Holistic Management of Adult ADHD with a History of Addiction: Emphasis on Low-Addiction-Risk Psychopharmacotherapy
Abstract
1. Introduction
2. ADHD Characteristics in Adults
2.1. Epidemiology of Adult ADHD
2.2. Symptoms of ADHD in Adulthood
2.3. Adult ADHD Diagnosis
2.4. Consequences of Untreated ADHD
2.5. The History of the Term “ADHD”
3. The Impact of Personality Traits and ADHD Symptoms on the Frequency of Addiction
4. ADHD Psychopharmacotherapy
4.1. A Modern Approach to ADHD
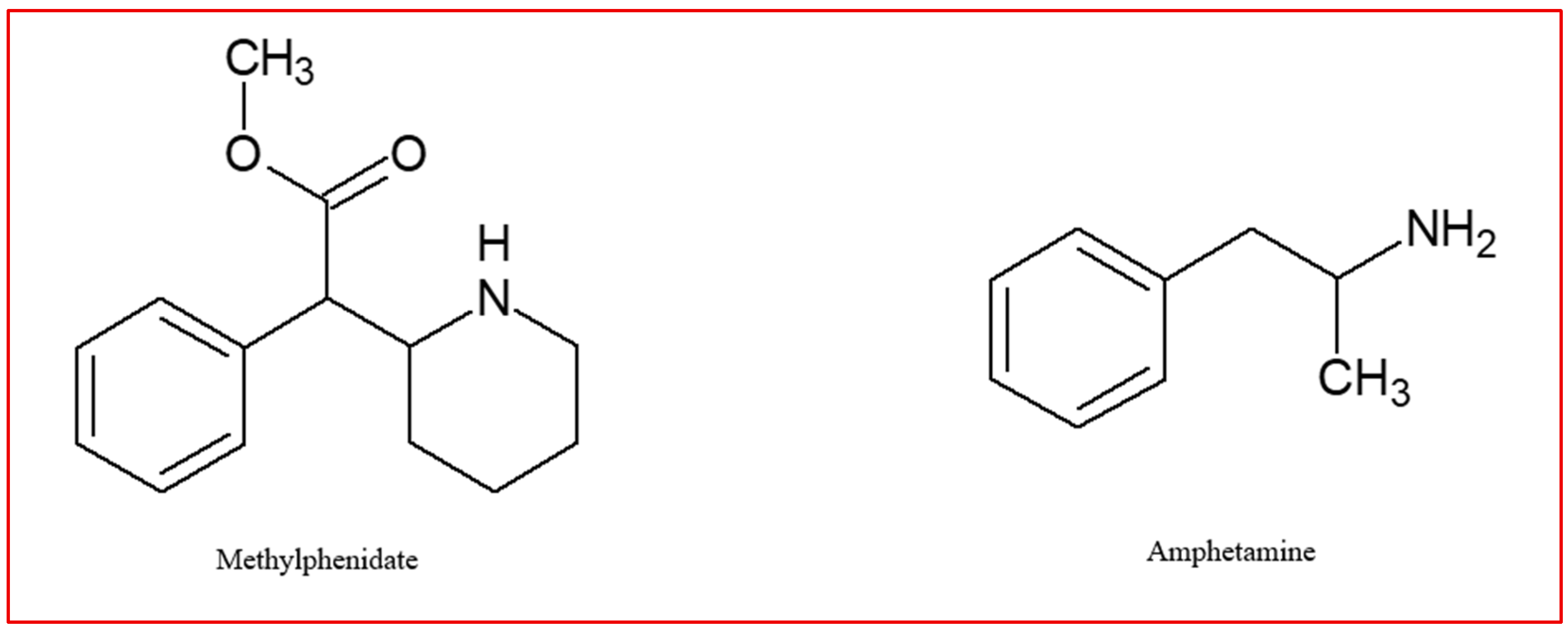
4.2. Methylphenidate
4.3. Amphetamines
4.4. Dopaminergic Psychostimulants and the Risk of Addiction
4.5. Non-Stimulants in ADHD Therapy
4.5.1. Atomoxetine
4.5.2. Bupropion
5. Non-Pharmacological Treatment
5.1. Cognitive-Behavioral Therapy (CBT)
5.2. Dietary Interventions and Supplementation
5.3. Neurofeedback
5.4. Brain Stimulation
5.5. Music Therapy
5.6. Physical Exercise
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADHD | Attention-deficit/hyperactivity disorder |
| SUD | Substance Use Disorder |
| NICE | National Institute for Health and Care Excellence |
| CNS | Central Nervous System |
| CIUS | Compulsive Internet Use Scale |
| EEG | Electroencephalogram |
| MPH | Methylphenidate |
| LDX | Lisdexamphetamine |
| SDX | Serdexmethylphenidate |
| ATX | Atomoxetine |
| NRI | Norepinephrine reuptake inhibitors |
| fMRI | magnetic resonance imaging |
References
- Ayano, G.; Demelash, S.; Gizachew, Y.; Tsegay, L.; Alati, R. The global prevalence of attention deficit hyperactivity disorder in children and adolescents: An umbrella review of meta-analyses. J. Affect. Disord. 2023, 339, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Buczyłowska, D.; Singh, N.; Baumbach, C.; Bratkowski, J.; Mysak, Y.; Wierzba-Łukaszyk, M.; Sitnik-Warchulska, K.; Skotak, K.; Lipowska, M.; Izydorczyk, B.; et al. Lifelong greenspace exposure and ADHD in Polish children: Role of physical activity and perceived neighbourhood characteristics. J. Environ. Psychol. 2024, 96, 102313. [Google Scholar] [CrossRef]
- Staley, B.S.; Robinson, L.R.; Claussen, A.H.; Katz, S.M.; Danielson, M.L.; Summers, A.D.; Farr, S.L.; Blumberg, S.J.; Tinker, S.C. Attention-Deficit/Hyperactivity Disorder Diagnosis, Treatment, and Telehealth Use in Adults—National Center for Health Statistics Rapid Surveys System, United States, October–November 2023. Morb. Mortal. Wkly. Rep. 2024, 73, 890–895. [Google Scholar] [CrossRef]
- Olatunji, G.; Faturoti, O.; Jaiyeoba, B.; Toluwabori, A.V.; Adefusi, T.; Olaniyi, P.; Aderinto, N.; Abdulbasit, M.O. Navigating unique challenges and advancing equitable care for children with ADHD in Africa: A review. Ann. Med. Surg. 2023, 85, 4939. [Google Scholar] [CrossRef] [PubMed]
- Rocco, I.; Corso, B.; Bonati, M.; Minicuci, N. Time of onset and/or diagnosis of ADHD in European children: A systematic review. BMC Psychiatry 2021, 21, 575. [Google Scholar] [CrossRef]
- Barbuti, M.; Maiello, M.; Spera, V.; Pallucchini, A.; Brancati, G.E.; Maremmani, A.G.I.; Perugi, G.; Maremmani, I. Challenges of treating ADHD with comorbid substance use disorder: Considerations for the clinician. J. Clin. Med. 2023, 12, 3096. [Google Scholar] [CrossRef] [PubMed]
- Waltereit, R.; Ehrlich, S.; Roessner, V. First-time diagnosis of ADHD in adults: Challenge to retrospectively assess childhood symptoms of ADHD from long-term memory. Eur. Child Adolesc. Psychiatry 2023, 32, 1333–1335. [Google Scholar] [CrossRef]
- de Souza, I.; Mattos, P.; Pina, C.; Fortes, D. ADHD: The impact when not diagnosed. J. Bras. Psiquiatr. 2008, 57, 139–141. [Google Scholar] [CrossRef]
- Drechsler, R.; Brem, S.; Brandeis, D.; Grünblatt, E.; Berger, G.; Walitza, S. ADHD: Current concepts and treatments in children and adolescents. Neuropediatrics 2020, 51, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Groom, M.J.; Cortese, S. Current pharmacological treatments for ADHD. Curr. Top. Behav. Neurosci. 2022, 57, 19–50. [Google Scholar] [CrossRef]
- Choi, W.-S.; Woo, Y.S.; Wang, S.-M.; Lim, H.K.; Bahk, W.-M. The prevalence of psychiatric comorbidities in adult ADHD compared with non-ADHD populations: A systematic literature review. PLoS ONE 2022, 17, e0277175. [Google Scholar] [CrossRef] [PubMed]
- Correll, C.U.; Starling, B.R.; Huss, M. Systematic review of transdermal treatment options in attention-deficit/hyperactivity disorder: Implications for use in adult patients. CNS Spectr. 2022, 27, 437–449. [Google Scholar] [CrossRef]
- Salvi, V.; Ribuoli, E.; Servasi, M.; Orsolini, L.; Volpe, U. ADHD and bipolar disorder in adulthood: Clinical and treatment implications. Med. Kaunas Lith. 2021, 57, 466. [Google Scholar] [CrossRef]
- da Silva, A.G.; Malloy-Diniz, L.F.; Garcia, M.S.; Rocha, R. Attention-Deficit/Hyperactivity Disorder and Women. In Women’s Mental Health; Springer: Cham, Switzerland, 2020; pp. 215–219. [Google Scholar] [CrossRef]
- Attoe, D.E.; Climie, E.A. Miss. Diagnosis: A systematic review of ADHD in adult women. J. Atten. Disord. 2023, 27, 645–657. [Google Scholar] [CrossRef]
- Hinshaw, S.P.; Nguyen, P.T.; O’Grady, S.M.; Rosenthal, E.A. Annual Research Review: Attention-deficit/hyperactivity disorder in girls and women: Underrepresentation, longitudinal processes, and key directions. J. Child Psychol. Psychiatry 2022, 63, 484–496. [Google Scholar] [CrossRef]
- Koumoula, A. The course of attention deficit hyperactivity disorder (ADHD) over the life span. Psychiatriki 2012, 23 (Suppl. S1), 49–59. [Google Scholar] [PubMed]
- Tapes, V.; Cosciug, I.; Deliv, I. Attention deficit hyperactivity disorder in the context of substance use disorder: A research perspective. Neurosci. Appl. 2024, 3, 104498. [Google Scholar] [CrossRef]
- Olagunju, A.E.; Ghoddusi, F. Attention-Deficit/Hyperactivity Disorder in Adults. Am. Fam. Physician 2024, 110, 157–166. [Google Scholar] [PubMed]
- Levy, F. DSM-5, ICD-11, RDoC and ADHD diagnosis. Aust. N. Z. J. Psychiatry 2014, 48, 1163–1164. [Google Scholar] [CrossRef]
- Krawczyk, P.; Święcicki, Ł. ICD-11 vs. ICD-10—A review of updates and novelties introduced in the latest version of the WHO International Classification of Diseases. Psychiatr. Pol. 2020, 54, 7–20. [Google Scholar] [CrossRef]
- Surman, C.B.H.; Walsh, D.M. Do ADHD treatments improve executive behavior beyond core ADHD symptoms in adults? Evidence from systematic analysis of clinical trials. J. Clin. Pharmacol. 2023, 63, 640–653. [Google Scholar] [CrossRef]
