, B.; Hapa, A.; Mutlu, E. Isotretinoin Treatment for Folliculitis Decalvans: A Retrospective Case-series Study. Int. J. Dermatol. 2018, 57, 250–253. [Google Scholar] [CrossRef]
- Wong, W.Y.L.; Kolbusz, R.V.; Goldberg, L.H.; Guana, A. Treatment oe a Recurrent Keratoacanthoma with Oral Isotretinoin. Int. J. Dermatol. 1994, 33, 579–583. [Google Scholar] [CrossRef]
- Lippman, S.M.; Itri, L.M.; Weber, R.S.; Ota, D.M.; Krakoff, I.H.; Gutterman, J.U.; Ki, W. 13-Cw-Retinoic Acid and Interferon a-2a: Effective Combination Therapy for Advanced Squamous Cell Carcinoma of the Skin. J. Natl. Cancer Inst. 1992, 84, 235–241. [Google Scholar] [CrossRef]
- Masetti, R.; Biagi, C.; Zama, D.; Vendemini, F.; Martoni, A.; Morello, W.; Gasperini, P.; Pession, A. Retinoids in Pediatric Onco-Hematology: The Model of Acute Promyelocytic Leukemia and Neuroblastoma. Adv. Ther. 2012, 29, 747–762. [Google Scholar] [CrossRef]
- Castleberry, R.P.; Emanuel, P.D.; Zuckerman, K.S.; Cohn, S.; Strauss, L.; Byrd, R.L.; Homans, A.; Chaffee, S.; Nitschke, R.; Gualtieri, R.J. A Pilot Study of Isotretinoin in the Treatment of Juvenile Chronic Myelogenous Leukemia. N. Engl. J. Med. 1994, 331, 1680–1684. [Google Scholar] [CrossRef]
- Tsimberidou, A.-M.; Giles, F.; Romaguera, J.; Duvic, M.; Kurzrock, R. Activity of Interferon-α and Isotretinoin in Patients with Advanced, Refractory Lymphoid Malignancies. Cancer 2004, 100, 574–580. [Google Scholar] [CrossRef]
- Scarisbrick, J.J.; Kim, Y.H.; Whittaker, S.J.; Wood, G.S.; Vermeer, M.H.; Prince, H.M.; Quaglino, P. Prognostic Factors, Prognostic Indices and Staging in Mycosis Fungoides and Sézary Syndrome: Where Are We Now? Br. J. Dermatol. 2014, 170, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Sethi, T.K.; Montanari, F.; Foss, F.; Reddy, N. How We Treat Advanced Stage Cutaneous T-cell Lymphoma—Mycosis Fungoides and Sézary Syndrome. Br. J. Haematol. 2021, 195, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Kapała, J.; Lewandowska, J.; Placek, W.; Owczarczyk-Saczonek, A. Adverse Events in Isotretinoin Therapy: A Single-Arm Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 6463. [Google Scholar] [CrossRef] [PubMed]
- Brzezinski, P.; Borowska, K.; Chiriac, A.; Smigielski, J. Adverse Effects of Isotretinoin: A Large, Retrospective Review. Dermatol. Ther. 2017, 30, e12483. [Google Scholar] [CrossRef]
- Marqueling, A.L.; Zane, L.T. Depression and Suicidal Behavior in Acne Patients Treated with Isotretinoin: A Systematic Review. Subscr. Semin. Cutan. Med. Surg. 2005, 24, 92–102. [Google Scholar] [CrossRef]
- Prabha, K.R.R.; Raja, S.K.; Dulvi, M.; Karthikeyan, V.U. Risk of Depression and Suicidal Behaviour in Acne Patients Treated with Isotretinoin: A Systematic Review and Meta-Analysis. J. Popul. Ther. Clin. Pharmacol. 2024, 31, 2233–2243. [Google Scholar] [CrossRef]
- Patraquim, C.; Silva, A.; Pereira, Â.; Gonçalves-Rocha, M.; Fernandes, J.; Pereira, A. Isotretinoin Embryopathy: Report of One Case. J. Pediatr. Neonatal Individ. Med. 2016, 5, e050108. [Google Scholar] [CrossRef]