- Faraone, S.V. ADHD in adults—A familiar disease with unfamiliar challenges. CNS Spectr. 2007, 12, 14–17. [Google Scholar] [CrossRef]
- Adamou, M.; Arif, M.; Asherson, P.; Aw, T.-C.; Bolea, B.; Coghill, D.; Guðjónsson, G.; Halmøy, A.; Hodgkins, P.; Müller, U.; et al. Occupational issues of adults with ADHD. BMC Psychiatry 2013, 13, 59. [Google Scholar] [CrossRef]
- Kosheleff, A.R.; Mason, O.; Jain, R.; Koch, J.; Rubin, J. Functional Impairments Associated with ADHD in Adulthood and the Impact of Pharmacological Treatment. J. Atten. Disord. 2023, 27, 669–697. [Google Scholar] [CrossRef]
- Retz, W.; Ginsberg, Y.; Turner, D.; Barra, S.; Retz-Junginger, P.; Larsson, H.; Asherson, P. Attention-Deficit/Hyperactivity Disorder (ADHD), antisociality and delinquent behavior over the lifespan. Neurosci. Biobehav. Rev. 2021, 120, 236–248. [Google Scholar] [CrossRef]
- Young, S.; Cocallis, K. ADHD and offending. J. Neural Transm. 2021, 128, 1009–1019. [Google Scholar] [CrossRef]
- Research Consortium for Non-Pharmacological Interventions on ADHD; Dentz, A.; Soelch, C.M.; Fahim, C.; Torsello, A.; Parent, V.; Ponsioen, A.; Guay, M.-C.; Bioulac-Rogier, S.; Clément, C.; et al. Non-pharmacological treatment of Attention Deficit Disorder with or without Hyperactivity (ADHD). Overview and report of the first international symposium on the non-pharmacological management of ADHD. L’Encephale 2024, 50, 309–328. [Google Scholar] [CrossRef]
- Nimmo-Smith, V.; Merwood, A.; Hank, D.; Brandling, J.; Greenwood, R.; Skinner, L.; Law, S.; Patel, V.; Rai, D. Non-pharmacological interventions for adult ADHD: A systematic review. Psychol. Med. 2020, 50, 529–541. [Google Scholar] [CrossRef]
- Lange, K.W.; Reichl, S.; Lange, K.M.; Tucha, L.; Tucha, O. The history of attention deficit hyperactivity disorder. Atten. Deficit Hyperact. Disord. 2010, 2, 241–255. [Google Scholar] [CrossRef]
- Goldstein, S.; DeVries, M. Handbook of DSM-5 Disorders in Children and Adolescents; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 978-3-319-57196-6. [Google Scholar]
- Ortal, S.; van de Glind, G.; Johan, F.; Itai, B.; Nir, Y.; Iliyan, I.; van den Brink, W. The Role of Different Aspects of Impulsivity as Independent Risk Factors for Substance Use Disorders in Patients with ADHD: A Review. Curr. Drug Abus. Rev. 2015, 8, 119–133. [Google Scholar] [CrossRef]
- Molina, B.S.G.; Pelham, W.E. Attention-Deficit/Hyperactivity Disorder and Risk of Substance Use Disorder: Developmental Considerations, Potential Pathways, and Opportunities for Research. Annu. Rev. Clin. Psychol. 2014, 10, 607–639. [Google Scholar] [CrossRef]
- Verdejo-García, A.; Bechara, A.; Recknor, E.C.; Pérez-García, M. Negative emotion-driven impulsivity predicts substance dependence problems. Drug Alcohol Depend. 2007, 91, 213–219. [Google Scholar] [CrossRef]
- Whelan, R.; Conrod, P.J.; Poline, J.-B.; Lourdusamy, A.; Banaschewski, T.; Barker, G.J.; Bellgrove, M.A.; Büchel, C.; Byrne, M.; Cummins, T.D.R.; et al. Adolescent impulsivity phenotypes characterized by distinct brain networks. Nat. Neurosci. 2012, 15, 920–925. [Google Scholar] [CrossRef]
- Costumero, V.; Barrós-Loscertales, A.; Bustamante, J.C.; Ventura-Campos, N.; Fuentes, P.; Ávila, C. Reward sensitivity modulates connectivity among reward brain areas during processing of anticipatory reward cues. Eur. J. Neurosci. 2013, 38, 2399–2407. [Google Scholar] [CrossRef]
- van Ewijk, H.; Groenman, A.P.; Zwiers, M.P.; Heslenfeld, D.J.; Faraone, S.V.; Hartman, C.A.; Luman, M.; Greven, C.U.; Hoekstra, P.J.; Franke, B.; et al. Smoking and the developing brain: Altered white matter microstructure in attention-deficit/hyperactivity disorder and healthy controls. Hum. Brain Mapp. 2015, 36, 1180–1189. [Google Scholar] [CrossRef]
- Newman, E.; Jernigan, T.L.; Lisdahl, K.M.; Tamm, L.; Tapert, S.F.; Potkin, S.G.; Mathalon, D.; Molina, B.; Bjork, J.; Castellanos, F.X.; et al. Go/No Go task performance predicts cortical thickness in the caudal inferior frontal gyrus in young adults with and without ADHD. Brain Imaging Behav. 2016, 10, 880–892. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.J.; Fowler, J.S.; Logan, J.; Gatley, S.J.; Wong, C.; Hitzemann, R.; Pappas, N.R. Reinforcing effects of psychostimulants in humans are associated with increases in brain dopamine and occupancy of D(2) receptors. J. Pharmacol. Exp. Ther. 1999, 291, 409–415. [Google Scholar] [CrossRef]
- Groen, Y.; Gaastra, G.F.; Lewis-Evans, B.; Tucha, O. Risky Behavior in Gambling Tasks in Individuals with ADHD—A Systematic Literature Review. PLoS ONE 2013, 8, e74909. [Google Scholar] [CrossRef]
- Verdejo-Garcia, A.; Albein-Urios, N. Impulsivity traits and neurocognitive mechanisms conferring vulnerability to substance use disorders. Neuropharmacology 2021, 183, 108402. [Google Scholar] [CrossRef]
- Luigjes, J.; Lorenzetti, V.; de Haan, S.; Youssef, G.J.; Murawski, C.; Sjoerds, Z.; van den Brink, W.; Denys, D.; Fontenelle, L.F.; Yücel, M. Defining Compulsive Behavior. Neuropsychol. Rev. 2019, 29, 4–13. [Google Scholar] [CrossRef]
- Dalley, J.W.; Everitt, B.J.; Robbins, T.W. Impulsivity, Compulsivity, and Top-Down Cognitive Control. Neuron 2011, 69, 680–694. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F.; Volkow, N.D. Neurobiology of addiction: A neurocircuitry analysis. Lancet Psychiatry 2016, 3, 760–773. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, H.; Lee, P.H.; Tsetsos, F.; Davis, L.K.; Yu, D.; Lee, S.H.; Dalsgaard, S.; Haavik, J.; Barta, C.; et al. Investigating Shared Genetic Basis Across Tourette Syndrome and Comorbid Neurodevelopmental Disorders Along the Impulsivity-Compulsivity Spectrum. Biol. Psychiatry 2021, 90, 317–327. [Google Scholar] [CrossRef]
- Milberger, S.; Biederman, J.; Faraone, S.V.; Chen, L.; Jones, J. ADHD Is Associated with Early Initiation of Cigarette Smoking in Children and Adolescents. J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 37–44. [Google Scholar] [CrossRef]
- Biederman, J.; Faraone, S.V.; Monuteaux, M.C.; Feighner, J.A. Patterns of Alcohol and Drug Use in Adolescents Can Be Predicted by Parental Substance Use Disorders. Pediatrics 2000, 106, 792–797. [Google Scholar] [CrossRef]
- Trifilieff, P.; Martinez, D. Imaging addiction: D2 receptors and dopamine signaling in the striatum as biomarkers for impulsivity. Neuropharmacology 2014, 76, 498–509. [Google Scholar] [CrossRef]
- Xiao, C.; Zhou, C.; Jiang, J.; Yin, C. Neural circuits and nicotinic acetylcholine receptors mediate the cholinergic regulation of midbrain dopaminergic neurons and nicotine dependence. Acta Pharmacol. Sin. 2020, 41, 1–9. [Google Scholar] [CrossRef]
- Fenster, C.P.; Whitworth, T.L.; Sheffield, E.B.; Quick, M.W.; Lester, R.A.J. Upregulation of Surface α4β2 Nicotinic Receptors Is Initiated by Receptor Desensitization After Chronic Exposure to Nicotine. J. Neurosci. 1999, 19, 4804–4814. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.-J.; Fowler, J.S.; Tomasi, D.; Telang, F.; Baler, R. Addiction: Decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain’s control circuit. BioEssays 2010, 32, 748–755. [Google Scholar] [CrossRef]
- Kandel, D. Sequence and Stages in Patterns of Adolescent Drug Use. Arch. Gen. Psychiatry 1975, 32, 923. [Google Scholar] [CrossRef]
- Wilens, T.E.; Adler, L.A.; Adams, J.; Sgambati, S.; Rotrosen, J.; Sawtelle, R.; Utzinger, L.; Fusillo, S. Misuse and Diversion of Stimulants Prescribed for ADHD: A Systematic Review of the Literature. J. Am. Acad. Child Adolesc. Psychiatry 2008, 47, 21–31. [Google Scholar] [CrossRef]
- Mohammadian, M.; Khachatryan, L.G.; Vadiyan, F.V.; Maleki, M.; Fatahian, F.; Mohammadian-Hafshejani, A. The association between maternal tobacco smoking during pregnancy and the risk of attention-deficit/hyperactivity disorder (ADHD) in offspring: A systematic review and meta-analysis. PLoS ONE 2025, 20, e0317112. [Google Scholar] [CrossRef] [PubMed]
- Desaunay, P.; Eude, L.-G.; Dreyfus, M.; Alexandre, C.; Fedrizzi, S.; Alexandre, J.; Uguz, F.; Guénolé, F. Benefits and Risks of Antidepressant Drugs During Pregnancy: A Systematic Review of Meta-Analyses. Pediatr. Drugs 2023, 25, 247–265. [Google Scholar] [CrossRef] [PubMed]
- Quintero, J.; Gutiérrez-Casares, J.R.; Álamo, C. Molecular Characterisation of the Mechanism of Action of Stimulant Drugs Lisdexamfetamine and Methylphenidate on ADHD Neurobiology: A Review. Neurol. Ther. 2022, 11, 1489–1517. [Google Scholar] [CrossRef] [PubMed]
- Biederman, J.; Spencer, T. Attention-deficit/hyperactivity disorder (adhd) as a noradrenergic disorder. Biol. Psychiatry 1999, 46, 1234–1242. [Google Scholar] [CrossRef]
- Ulke, C.; Rullmann, M.; Huang, J.; Luthardt, J.; Becker, G.-A.; Patt, M.; Meyer, P.M.; Tiepolt, S.; Hesse, S.; Sabri, O.; et al. Adult attention-deficit/hyperactivity disorder is associated with reduced norepinephrine transporter availability in right attention networks: A (S,S)-O-[11C]methylreboxetine positron emission tomography study. Transl. Psychiatry 2019, 9, 301. [Google Scholar] [CrossRef]
- Odgers, C.L.; Caspi, A.; Nagin, D.S.; Piquero, A.R.; Slutske, W.S.; Milne, B.J.; Dickson, N.; Poulton, R.; Moffitt, T.E. Is It Important to Prevent Early Exposure to Drugs and Alcohol Among Adolescents? Psychol. Sci. 2008, 19, 1037–1044. [Google Scholar] [CrossRef]
- Khantzian, E.J. The Self-Medication Hypothesis of Substance Use Disorders: A Reconsideration and Recent Applications. Harv. Rev. Psychiatry 1997, 4, 231–244. [Google Scholar] [CrossRef]
- Wilens, T.E.; Biederman, J.; Faraone, S.V.; Martelon, M.; Westerberg, D.; Spencer, T.J. Presenting ADHD Symptoms, Subtypes, and Comorbid Disorders in Clinically Referred Adults with ADHD. J. Clin. Psychiatry 2009, 70, 1557–1562. [Google Scholar] [CrossRef]
- First, M.B. Diagnostic and Statistical Manual of Mental Disorders, 5th Edition, and Clinical Utility. J. Nerv. Ment. Dis. 2013, 201, 727–729. [Google Scholar] [CrossRef]
- Romo, L.; Rémond, J.J.; Coeffec, A.; Kotbagi, G.; Plantey, S.; Boz, F.; Kern, L. Gambling and Attention Deficit Hyperactivity Disorders (ADHD) in a Population of French Students. J. Gambl. Stud. 2015, 31, 1261–1272. [Google Scholar] [CrossRef]
- Szerman, N.; Ferre, F.; Basurte-Villamor, I.; Vega, P.; Mesias, B.; Marín-Navarrete, R.; Arango, C. Gambling Dual Disorder: A Dual Disorder and Clinical Neuroscience Perspective. Front. Psychiatry 2020, 11, 589155. [Google Scholar] [CrossRef]
- Chen, C.; Dai, S.; Shi, L.; Shen, Y.; Ou, J. Associations Between Attention Deficit/Hyperactivity Disorder and Internet Gaming Disorder Symptoms Mediated by Depressive Symptoms and Hopelessness Among College Students. Neuropsychiatr. Dis. Treat. 2021, 17, 2775–2782. [Google Scholar] [CrossRef]
- Romo, L.; Ladner, J.; Kotbagi, G.; Morvan, Y.; Saleh, D.; Tavolacci, M.P.; Kern, L. Attention-deficit hyperactivity disorder and addictions (substance and behavioral): Prevalence and characteristics in a multicenter study in France. J. Behav. Addict. 2018, 7, 743–751. [Google Scholar] [CrossRef]
- Ho, R.C.; Zhang, M.W.; Tsang, T.Y.; Toh, A.H.; Pan, F.; Lu, Y.; Cheng, C.; Yip, P.S.; Lam, L.T.; Lai, C.-M.; et al. The association between internet addiction and psychiatric co-morbidity: A meta-analysis. BMC Psychiatry 2014, 14, 183. [Google Scholar] [CrossRef] [PubMed]
- Findon, J.L.; Muck, A.; Davison, B.T.; Dommett, E.J. Investigating behavioural addictions in adults with and without attention deficit hyperactivity disorder. PLoS ONE 2025, 20, e0317525. [Google Scholar] [CrossRef]
- Li, W.; Zhang, W.; Xiao, L.; Nie, J. The association of Internet addiction symptoms with impulsiveness, loneliness, novelty seeking and behavioral inhibition system among adults with attention-deficit/hyperactivity disorder (ADHD). Psychiatry Res. 2016, 243, 357–364. [Google Scholar] [CrossRef]
- Faraone, S.V.; Banaschewski, T.; Coghill, D.; Zheng, Y.; Biederman, J.; Bellgrove, M.A.; Newcorn, J.H.; Gignac, M.; Al Saud, N.M.; Manor, I.; et al. The World Federation of ADHD International Consensus Statement: 208 Evidence-based conclusions about the disorder. Neurosci. Biobehav. Rev. 2021, 128, 789–818. [Google Scholar] [CrossRef]
- Taylor, E.; Döpfner, M.; Sergeant, J.; Asherson, P.; Banaschewski, T.; Buitelaar, J.; Coghill, D.; Danckaerts, M.; Rothenberger, A.; Sonuga-Barke, E.; et al. European clinical guidelines forhyperkinetic disorder—First upgrade. Eur. Child Adolesc. Psychiatry 2004, 13, i7–i30. [Google Scholar] [CrossRef]
- Wolraich, M.L.; Hagan, J.F., Jr.; Allan, C.; Chan, E.; Davison, D.; Earls, M.; Evans, S.W.; Flinn, S.K.; Froehlich, T.; Frost, J.; et al. Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents. Pediatrics 2019, 144, e20192528. [Google Scholar] [CrossRef] [PubMed]
- NICE. Attention Deficit Hyperactivity Disorder: Diagnosis and Management. Available online: https://www.nice.org.uk/guidance/ng87 (accessed on 7 September 2025).