- Choi, E.J.; Kim, N.; Kwak, H.-S.; Han, H.J.; Chun, K.-C.; Kim, Y.-A.; Koh, J.-W.; Han, J.Y.; Joo, S.H.; Lee, J.S.; et al. The Rates of Major Malformations after Gestational Exposure to Isotretinoin: A Systematic Review and Meta-Analysis. Obs. Gynecol. Sci. 2021, 64, 364–373. [Google Scholar] [CrossRef]
- Sladden, M.J.; Harman, K.E. What Is the Chance of a Normal Pregnancy in a Woman Whose Fetus Has Been Exposed to Isotretinoin? Arch. Dermatol. 2007, 143, 1187–1188. [Google Scholar] [CrossRef] [PubMed]
- Brzeziński, P.; Feszak, I.J.; Śmigielski, J.; Kawczak, P.; Bączek, T. Compliance with the European Pregnancy Prevention Programme in Isotretinoin Treatment: Safety Outcomes and Dose-Related Correlations. JCM 2025, 14, 3497. [Google Scholar] [CrossRef] [PubMed]
- Al Dhafiri, M.; Kaliyadan, F.; Almukhaimar, S.; Alsultan, F.; Al Hayim, E.; Alnaim, R.; Aldossari, A. Isotretinoin Use and Liver Enzymes Changes: A Single-Center Study in Saudi Arabia. Cureus 2023, 15, e51263. [Google Scholar] [CrossRef] [PubMed]
- De Marchi, M.Â.; Maranhão, R.C.; Brandizzi, L.I.V.; Souza, D.R.S. Effects of Isotretinoin on the Metabolism of Triglyceride-Rich Lipoproteins and on the Lipid Profile in Patients with Acne. Arch. Dermatol. Res. 2006, 297, 403–408. [Google Scholar] [CrossRef]
- Salem Hareedy, M.; Mahmoud, W.A.; Tawfik, K.M. Patterns of Thyroid Dysfunctions in Adolescent Patients Suffering from Severe Acne during Isotretinoin Treatment. Clin. Exp. Pharmacol. Physiol. 2021, 48, 1317–1326. [Google Scholar] [CrossRef]
- Karadag, A.S.; Takci, Z.; Ertugrul, D.T.; Bilgili, S.G.; Balahoroglu, R.; Takir, M. The Effect of Different Doses of Isotretinoin on Pituitary Hormones. Dermatology 2015, 230, 354–359. [Google Scholar] [CrossRef]
- Karadag, A.; Ertugrul, D.; Tutal, E.; Akin, K. Isotretinoin Influences Pituitary Hormone Levels in Acne Patients. Acta Derm.-Venereol. 2011, 91, 31–34. [Google Scholar] [CrossRef]
- Retinoid-Containing Medicinal Products—Referral | European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/medicines/human/referrals/retinoid-containing-medicinal-products (accessed on 3 July 2025).
- Updated Measures for Pregnancy Prevention during Retinoid Use | European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/news/updated-measures-pregnancy-prevention-during-retinoid-use (accessed on 3 July 2025).
- Almarri, F.H.; Dhafiri, M.A.; Albejais, R.A.; Albaqshi, M.A.; Alotaibi, W.D.; Almarri, F.; Dhafiri, M.A.; Albejais, R.A.; Albaqshi, M.A.H.; Alotaibi, W.D. The Perception of Contraceptive Practice Among Female Patients Treated with Isotretinoin in Saudi Arabia. Cureus 2024, 16, e69390. [Google Scholar] [CrossRef]
- Rademaker, M.; Wishart, J.M.; Birchall, N.M. Isotretinoin 5 Mg Daily for Low-grade Adult Acne Vulgaris—A Placebo-controlled, Randomized Double-blind Study. Acad. Dermatol. Venereol. 2014, 28, 747–754. [Google Scholar] [CrossRef]
- Rademaker, M.; Wishart, J.; Birchall, N. Long Term Remission of Persistent Adult Acne Following Very Low-dose (5 Mg/Day) Isotretinoin. Aust. J. Dermatol. 2017, 58, 69. [Google Scholar] [CrossRef]