- Asherson, P.; Balazs, J.; Bitter, I.; Carpentier, P.-J.; Jaeschke, R.; Mohr, P.; Ramos-Quiroga, J.A.; Rossi, P.D.; Semerci, B.; Kooij, S. Attention Deficit Hyperactivity Disorder in Adults. In Mental Health Research and Practice; Cambridge University Press: Cambridge, UK, 2024; pp. 135–157. [Google Scholar] [CrossRef]
- Kooij, J.J.S.; Bijlenga, D.; Salerno, L.; Jaeschke, R.; Bitter, I.; Balázs, J.; Thome, J.; Dom, G.; Kasper, S.; Filipe, C.N.; et al. Updated European Consensus Statement on diagnosis and treatment of adult ADHD. Eur. Psychiatry 2019, 56, 14–34. [Google Scholar] [CrossRef]
- Cortese, S.; Adamo, N.; Giovane, C.D.; Mohr-Jensen, C.; Hayes, A.J.; Carucci, S.; Atkinson, L.Z.; Tessari, L.; Banaschewski, T.; Coghill, D.; et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: A systematic review and network meta-analysis. Lancet Psychiatry 2018, 5, 727–738. [Google Scholar] [CrossRef]
- Bradley, C. The behavior of children receiving benzedrine. Am. J. Psychiatry 1937, 94, 577–585. [Google Scholar] [CrossRef]
- The MTA Cooperative Group; Multimodal Treatment Study of Children with ADHD. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry 1999, 56, 1073–1086. [Google Scholar] [CrossRef]
- Michelson, D.; Allen, A.J.; Busner, J.; Casat, C.; Dunn, D.; Kratochvil, C.; Newcorn, J.; Sallee, F.R.; Sangal, R.B.; Saylor, K.; et al. Once-Daily Atomoxetine Treatment for Children and Adolescents with Attention Deficit Hyperactivity Disorder: A Randomized, Placebo-Controlled Study. Am. J. Psychiatry 2002, 159, 1896–1901. [Google Scholar] [CrossRef] [PubMed]
- Sallee, F.R.; Mcgough, J.; Wigal, T.; Donahue, J.; Lyne, A.; Biederman, J. Guanfacine Extended Release in Children and Adolescents with Attention-Deficit/Hyperactivity Disorder: A Placebo-Controlled Trial. J. Am. Acad. Child Adolesc. Psychiatry 2009, 48, 155–165. [Google Scholar] [CrossRef]
- Caye, A.; Swanson, J.M.; Coghill, D.; Rohde, L.A. Treatment strategies for ADHD: An evidence-based guide to select optimal treatment. Mol. Psychiatry 2018, 24, 390–408. [Google Scholar] [CrossRef]
- Childress, A.C.; Komolova, M.; Sallee, F.R. An update on the pharmacokinetic considerations in the treatment of ADHD with long-acting methylphenidate and amphetamine formulations. Expert Opin. Drug Metab. Toxicol. 2019, 15, 937–974. [Google Scholar] [CrossRef]
- Markowitz, J.S.; Patrick, K.S. The Clinical Pharmacokinetics of Amphetamines Utilized in the Treatment of Attention-Deficit/Hyperactivity Disorder. J. Child Adolesc. Psychopharmacol. 2017, 27, 678–689. [Google Scholar] [CrossRef]
- Wenthur, C.J. Classics in Chemical Neuroscience: Methylphenidate. ACS Chem. Neurosci. 2016, 7, 1030–1040. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Li, R.; Wong, S.H.S.; Sum, R.K.W.; Sit, C.H.P. The impact of exercise interventions concerning executive functions of children and adolescents with attention-deficit/hyperactive disorder: A systematic review and meta-analysis. Int. J. Behav. Nutr. Phys. Act. 2021, 18, 68. [Google Scholar] [CrossRef]
- Cantelon, J.A.; Giles, G.E. A Review of Cognitive Changes During Acute Aerobic Exercise. Front. Psychol. 2021, 12, 653158. [Google Scholar] [CrossRef]
- Dinoff, A.; Herrmann, N.; Swardfager, W.; Liu, C.S.; Sherman, C.; Chan, S.; Lanctôt, K.L. The Effect of Exercise Training on Resting Concentrations of Peripheral Brain-Derived Neurotrophic Factor (BDNF): A Meta-Analysis. PLoS ONE 2016, 11, e0163037. [Google Scholar] [CrossRef]
- Bustamante, E.E.; Davis, C.L.; Frazier, S.L.; Rusch, D.; Fogg, L.F.; Atkins, M.S.; Marquez, D.X. Randomized Controlled Trial of Exercise for ADHD and Disruptive Behavior Disorders. Med. Sci. Sports Exerc. 2016, 48, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Childress, A.; Tran, C. Current Investigational Drugs for the Treatment of Attention-Deficit/Hyperactivity Disorder. Expert Opin. Investig. Drugs 2016, 25, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Li, M.J.; Shoptaw, S.J. Clinical management of psychostimulant withdrawal: Review of the evidence. Addiction 2023, 118, 750–762. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, R.R.; Sujkowska, E.; Sowa-Kućma, M. Methylphenidate for attention-deficit/hyperactivity disorder in adults: A narrative review. Psychopharmacology 2021, 238, 2667–2691. [Google Scholar] [CrossRef] [PubMed]
- Bieś, R.; Fojcik, J.; Warchala, A.; Trędzbor, B.; Krysta, K.; Piekarska-Bugiel, K.; Krzystanek, M. The Risk of Methylphenidate Pharmacotherapy for Adults with ADHD. Pharmaceuticals 2023, 16, 1292. [Google Scholar] [CrossRef]
- Docherty, J.R.; Alsufyani, H.A. Cardiovascular and temperature adverse actions of stimulants. Br. J. Pharmacol. 2021, 178, 2551–2568. [Google Scholar] [CrossRef]
- Gandhi, A.; Beekman, C.; Parker, R.; Fang, L.; Babiskin, A.; Matta, M.K. Novel and rapid LC-MS/MS method for quantitative analysis of methylphenidate in dried blood spots. Bioanalysis 2018, 10, 839–850. [Google Scholar] [CrossRef]
- Brumbaugh, S.; Tuan, W.J.; Scott, A.; Latronica, J.R.; Bone, C. Trends in characteristics of the recipients of new prescription stimulants between years 2010 and 2020 in the United States: An observational cohort study. eClinicalMedicine 2022, 50, 101524. [Google Scholar] [CrossRef]
- Sluiter, M.N.; de Vries, Y.A.; Koning, L.G.; Hak, E.; Bos, J.H.J.; Schuiling-Veninga, C.C.M.; Batstra, L.; Doornenbal, J.M.; de Jonge, P. A Prescription Trend Analysis of Methylphenidate: Relation to Study Reports on Efficacy. Adm. Policy Ment. Health Ment. Health Serv. Res. 2020, 47, 291–299. [Google Scholar] [CrossRef]
- Hartz, I.; Madsstuen, N.H.H.; Andersen, P.N.; Handal, M.; Odsbu, I. Nationwide trends in the use of ADHD medications in the period 2006–2022: A study from the Norwegian prescription database. BMC Psychiatry 2024, 24, 767. [Google Scholar] [CrossRef]
- Hasan, S.S.; Bal, N.; Baker, I.; Kow, C.S.; Khan, M.U. Adverse Drug Reaction Reporting and Prescribing Trends of Drugs for Attention Deficit Hyperactivity Disorder in Primary Care England, 2010–2019. J. Atten. Disord. 2022, 26, 467–475. [Google Scholar] [CrossRef]
- Kimko, H.C.; Cross, J.T.; Abernethy, D.R. Pharmacokinetics and clinical effectiveness of methylphenidate. Clin. Pharmacokinet. 1999, 37, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Cutler, A.J.; Hanaie, J. Understanding the delivery technology used in ADHD stimulant medications can help to individualize treatment. CNS Spectr. 2025, 30, e30. [Google Scholar] [CrossRef] [PubMed]
- Charach, G.; Karniel, E.; Grosskopf, I.; Rabinovich, A.; Charach, L.; Sayil, C. Methylphenidate has mild hyperglycemic and hypokalemia effects and increases leukocyte and neutrophil counts. Medicine 2020, 99, E20931. [Google Scholar] [CrossRef] [PubMed]
- Patrick, K.S.; Radke, J.L.; Raymond, J.R.; Koller, L.; Nguyen, L.V.; Rodriguez, W.; Straughn, A.B. Drug Regimen Individualization for Attention-Deficit/Hyperactivity Disorder: Guidance for Methylphenidate and Dexmethylphenidate Formulations. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2019, 39, 677–688. [Google Scholar] [CrossRef]