- Rasi, A.; Behrangi, E.; Rohaninasab, M.; Nahad, Z. Efficacy of Fixed Daily 20 Mg of Isotretinoin in Moderate to Severe Scar Prone Acne. Adv. Biomed. Res. 2014, 3, 103. [Google Scholar] [CrossRef]
- Daly, A.U.; Baptista Gonçalves, R.; Lau, E.; Bowers, J.; Hussaini, N.; Charalambides, M.; Coumbe, J.; Flohr, C.; Layton, A.M. A Systematic Review of Isotretinoin Dosing in Acne Vulgaris. JEADV Clin. Pract. 2023, 2, 432–449. [Google Scholar] [CrossRef]
- Sadeghzadeh-Bazargan, A.; Ghassemi, M.; Goodarzi, A.; Roohaninasab, M.; Najar Nobari, N.; Behrangi, E. Systematic Review of Low-dose Isotretinoin for Treatment of Acne Vulgaris: Focus on Indication, Dosage, Regimen, Efficacy, Safety, Satisfaction, and Follow up, Based on Clinical Studies. Dermatol. Ther. 2021, 34, e14438. [Google Scholar] [CrossRef] [PubMed]
- Strauss, J.S.; Leyden, J.J.; Lucky, A.W.; Lookingbill, D.P.; Drake, L.A.; Hanifin, J.M.; Lowe, N.J.; Jones, T.M.; Stewart, D.M.; Jarratt, M.T.; et al. Safety of a New Micronized Formulation of Isotretinoin in Patients with Severe Recalcitrant Nodular Acne: A Randomized Trial Comparing Micronized Isotretinoin with Standard Isotretinoin. J. Am. Acad. Dermatol. 2001, 45, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Bagatin, E.; Costa, C.S. The Use of Isotretinoin for Acne—An Update on Optimal Dosing, Surveillance, and Adverse Effects. Expert Rev. Clin. Pharmacol. 2020, 13, 885–897. [Google Scholar] [CrossRef]
- Alrasheed, A.; Alsadhan, K.; Alfawzan, N.; AbuDujain, N.; Alnasser, A.; Almousa, H. Impact of Isotretinoin on Blood Lipids and Liver Enzymes: A Retrospective Cohort Study in Saudi Arabia. TCRM 2024, 20, 567–575. [Google Scholar] [CrossRef]
- Gharaei Nejad, K.; Darjani, A.; Alizadeh, N.; Hakemzadeh, S.T.; Hassanzadeh Rad, A.; Kazemnejad-Leili, E.; Ghadarjani, R.; Eftekhari, H.; Rafiei, R.; Dalili, S. Serum Lipid Profile in Adolescents and Adults with Acne Vulgaris Receiving Isotretinoin. Casp. J. Intern. Med. 2024, 15, 659–665. [Google Scholar] [CrossRef]
- Sibi Krishna, T.; Kaur, R.; Malhotra, V.; Fatima, Z.; Mustajab, M.; Singh, T.; Iqbal, M.; Kesavan, T.; Ajeya, S.P.; Mathew, S.G.; et al. The Impact of Isotretinoin on Lipid Profile: A Systematic Review. Ann. Med. Surg. 2025, 87, 4395–4403. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.S.; Beijamini, V.; Melchiors, A.C. The Effect of Isotretinoin on Triglycerides and Liver Aminotransferases. An. Bras. Dermatol. 2012, 87, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Scharnitz, T.P.; Muscat, J.; Chen, A.; Gupta-Elera, G.; Kirby, J.S. Laboratory Monitoring During Isotretinoin Therapy for Acne: A Systematic Review and Meta-Analysis. JAMA Dermatol. 2016, 152, 35. [Google Scholar] [CrossRef] [PubMed]
- Blasiak, R.C.; Stamey, C.R.; Burkhart, C.N.; Lugo-Somolinos, A.; Morrell, D.S. High-Dose Isotretinoin Treatment and the Rate of Retrial, Relapse, and Adverse Effects in Patients with Acne Vulgaris. JAMA Dermatol. 2013, 149, 1392. [Google Scholar] [CrossRef]
- Tawanwongsri, W.; Kanchanasuwan, T.; Eden, C. Isotretinoin and Hepatotoxicity in Patients with Acne: A Narrative Review. Cosmetics 2025, 12, 17. [Google Scholar] [CrossRef]
- Uyar, B.; Solak, A.; Saklamaz, A.; Akyildiz, M.; Genç, B.; Gökduman, A. Effects of Isotretinoin on the Thyroid Gland and Thyroid Function Tests in Acne Patients: A Preliminary Study. Indian J. Dermatol. Venereol. Leprol. 2016, 82, 587. [Google Scholar] [CrossRef]
- Yıldırım, N.; Doğan, S.; Atakan, N. Evaluation of Thyroid Function Tests of Acne Vulgaris Patients Treated with Systemic Isotretinoin. J. Dermatol. Treat. 2017, 28, 141–144. [Google Scholar] [CrossRef]
- Feily, A.; Taheri, T.; Meier-Schiesser, B.; Rhinehart, D.P.; Sobhanian, S.; Colon-Diaz, M.; Feily, A.; Ramirez-Fort, M.K. The Effect of Low-Dose Isotretinoin Therapy on Serum Androgen Levels in Women with Acne Vulgaris. Int. J. Women’s Dermatol. 2020, 6, 102–104. [Google Scholar] [CrossRef]
- Siddiqi, A.I. A Case of False Positive Macro-Prolactin. J. Coll. Physicians Surg. Pak. 2021, 31, 749. [Google Scholar] [CrossRef]
- Park, Y.J.; Shin, H.Y.; Choi, W.K.; Lee, A.-Y.; Lee, S.H.; Hong, J.S. Optimal Laboratory Testing Protocol for Patients with Acne Taking Oral Isotretinoin. World J. Clin. Cases 2023, 11, 2435–2442. [Google Scholar] [CrossRef]
- Zhu, Y.-X.; Wu, L.; Zhu, S.-C.; Wang, X.-P.; Zhang, D.; Tan, Y.-P.; Ouyang, X.-L.; Li, C.-M. Re-Evaluating the Necessity of Routine Laboratory Monitoring during Isotretinoin Therapy for Acne. World J. Clin. Cases 2024, 12, 6237–6240. [Google Scholar] [CrossRef]
- Wright, S.; Strunk, A.; Garg, A. Risk of New-Onset Inflammatory Bowel Disease among Patients with Acne Vulgaris Exposed to Isotretinoin. J. Am. Acad. Dermatol. 2021, 84, 41–45. [Google Scholar] [CrossRef]
- Crockett, S.D.; Porter, C.Q.; Martin, C.F.; Sandler, R.S.; Kappelman, M.D. Isotretinoin Use and the Risk of Inflammatory Bowel Disease: A Case–Control Study. Am. J. Gastroenterol. 2010, 105, 1986–1993. [Google Scholar] [CrossRef]

| Applications of Isotretinoin | |||
|---|---|---|---|
| Main Application | Other Dermatological Applications | Cancers | Leukemias/Lymphomas |
| Severe acne | Rosacea Seborrheic dermatitis Hidradenitis suppurativa Lichen planus Dissecting cellulitis Ichthyosis Pityriasis rubra pilaris Psoriasis Folliculitis decalvans | Keratoacanthoma (KA) Squamous cell carcinoma (SCC) | Acute promyelocytic leukemia (APL) Juvenile chronic myelogenous leukemia (CML) Recurrent non-Hodgkin lymphoma (NHL) Mycosis fungoides/Sézary syndrome (MF/SS) |
| Adverse Effects of Isotretinoin | ||
|---|---|---|
| Common | Less Common | Biomarkers and Hormones |
| Xerosis Erythema Epistaxis Cheilitis Myalgias Pruritus (itching) Skin exfoliation Arthralgias Retinoid dermatitis | Mood alterations Depression exacerbation Dry eyes Photosensitivity Seborrheic dermatitis Dry dandruff Xerophthalmia Polydipsia Abdominal pain | Liver: ↑ AST, ↑ ALT, ↑ GGTP, ↑ ALP Lipid profile: ↑ TC, ↑ LDL, ↑ TGs, ↓ HDL Thyroid: ↓ fT3, ↓ fT4, ⇅ TSH. Pituitary: ↓ LH, ↓ PRL, ↓ ACTH, ↓ GH, Peripheral: ↓ testosterone, ↓ cortisol, ↓ IGF-1 |
| Variable | Value |
|---|---|
| Age | Mean: 28.1; SD: 12.2; Median: 24; IQR: 19–34 |
| Sex | Female: 263 (71.1%); Male: 107 (28.9%) |
| Age bands | 15–24: 196 (53.0%); 25–34: 85 (23.0%); ≥35: 89 (24.1%) |