- Duong, S.; Chung, K.; Wigal, S.B. Metabolic, toxicological, and safety considerations for drugs used to treat ADHD. Expert Opin. Drug Metab. Toxicol. 2012, 8, 543–552. [Google Scholar] [CrossRef]
- Verma, R.K.; Krishna, D.M.; Garg, S. Formulation aspects in the development of osmotically controlled oral drug delivery systems. J. Control. Release 2002, 79, 7–27. [Google Scholar] [CrossRef]
- Young, S.; Abbasian, C.; Al-Attar, Z.; Branney, P.; Colley, B.; Cortese, S.; Cubbin, S.; Deeley, Q.; Gudjonsson, G.H.; Hill, P.; et al. Identification and treatment of individuals with attention-deficit/hyperactivity disorder and substance use disorder: An expert consensus statement. World J. Psychiatry 2023, 13, 84–112. [Google Scholar] [CrossRef]
- Findling, R.L.; Dinh, S. Transdermal Therapy for Attention-Deficit Hyperactivity Disorder with the Methylphenidate Patch (MTS). CNS Drugs 2014, 28, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, F.; Kim, B.S. Recent advances in polymeric transdermal drug delivery systems. J. Control. Release 2022, 341, 132–146. [Google Scholar] [CrossRef]
- McRae-Clark, A.L.; Brady, K.T.; Hartwell, K.J.; White, K.; Carter, R.E. Methylphenidate Transdermal System in Adults with Past Stimulant Misuse. J. Atten. Disord. 2011, 15, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Castells, X.; Blanco-Silvente, L.; Cunill, R. Amphetamines for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst. Rev. 2018, 8, CD007813. [Google Scholar] [CrossRef]
- Broadley, K.J. Reappraisal of the mechanism of cardiovascular responses to sympathomimetic amines in anaesthetised rats: Dual α1-adrenoceptor and trace amine receptor mechanisms. Naunyn. Schmiedebergs Arch. Pharmacol. 2024, 398, 2627–2639. [Google Scholar] [CrossRef]
- National Institute of Diabetes and Digestive and Kidney Diseases. Amphetamines. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2021. [Google Scholar]
- Pires, B.; Rosendo, L.M.; Brinca, A.T.; Simão, A.Y.; Barroso, M.; Rosado, T.; Gallardo, E. The Therapeutic Potential of Amphetamine-like Psychostimulants. Life 2023, 13, 2180. [Google Scholar] [CrossRef]
- Steingard, R.; Taskiran, S.; Connor, D.F.; Markowitz, J.S.; Stein, M.A. New Formulations of Stimulants: An Update for Clinicians. J. Child Adolesc. Psychopharmacol. 2019, 29, 324–339. [Google Scholar] [CrossRef]
- Heal, D.J.; Gosden, J.; Smith, S.L. Stimulant prodrugs: A pharmacological and clinical assessment of their role in treating ADHD and binge-eating disorder. Adv. Pharmacol. 2024, 99, 251–286. [Google Scholar] [CrossRef] [PubMed]
- Kämmerer, W. Comparative pharmacology and abuse potential of oral dexamphetamine and lisdexamfetamine—A literature review. Hum. Psychopharmacol. Clin. Exp. 2024, 39, e2910. [Google Scholar] [CrossRef] [PubMed]
- Janetzki, J.; Kalisch, L.; Hall, K.; Pratt, N. Utilisation Trends of Lisdexamfetamine: Insights from Recent Medicine Shortages in Australia. Pharmacoepidemiol. Drug Saf. 2025, 34, e70113. [Google Scholar] [CrossRef]
- Haedener, M.; Weinmann, W.; Eich, D.; Liebrenz, M.; Wuethrich, T.; Buadze, A. Evaluating the reliability of hair analysis in monitoring the compliance of ADHD patients under treatment with Lisdexamphetamine. PLoS ONE 2021, 16, e0248747. [Google Scholar] [CrossRef]
- Goodman, D.W. Lisdexamfetamine Dimesylate: The First Prodrug Stimulant. Psychiatry Edgmont 2007, 4, 39. [Google Scholar]
- Jasinski, D.R.; Krishnan, S.S. Abuse liability and safety of oral lisdexamfetamine dimesylate in individuals with a history of stimulant abuse. J. Psychopharmacol. 2009, 23, 419–427. [Google Scholar] [CrossRef]
- Jasinski, D.R.; Krishnan, S. Human pharmacology of intravenous lisdexamfetamine dimesylate: Abuse liability in adult stimulant abusers. J. Psychopharmacol. 2009, 23, 410–418. [Google Scholar] [CrossRef]
- Dolder, P.C.; Strajhar, P.; Vizeli, P.; Hammann, F.; Odermatt, A.; Liechti, M.E. Pharmacokinetics and pharmacodynamics of lisdexamfetamine compared with D-amphetamine in healthy subjects. Front. Pharmacol. 2017, 8, 289246. [Google Scholar] [CrossRef]
- Barnhardt, E.A.; Narayanan, A.R.; Coury, D.L. Evaluating serdexmethylphenidate and dexmethylphenidate capsules as a once-daily treatment option for ADHD. Expert Opin. Pharmacother. 2023, 24, 1215–1219. [Google Scholar] [CrossRef] [PubMed]
- Rohner, H.; Gaspar, N.; Philipsen, A.; Schulze, M. Prevalence of Attention Deficit Hyperactivity Disorder (ADHD) Among Substance Use Disorder (SUD) Populations: Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 1275. [Google Scholar] [CrossRef]
- Darke, S.; Peacock, A.; Duflou, J.A.; Farrell, M.; Lappin, J. Methylphenidate and (lis)dexamfetamine toxicity-related deaths of adults, Australia, 2000–24: Analysis of NCIS data. Med. J. Aust. 2025, 222, 259–261. [Google Scholar] [CrossRef]
- Taşkan, M.; Tufan, A.E.; Öztürk, Y.; Kesikbaş, B.B.; Imrek, Y.; Akinci, B.; Koçak, G. Drug Holidays May Attenuate Beneficial Effects of Treatment on Emotion Regulation and Recognition Among Children with ADHD: A Single-Center, Prospective Study. Psychiatry Clin. Psychopharmacol. 2024, 34, 285–293. [Google Scholar] [CrossRef]
- Swanson, J.M. Long-acting stimulants: Development and dosing. Can. Child Adolesc. Psychiatry Rev. 2005, 14, 4. [Google Scholar]
- Ibrahim, K.; Donyai, P. Drug Holidays from ADHD Medication: International Experience Over the Past Four Decades. J. Atten. Disord. 2015, 19, 551–568. [Google Scholar] [CrossRef]
- Göl Özcan, G.; Öztürk, Y.; Sari, M.; İmrek, Y.; Özyurt, G.; Tufan, A.E.; Akay, A.P. Drug holidays may not affect processing speed while they may reduce beneficial effects on resistance to interference among children with treated with methylphenidate: A single-center, prospective study. Nord. J. Psychiatry 2021, 75, 323–329. [Google Scholar] [CrossRef]
- Molina, B.S.G.; Kennedy, T.M.; Howard, A.L.; Swanson, J.M.; Arnold, L.E.; Mitchell, J.T.; Stehli, A.; Kennedy, E.H.; Epstein, J.N.; Hechtman, L.T.; et al. Association Between Stimulant Treatment and Substance Use Through Adolescence into Early Adulthood. JAMA Psychiatry 2023, 80, 933. [Google Scholar] [CrossRef] [PubMed]
- Levin, F.R.; Choi, C.J.; Pavlicova, M.; Mariani, J.J.; Mahony, A.; Brooks, D.J.; Nunes, E.V.; Grabowski, J. How treatment improvement in ADHD and cocaine dependence are related to one another: A secondary analysis. Drug Alcohol Depend. 2018, 188, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Levin, F.R.; Mariani, J.J.; Specker, S.; Mooney, M.; Mahony, A.; Brooks, D.J.; Babb, D.; Bai, Y.; Eberly, L.E.; Nunes, E.V.; et al. Extended-Release Mixed Amphetamine Salts vs Placebo for Comorbid Adult Attention-Deficit/Hyperactivity Disorder and Cocaine Use Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2015, 72, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Tardelli, V.S.; Bisaga, A.; Arcadepani, F.B.; Gerra, G.; Levin, F.R.; Fidalgo, T.M. Prescription psychostimulants for the treatment of stimulant use disorder: A systematic review and meta-analysis. Psychopharmacology 2020, 237, 2233–2255. [Google Scholar] [CrossRef]