| Height (cm) | Mean: 171.2; SD: 7.8; Median: 171; IQR: 166–177 |
| Weight (kg) | Mean: 71.7; SD: 14.9; Median: 70.0; IQR: 61.2–81.6 |
| BMI (kg/m2) | Mean: 24.4; SD: 4.4; Median: 24.1; IQR: 21.2–27.5 |
| Daily dose (mg/day) | Mean: 23.4; SD: 9.1; Median: 20.0; IQR: 20.0–30.0 |
| Cumulative dose (mg/kg) | Mean: 88.3; SD: 31.5; Median: 80.0; IQR: 50.0–120.0 |
| Variable | Value |
|---|---|
| Age | Mean: 29.44; SD: 6.31; Median: 29; IQR: 24–35 |
| Sex | Female: 204 (68.0%); Male: 96 (32.0%) |
| Age bands | 15–24: 85 (28.3%); 25–34: 130 (43.3%); ≥35: 85 (28.3%) |
| Height (cm) | Mean: 176.0; SD: 9.5; Median: 176; IQR: 170–182 |
| Weight (kg) | Mean: 70.5; SD: 13.8; Median: 69.0; IQR: 61.0–78.0 |
| BMI (kg/m2) | Mean: 22.7; SD: 3.9; Median: 22.5; IQR: 20.5–24.9 |
| (A) | |
| Adverse Effect | Percentage [%] (n) |
| Xerosis | 70% (259) |
| Retinoid dermatitis | 20% (77) |
| Hair loss | 5.9% (22) |
| Pruritus (itching) | 8.4% (31) |
| Seborrhea | 3.5% (13) |
| Hand eczema | 3.5% (13) |
| Desquamation | 3.25% (12) |
| Pityriasis capitis | 3% (11) |
| Onycholysis | 1.6% (6) |
| Facial edema | 1.1% (4) |
| Dermatographic urticaria | 1.1% (4) |
| Lichen planus | <1% (3) |
| Folliculitis decalvans | <1% (2) |
| Photosensitivity | <1% (2) |
| Trachyonychia | <1% (2) |
| Skin maceration | <1% (2) |
| (B) | |
| Adverse Effect | Percentage [%] (n) |
| Cheilitis | 15.5% (57) |
| Glossitis | <1% (1) |
| (C) | |
| Adverse Effect | Percentage [%] (n) |
| Epistaxis | 3.7% (14) |
| Sore throat | 1.1% (4) |
| Sinus pain | <1% (2) |
| (D) | |
| Adverse Effect | Percentage [%] (n) |
| Xerophthalmia | 4.3% (16) |
| Vision changes | <1% (3) |
| Conjunctivitis | <1% (2) |
| (E) | |
| Adverse Effect | Percentage [%] (n) |
| Arthralgia | 6.75% (25) |
| Myalgia | 4.9% (18) |
| Back pain | 3.25% (12) |
| (F) | |
| Adverse Effect | Percentage [%] (n) |
| Abdominal pain | 3.7% (14) |
| Nausea | 1.6% (6) |
| (G) | |
| Adverse Effect | Percentage [%] (n) |
| Impetigo contagiosa | <1% (3) |
| Furunculus | <1% (2) |
| Herpes simplex | <1% (2) |
| (H) | |
| Adverse Event | Percentage (%) (n) |
| Fatigue, drowsiness | 7.8% (29) |
| Headaches Exacerbation of depression | 4.6% (17) 1.4% (5) |
| Dizziness | 1.1% (4) |
| Emotional lability | <1% (3) |
| Tremors | <1% (2) |
| (I) | |
| Adverse Effect | Percentage [%] (n) |
| Menstrual disorders | 1.6% (6) |
| Angiogranuloma | <1% (2) |
| Cherry angioma | <1% (2) |
| Hematoma | <1% (2) |
| Polyuria | <1% (2) |
| Polydipsia | <1% (2) |
| Petechiae | <1% (1) |
| Adverse Effect | Variable | Spearman ρ | p-Value |
|---|---|---|---|
| Hand eczema | Daily dose | +0.082 | 0.0370 |
| Pruritus | Cumulative dose | −0.088 | 0.0367 |
| Ro-dermatitis | Age | −0.080 | 0.0286 |
| Desquamation | Age | +0.083 | 0.0228 |
| Parameter | Cases | Mean | SD | Median | Min | Max |
|---|---|---|---|---|---|---|
| TC | 303 | 173.9 | 38.0 | 169.0 | 65.0 | 327.0 |
| HDL | 206 | 58.7 | 18.0 | 55.1 | 28.7 | 183.0 |
| LDL | 195 | 104.4 | 33.4 | 98.0 | 42.2 | 227.0 |
| TGs | 295 | 96.3 | 49.0 | 82.4 | 33.0 | 387.0 |