- Allain, F.; Delignat-Lavaud, B.; Beaudoin, M.P.; Jacquemet, V.; Robinson, T.E.; Trudeau, L.E.; Samaha, A.N. Amphetamine maintenance therapy during intermittent cocaine self-administration in rats attenuates psychomotor and dopamine sensitization and reduces addiction-like behavior. Neuropsychopharmacology 2020, 46, 305–315. [Google Scholar] [CrossRef]
- Levin, F.R.; Mariani, J.J.; Pavlicova, M.; Choi, C.J.; Basaraba, C.; Mahony, A.L.; Brooks, D.J.; Brezing, C.A.; Naqvi, N. Extended-Release Mixed Amphetamine Salts for Comorbid Adult Attention-Deficit/Hyperactivity Disorder and Cannabis Use Disorder: A Pilot, Randomized Double-Blind, Placebo-Controlled Trial. J. Atten. Disord. 2024, 28, 1467–1481. [Google Scholar] [CrossRef]
- Groenman, A.P.; Schweren, L.J.S.; Weeda, W.; Luman, M.; Noordermeer, S.D.S.; Heslenfeld, D.J.; Franke, B.; Faraone, S.V.; Rommelse, N.; Hartman, C.A.; et al. Stimulant treatment profiles predicting co-occurring substance use disorders in individuals with attention-deficit/hyperactivity disorder. Eur. Child Adolesc. Psychiatry 2019, 28, 1213–1222. [Google Scholar] [CrossRef]
- Ezard, N.; Clifford, B.; Siefried, K.J.; Ali, R.; Dunlop, A.; McKetin, R.; Bruno, R.; Carr, A.; Ward, J.; Farrell, M.; et al. Lisdexamfetamine in the treatment of methamphetamine dependence: A randomised, placebo-controlled trial. Addiction 2024, 120, 1345–1359. [Google Scholar] [CrossRef]
- Acheson, L.S.; Ezard, N.; Lintzeris, N.; Dunlop, A.; Brett, J.; Rodgers, C.; Gill, A.; Christmass, M.; McKetin, R.; Farrell, M.; et al. Trial protocol of an open label pilot study of lisdexamfetamine for the treatment of acute methamphetamine withdrawal. PLoS ONE 2022, 17, e0275371. [Google Scholar] [CrossRef] [PubMed]
- Wilens, T.E.; Faraone, S.V.; Biederman, J.; Gunawardene, S. Does Stimulant Therapy of Attention-Deficit/Hyperactivity Disorder Beget Later Substance Abuse? A Meta-Analytic Review of the Literature. Pediatrics 2003, 111, 179–185. [Google Scholar] [CrossRef]
- Quinn, P.D.; Chang, Z.; Hur, K.; Gibbons, R.D.; Lahey, B.B.; Rickert, M.E.; Sjölander, A.; Lichtenstein, P.; Larsson, H.; D’Onofrio, B.M. ADHD medication and substance-related problems. Am. J. Psychiatry 2017, 174, 877–885. [Google Scholar] [CrossRef]
- Vilus, J.T.; Engelhard, C.; Ann, F.; Lurie, R.H. Nonstimulant Medications for Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents. Pediatr. Ann. 2025, 54, e27–e33. [Google Scholar] [CrossRef]
- Ilipilla, G.; Arnold, L.E. The role of adrenergic neurotransmitter reuptake inhibitors in the ADHD armamentarium. Expert Opin. Pharmacother. 2024, 25, 945–956. [Google Scholar] [CrossRef]
- Newcorn, J.H.; Krone, B.; Dittmann, R.W. Nonstimulant Treatments for ADHD. Child Adolesc. Psychiatr. Clin. N. Am. 2022, 31, 417–435. [Google Scholar] [CrossRef]
- Zaso, M.J.; Park, A.; Antshel, K.M. Treatments for Adolescents with Comorbid ADHD and Substance Use Disorder: A Systematic Review. J. Atten. Disord. 2020, 24, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Wu, D.D.; Guo, H.L.; Hu, Y.H.; Xia, Y.; Ji, X.; Fang, W.R.; Li, Y.M.; Xu, J.; Chen, F.; et al. The Mechanism, Clinical Efficacy, Safety, and Dosage Regimen of Atomoxetine for ADHD Therapy in Children: A Narrative Review. Front. Psychiatry 2022, 12, 780921. [Google Scholar] [CrossRef] [PubMed]
- Thurstone, C.; Riggs, P.D.; Salomonsen-Sautel, S.; Mikulich-Gilbertson, S.K. Randomized, Controlled Trial of Atomoxetine for Attention-Deficit/Hyperactivity Disorder in Adolescents with Substance Use Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2010, 49, 573–582. [Google Scholar] [CrossRef]
- Mechler, K.; Banaschewski, T.; Hohmann, S.; Häge, A. Evidence-based pharmacological treatment options for ADHD in children and adolescents. Pharmacol. Ther. 2022, 230, 107940. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, S.; Lee, Y.J. Systematic Review of Suicidal Behaviors Related to Methylphenidate and Atomoxetine in Patients with Attention Deficit Hyperactivity Disorder. J. Korean Acad. Child Adolesc. Psychiatry 2023, 34, 125–132. [Google Scholar] [CrossRef]
- Chen, L.; Du, W. Multidimensional Comparison of Methylphenidate and Atomoxetine in the Treatment of Attention-Deficit/Hyperactivity Disorder in Children, a 12-Week, Open-Label, Head-to-Head Clinical Trial. Psychiatry Investig. 2025, 22, 140–147. [Google Scholar] [CrossRef]
- Khoodoruth, M.A.S.; Ouanes, S.; Khan, Y.S. A systematic review of the use of atomoxetine for management of comorbid anxiety disorders in children and adolescents with attention-deficit hyperactivity disorder. Res. Dev. Disabil. 2022, 128, 104275. [Google Scholar] [CrossRef]
- Huecker, M.R.; Smiley, A.; Saadabadi, A. Bupropion. In XPharm: The Comprehensive Pharmacology Reference; Elsevier: Amsterdam, The Netherlands, 2024; pp. 1–4. [Google Scholar]
- Elmarasi, M.; Fuehrlein, B. US Medicaid program: An analysis of the spending and utilization patterns for antidepressants from 2017 to 2021. Explor. Res. Clin. Soc. Pharm. 2024, 13, 100392. [Google Scholar] [CrossRef]
- Stahl, S.M.; Pradko, J.; Haight, B.R.; Modell, J.G.; Rockett, C.B.; Learned-Coughlin, S. A Review of the Neuropharmacology of Bupropion, a Dual Norepinephrine and Dopamine Reuptake Inhibitor. Prim. Care Companion J. Clin. Psychiatry 2004, 6, 159–166. [Google Scholar] [CrossRef]
- Verbeeck, W.; Bekkering, G.E.; den Noortgate, W.V.; Kramers, C. Bupropion for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst. Rev. 2017, 10. [Google Scholar] [CrossRef]
- Riggs, P.D.; Leon, S.L.; Mikulich, S.K.; Pottle, L.C. An open trial of bupropion for ADHD in adolescents with substance use disorders and conduct disorder. J. Am. Acad. Child Adolesc. Psychiatry 1998, 37, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Solhkhah, R.; Wilens, T.E.; Daly, J.; Prince, J.B.; Patten, S.L.V.; Biederman, J. Bupropion SR for the Treatment of Substance- Abusing Outpatient Adolescents with Attention- Deficit/Hyperactivity Disorder and Mood Disorders. J. Child Adolesc. Psychopharmacol. 2005, 15, 777–786. [Google Scholar] [CrossRef]
- Detyniecki, K. Do Psychotropic Drugs Cause Epileptic Seizures? A Review of the Available Evidence. Curr. Top. Behav. Neurosci. 2021, 55, 267–279. [Google Scholar] [CrossRef]
- Schmitz, A.; Botner, B.; Hund, M. Bupropion with Clozapine: Case Reports of Seizure After Coadministration. J. Pharm. Pract. 2021, 34, 497–502. [Google Scholar] [CrossRef]
- Adamou, M.; Asherson, P.; Arif, M.; Buckenham, L.; Cubbin, S.; Dancza, K.; Gorman, K.; Gudjonsson, G.; Gutman, S.; Kustow, J.; et al. Recommendations for occupational therapy interventions for adults with ADHD: A consensus statement from the UK adult ADHD network. BMC Psychiatry 2021, 21, 72. [Google Scholar] [CrossRef]
- Mattingly, G.W.; Wilson, J.; Ugarte, L.; Glaser, P. Individualization of attention-deficit/hyperactivity disorder treatment: Pharmacotherapy considerations by age and co-occurring conditions. CNS Spectr. 2021, 26, 202–221. [Google Scholar] [CrossRef]