| Parameter | Cases (+) | Cases (All) | Controls (+) | Controls (All) | OR | OR (95% CI) | RR | RR (95% CI) | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| TC | 104 | 303 | 64 | 300 | 1.93 | 1.34–2.77 | 1.61 | 1.23–2.10 | 0.0004 |
| HDL | 80 | 206 | 38 | 300 | 2.68 | 1.75–4.10 | 2.2 | 1.55–3.12 | <0.0001 |
| LDL | 105 | 195 | 41 | 300 | 3.4 | 2.26–5.10 | 2.55 | 1.87–3.47 | <0.0001 |
| TGs | 51 | 295 | 29 | 300 | 1.95 | 1.20–3.17 | 1.78 | 1.16–2.72 | 0.0062 |
| Dyslipidemia | 195 | 303 | 121 | 300 | 2.67 | 1.92–3.71 | 1.6 | 1.36–1.88 | <0.0001 |
| Parameter | Cases (+) | Cases (All) | Controls (+) | Controls (All) | OR | OR (95% CI) | RR | RR (95% CI) | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| AST | 17 | 310 | 5 | 200 | 2.26 | 0.82–6.23 | 2.19 | 0.82–5.84 | 0.1 |
| ALT | 20 | 309 | 13 | 200 | 1.51 | 0.73–3.12 | 1.46 | 0.75–2.85 | 0.27 |
| Parameter | Cases (+) | Cases (All) | Controls (+) | Controls (All) | OR | OR (95% CI) | RR | RR (95% CI) | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| TSH | 17 | 227 | 10 | 200 | 1.54 | 0.69–3.45 | 1.5 | 0.70–3.20 | 0.29 |
| Parameter | Cases (+) | Cases (All) | Controls (+) | Controls (All) | OR | OR (95% CI) | RR | RR (95% CI) | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| PRL | 39 | 127 | 5 | 100 | 8.42 | 2.97–23.84 | 6.14 | 2.51–15.05 | <0.00001 |
| Adverse Event | Typical Time to First Onset | Clinical Note |
|---|---|---|
| Cheilitis | within 7 days | Usually the earliest signal of intolerance. |
| Xerosis | 2–3 weeks | Dose-dependent; emollient prophylaxis recommended. |
| Epistaxis | by month 1 | Often follows nasal dryness; topical ointment useful for prevention. |
| Xerophthalmia | 6 weeks–3 months | Managed with lubricating drops; contact lens intolerance should be considered. |
| Dyslipidemia | by week 4 | Detected on laboratory testing. |
| Musculoskeletal pain | 2–3 months | Usually mild. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feszak, I.J.; Brzeziński, P.; Feszak, S.; Kitowska, A.; Waśkow, M.; Kawczak, P.; Bączek, T. Isotretinoin Treatment for Acne Vulgaris: A Five-Year Retrospective Analysis of Clinical and Biochemical Adverse Effects. J. Clin. Med. 2025, 14, 6473. https://doi.org/10.3390/jcm14186473
Feszak IJ, Brzeziński P, Feszak S, Kitowska A, Waśkow M, Kawczak P, Bączek T. Isotretinoin Treatment for Acne Vulgaris: A Five-Year Retrospective Analysis of Clinical and Biochemical Adverse Effects. Journal of Clinical Medicine. 2025; 14(18):6473. https://doi.org/10.3390/jcm14186473
Chicago/Turabian StyleFeszak, Igor Jarosław, Piotr Brzeziński, Sylwia Feszak, Aleksandra Kitowska, Monika Waśkow, Piotr Kawczak, and Tomasz Bączek. 2025. "Isotretinoin Treatment for Acne Vulgaris: A Five-Year Retrospective Analysis of Clinical and Biochemical Adverse Effects" Journal of Clinical Medicine 14, no. 18: 6473. https://doi.org/10.3390/jcm14186473
APA StyleFeszak, I. J., Brzeziński, P., Feszak, S., Kitowska, A., Waśkow, M., Kawczak, P., & Bączek, T. (2025). Isotretinoin Treatment for Acne Vulgaris: A Five-Year Retrospective Analysis of Clinical and Biochemical Adverse Effects. Journal of Clinical Medicine, 14(18), 6473. https://doi.org/10.3390/jcm14186473