- Sibley, M.H.; Bruton, A.M.; Zhao, X.; Johnstone, J.M.; Mitchell, J.; Hatsu, I.; Arnold, L.E.; Basu, H.H.; Levy, L.; Vyas, P.; et al. Non-pharmacological interventions for attention-deficit hyperactivity disorder in children and adolescents. Lancet Child Adolesc. Health 2023, 7, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Shareghfarid, E.; Sangsefidi, Z.S.; Salehi-Abargouei, A.; Hosseinzadeh, M. Empirically derived dietary patterns and food groups intake in relation with Attention Deficit/Hyperactivity Disorder (ADHD): A systematic review and meta-analysis. Clin. Nutr. ESPEN 2020, 36, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.; Kim, D.; Hong, Y.-S.; Kim, Y.-M.; Seo, J.-H.; Choe, B.; Park, J.; Kang, J.-W.; Yoo, J.-H.; Chueh, H.; et al. Dietary Patterns in Children with Attention Deficit/Hyperactivity Disorder (ADHD). Nutrients 2014, 6, 1539–1553. [Google Scholar] [CrossRef]
- Azadbakht, L.; Esmaillzadeh, A. Dietary patterns and attention deficit hyperactivity disorder among Iranian children. Nutrition 2012, 28, 242–249. [Google Scholar] [CrossRef]
- Kotsi, E.; Kotsi, E.; Perrea, D.N. Vitamin D levels in children and adolescents with attention-deficit hyperactivity disorder (ADHD): A meta-analysis. ADHD Atten. Deficit Hyperact. Disord. 2019, 11, 221–232. [Google Scholar] [CrossRef] [PubMed]
- der Heijden, K.B.V.; Smits, M.G.; Someren, E.J.W.V.; Ridderinkhof, K.R.; Gunning, W.B. Effect of Melatonin on Sleep, Behavior, and Cognition in ADHD and Chronic Sleep-Onset Insomnia. J. Am. Acad. Child Adolesc. Psychiatry 2007, 46, 233–241. [Google Scholar] [CrossRef]
- Hirayama, S.; Terasawa, K.; Rabeler, R.; Hirayama, T.; Inoue, T.; Tatsumi, Y.; Purpura, M.; Jäger, R. The effect of phosphatidylserine administration on memory and symptoms of attention-deficit hyperactivity disorder: A randomised, double-blind, placebo-controlled clinical trial. J. Hum. Nutr. Diet. 2014, 27, 284–291. [Google Scholar] [CrossRef]
- Hirayama, S.; Hamazaki, T.; Terasawa, K. Effect of docosahexaenoic acid-containing food administration on symptoms of attention-deficit/hyperactivity disorder—A placebo-controlled double-blind study. Eur. J. Clin. Nutr. 2004, 58, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Micoulaud-Franchi, J.A.; Jeunet, C.; Pelissolo, A.; Ros, T. EEG Neurofeedback for Anxiety Disorders and Post-Traumatic Stress Disorders: A Blueprint for a Promising Brain-Based Therapy. Curr. Psychiatry Rep. 2021, 23, 84. [Google Scholar] [CrossRef]
- Schönenberg, M.; Wiedemann, E.; Schneidt, A.; Scheeff, J.; Logemann, A.; Keune, P.M.; Hautzinger, M. Neurofeedback, sham neurofeedback, and cognitive-behavioural group therapy in adults with attention-deficit hyperactivity disorder: A triple-blind, randomised, controlled trial. Lancet Psychiatry 2017, 4, 673–684. [Google Scholar] [CrossRef] [PubMed]
- McGough, J.J.; Loo, S.K.; Sturm, A.; Cowen, J.; Leuchter, A.F.; Cook, I.A. An Eight-Week, Open-Trial, Pilot Feasibility Study of Trigeminal Nerve Stimulation in Youth with Attention-Deficit/Hyperactivity Disorder. Brain Stimulat. 2015, 8, 299–304. [Google Scholar] [CrossRef]
- McGough, J.J.; Sturm, A.; Cowen, J.; Tung, K.; Salgari, G.C.; Leuchter, A.F.; Cook, I.A.; Sugar, C.A.; Loo, S.K. Double-Blind, Sham-Controlled, Pilot Study of Trigeminal Nerve Stimulation for Attention-Deficit/Hyperactivity Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2019, 58, 403–411.e3. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, Y.; Qiu, H.; Zhang, Z.; Tan, X.; Huang, P.; Zhang, M.; Miao, D.; Zhu, X. A new perspective for evaluating the efficacy of tACS and tDCS in improving executive functions: A combined tES and fNIRS study. Hum. Brain Mapp. 2024, 45, e26559. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. FDA Permits Marketing of First Medical Device for Treatment of ADHD; FDA: Silver Spring, MD, USA, 2019.
- Beh, S.C. External trigeminal nerve stimulation: Potential rescue treatment for acute vestibular migraine. J. Neurol. Sci. 2020, 408, 116550. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Liang, S.-C.; Sun, C.-K.; Cheng, Y.-S.; Tzang, R.-F.; Chiu, H.-J.; Wang, M.-Y.; Cheng, Y.-C.; Hung, K.-C. A meta-analysis on the therapeutic efficacy of repetitive transcranial magnetic stimulation for cognitive functions in attention-deficit/hyperactivity disorders. BMC Psychiatry 2023, 23, 756. [Google Scholar] [CrossRef]
- Martin-Moratinos, M.; Bella-Fernández, M.; Blasco-Fontecilla, H. Effects of Music on Attention-Deficit/Hyperactivity Disorder (ADHD) and Potential Application in Serious Video Games: Systematic Review. J. Med. Internet Res. 2023, 25, e37742. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Jia, S.; Wang, X.; Wang, A.; Ma, T.; Li, S.; Chen, J.; Guo, Z.; Ding, F.; Ren, Y.; et al. Effects of physical exercise on anxiety depression and emotion regulation in children with attention deficit hyperactivity disorder: A systematic review and meta-analysis. Front. Pediatr. 2025, 12, 1479615. [Google Scholar] [CrossRef]
- Conners, C.K.; Epstein, J.N.; March, J.S.; Angold, A.; Wells, K.C.; Klaric, J.; Swanson, J.M.; Arnold, L.E.; Abikoff, H.B.; Elliott, G.R.; et al. Multimodal Treatment of ADHD in the MTA: An Alternative Outcome Analysis. J. Am. Acad. Child Adolesc. Psychiatry 2001, 40, 159–167. [Google Scholar] [CrossRef]
- Breitling, C.; Zaehle, T.; Dannhauer, M.; Bonath, B.; Tegelbeckers, J.; Flechtner, H.-H.; Krauel, K. Improving Interference Control in ADHD Patients with Transcranial Direct Current Stimulation (tDCS). Front. Cell. Neurosci. 2016, 10, 72. [Google Scholar] [CrossRef]
- Arnold, L.E.; Arns, M.; Barterian, J.; Bergman, R.; Black, S.; Conners, C.K.; Connor, S.; Dasgupta, S.; deBeus, R.; Higgins, T.; et al. Double-Blind Placebo-Controlled Randomized Clinical Trial of Neurofeedback for Attention-Deficit/Hyperactivity Disorder with 13-Month Follow-Up. J. Am. Acad. Child Adolesc. Psychiatry 2021, 60, 841–855. [Google Scholar] [CrossRef]
- Antshel, K.M.; Barkley, R. Psychosocial Interventions in Attention Deficit Hyperactivity Disorder. Child Adolesc. Psychiatr. Clin. N. Am. 2008, 17, 421–437. [Google Scholar] [CrossRef] [PubMed]
- Safren, S.A.; Otto, M.W.; Sprich, S.; Winett, C.L.; Wilens, T.E.; Biederman, J. Cognitive-behavioral therapy for ADHD in medication-treated adults with continued symptoms. Behav. Res. Ther. 2005, 43, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Kasuya-Ueba, Y.; Zhao, S.; Toichi, M. The Effect of Music Intervention on Attention in Children: Experimental Evidence. Front. Neurosci. 2020, 14, 757. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Huang, X.; Lin, S. Yoga and music intervention reduces inattention, hyperactivity/impulsivity, and oppositional defiant disorder in children’s consumer with comorbid ADHD and ODD. Front. Psychol. 2023, 14, 1150018. [Google Scholar] [CrossRef]




| Criteria | ICD-11 | DSM-V |
|---|---|---|
| Persistent time of symptoms | 6 months or longer | 6 months or longer (in two different settings) |
| Inattention | Symptoms are persistent and have a direct negative impact on functioning, including:
| Five or more symptoms including:
|
| Hyperactivity | Symptoms are persistent and direct negative impact on functioning, including:
| Five or more symptoms, including:
|
| Form | The Manner of Application | Benefits of This Form of Therapy | Weakness of This Form of Therapy | Risk of Addiction |
|---|---|---|---|---|
| Instant-release | Absorption in digestive system (oral administration) | Instant way of action | Brief time of action, Must be ingested multiple times per day, Daily fluctuation of active compound (resulting in irritability, mood swings, and cognitive impairment), Increases the risk of abuse | High (under revision) |
| The osmotic-controlled release oral delivery system (OROS) | Oral administration Osmotic releasement of active compound in digestive system | Reduction in daily administrated medicine to one per day | The risk of abuse (crushable, can be dissolved and delivered intravenously or intranasally) | Moderate |
| Transdermal patches | Skin absorption (applied on the hip) | Flexible modulation of the duration of the pharmaceutical agent, Can be used by patients with gastrointestinal problems and dysphagia, Averts possible irritation of digestive system, Hepatoprotective properties, Possible abuse reduction | Skin irritation | Low |
| Serdexmethylphenidate | Oral administration, biotransformation to methylphenidate | No data | Minimal utility in the treatment of ADHD | Low |
| Administrated Drug | Participants | Form and Dose of Administrated Drug | Duration of Admission | Results | Limitations of Study | References |
|---|---|---|---|---|---|---|
| Methylphenidate | 14 adults | Transdermal patches (1.1 mg/h—1st week; 1.6 mg/h—2nd week; 2.2 mg/h—rest of weeks) | 8 weeks | Stimulant substances positive in 0/107 urine samples (1 self-report); Presence of non-stimulants (marijuana, opioids, and benzodiazepines) in 5/107 urine samples | Limited number of participants, may be not representative | [108] |
| Amphetamine salts | 126 participants | 60 mg or 80 mg extended-release AMPs | 14 weeks of daily doses | 60 subjects achieved cocaine abstinence | Not large sample of subjects | [130] |
| 2889 patients | Modafinil, methylphenidate, or AMPSs—varying between 38 trials | Systematic review, meta-analysis—varying between trials | Cocaine-negative urine samples: 281 (AMP treatment) vs. 216 (placebo usage) | Subjects without ADHD; included modafinil in paper, in results only included AMP | [132] | |
| 28 patients | 80 mg | 12 week | AMP-administrated group—a significant decrease in cannabis use days over time compared to placebo | Small sample size | [134] | |
| Lisdexamphetamine | 36 adults | Orally: 50, 100 and 150 mg LDX; 40 mg dAMP and 200 mg diethylpropion; and placebo | 6 study treatments (with different drugs) with 48 h interval | DRQS-liking score: 100 mg dose of LDX << 40 mg dose of d-AMP 150 mg of LDX ≈ AMP | Results subjective to patients’ feelings; No long-term research; no inclusion of pharmacokinetic measures | [119] |
| 12 males | Intravenous: 25 mg LDX, 10 mg d-AMP or placebo | 3 study treatments with 48 h interval | On 5 five ARCI subscales LDX comparable to placebo | Enrolment of only males; Results subjective to patients’ feelings; No long-term research | [120] | |
| 24 subjects | Orally: 100 mg LDX, 40 mg AMP or placebo | One dose | Plasma concertation of active ingredient similar between LDX and AMP | Use of only high does; No long-term research | [89] | |
| 155 participants | Orally 250 mg LDX orally | 12 weeks, plus 1-week induction and 2-week taper | OR of previous days of use of previous 28 days at week 13 between ones treated with LDX and placebo is 0.68 | 43% of subjects did not remain on medication; mostly based on self-report; limited consumer engagement | [136] | |
| Not-stated stimulants | 579 children born between 1994 and 1996 | Not stated—cohort study | Data required at age of subject: 3 and 9 months, 2, 3, 6, 8, 10, 12, 14, and 16 years | Once subjects reached early adulthood number of patients misusing stimulants stopped increasing both ADHD patients and non-ADHD subjects | Administration of stimulants were only reported by parents (or self-reported once subject reached 18 years old) and are not backed by medical data | [129] |
| Stimulants | 303 patients | Mostly immediate-release MPH, but also extended-release MPH and d-AMP | Follow-up on average 4.2 years after enrolment | Patients treated intensively at early age are more 0.28 more likely to develop SUD compared to naïve treatment | Risk of endogeneity; no distinction of different SUD | [135] |
| Atomoxitine | 70 adolescents | Subjects’ weight < 70 kg: start at 0.5–0.75 mg/kg per day and increased by 25 mg per week until total dose 1.1–1.5 mg/kg. Participants’ weight > 70 kg: start at 50 mg per day and increased to 75 mg per day in the second week and 100 mg in the third week | 12 weeks | No difference between atomoxetine and placebo group in terms of days of non-nicotine substance use | Not tested in adult population; only include non-nicotine substance use | [145] |
| Bupropion | 14 adolescent boys | Start at 100 mg bupropion twice a day; Second week: 100 mg bupropion three times a day (if previous dose tolerated) | 5 weeks | Two subjects with positive urine drug screen test | Not tested in adult population; only male as participants small sample size, lack of; controls or blinded assessments; assessments performed only before and after treatment | [154] |
| 13 adolescent outpatients | Start at 100 mg bupropion once-daily and then increased in time to 400 mg bupropion | 6 months | 13 adolescents: 0 or 1 prescribed drug misuse self-report | Not tested in adult population; possible observatory bias; small sample size; limited medical information | [155] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żełabowski, K.; Petrov, W.; Ślebioda, D.; Rusinek, M.; Biedka, K.; Błaszczyk, K.; Wesołowski, M.; Wojtysiak, K.; Sroka, M.; Ratka, Z.; et al. Holistic Management of Adult ADHD with a History of Addiction: Emphasis on Low-Addiction-Risk Psychopharmacotherapy. J. Clin. Med. 2025, 14, 6470. https://doi.org/10.3390/jcm14186470
Żełabowski K, Petrov W, Ślebioda D, Rusinek M, Biedka K, Błaszczyk K, Wesołowski M, Wojtysiak K, Sroka M, Ratka Z, et al. Holistic Management of Adult ADHD with a History of Addiction: Emphasis on Low-Addiction-Risk Psychopharmacotherapy. Journal of Clinical Medicine. 2025; 14(18):6470. https://doi.org/10.3390/jcm14186470
Chicago/Turabian StyleŻełabowski, Kacper, Wiktor Petrov, Dawid Ślebioda, Malwina Rusinek, Kamil Biedka, Katarzyna Błaszczyk, Michał Wesołowski, Kacper Wojtysiak, Mateusz Sroka, Zuzanna Ratka, and et al. 2025. "Holistic Management of Adult ADHD with a History of Addiction: Emphasis on Low-Addiction-Risk Psychopharmacotherapy" Journal of Clinical Medicine 14, no. 18: 6470. https://doi.org/10.3390/jcm14186470
APA StyleŻełabowski, K., Petrov, W., Ślebioda, D., Rusinek, M., Biedka, K., Błaszczyk, K., Wesołowski, M., Wojtysiak, K., Sroka, M., Ratka, Z., Ilski, I., & Chłopaś-Konowałek, A. (2025). Holistic Management of Adult ADHD with a History of Addiction: Emphasis on Low-Addiction-Risk Psychopharmacotherapy. Journal of Clinical Medicine, 14(18), 6470. https://doi.org/10.3390/jcm14186470







