The Prediction of Early Neurological Outcomes in Out-of-Hospital Cardiac Arrest Patients: A Multicenter Prospective Cohort Study by the KORHN Registry
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Population
2.3. Variables and Endpoints
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics and Poor Neurological Outcomes
3.2. Key Variables and the KORHN Score
3.3. Comparison of the KORHN Score and Other Risk Scores
4. Discussion
4.1. The Overall Results
4.2. Promising Variables of the KORHN Score
4.3. Comparison Between the KORHN Score and Other Risk Scores
4.4. Clinical Implementation
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Pareek, N.; Kordis, P.; Beckley-Hoelscher, N.; Pimenta, D.; Kocjancic, S.T.; Jazbec, A.; Nevett, J.; Fothergill, R.; Kalra, S.; Lockie, T. A practical risk score for early prediction of neurological outcome after out-of-hospital cardiac arrest: MIRACLE2. Eur. Heart J. 2020, 41, 4508–4517. [Google Scholar] [CrossRef] [PubMed]
- Martinell, L.; Nielsen, N.; Herlitz, J.; Karlsson, T.; Horn, J.; Wise, M.P.; Undén, J.; Rylander, C. Early predictors of poor outcome after out-of-hospital cardiac arrest. Crit. Care 2017, 21, 96. [Google Scholar] [CrossRef] [PubMed]
- Maupain, C.; Bougouin, W.; Lamhaut, L.; Deye, N.; Diehl, J.L.; Geri, G.; Perier, M.C.; Beganton, F.; Marijon, E.; Jouven, X.; et al. The CAHP (Cardiac Arrest Hospital Prognosis) score: A tool for risk stratification after out-of-hospital cardiac arrest. Eur. Heart J. 2016, 37, 3222–3228. [Google Scholar] [CrossRef]
- Adrie, C.; Cariou, A.; Mourvillier, B.; Laurent, I.; Dabbane, H.; Hantala, F.; Rhaoui, A.; Thuong, M.; Monchi, M. Predicting survival with good neurological recovery at hospital admission after successful resuscitation of out-of-hospital cardiac arrest: The OHCA score. Eur. Heart J. 2006, 27, 2840–2845. [Google Scholar] [CrossRef]
- Kiehl, E.L.; Parker, A.M.; Matar, R.M.; Gottbrecht, M.F.; Johansen, M.C.; Adams, M.P.; Griffiths, L.A.; Dunn, S.P.; Bidwell, K.L.; Menon, V.; et al. C-GRApH: A Validated Scoring System for Early Stratification of Neurologic Outcome After Out-of-Hospital Cardiac Arrest Treated with Targeted Temperature Management. J. Am. Heart Assoc. 2017, 6, e003821. [Google Scholar] [CrossRef]
- Kang, C.; Min, J.H.; Park, J.S.; You, Y.; Jeong, W.; Ahn, H.J.; In, Y.N.; Lee, I.H.; Jeong, H.S.; Lee, B.K.; et al. Association of ultra-early diffusion-weighted magnetic resonance imaging with neurological outcomes after out-of-hospital cardiac arrest. Crit. Care 2023, 27, 16. [Google Scholar] [CrossRef]
- Lee, J.H. Early Neuroprognostication Using Frontal Spectrograms in Moderately Sedated Cardiac Arrest Patients. Clin. EEG Neurosci. 2023, 54, 281–288. [Google Scholar] [CrossRef]
- Dankiewicz, J.; Cronberg, T.; Lilja, G.; Jakobsen, J.C.; Levin, H.; Ullén, S.; Rylander, C.; Wise, M.P.; Oddo, M.; Cariou, A.; et al. Hypothermia versus Normothermia after Out-of-Hospital Cardiac Arrest. N. Engl. J. Med. 2021, 384, 2283–2294. [Google Scholar] [CrossRef]
- Callaway, C.W.; Coppler, P.J.; Faro, J.; Puyana, J.S.; Solanki, P.; Dezfulian, C.; Doshi, A.A.; Elmer, J.; Frisch, A.; Guyette, F.X.; et al. Association of Initial Illness Severity and Outcomes After Cardiac Arrest with Targeted Temperature Management at 36 degrees C or 33 degrees C. J. Am. Med. Assoc. Netw. Open. 2020, 3, e208215. [Google Scholar]
- Nutma, S.; Tjepkema-Cloostermans, M.C.; Ruijter, B.J.; Tromp, S.C.; van den Bergh, W.M.; Foudraine, N.A.; Kornips, F.H.M.; Drost, G.; Scholten, E.; Strang, A.; et al. Effects of targeted temperature management at 33 °C vs. 36 °C on comatose patients after cardiac arrest stratified by the severity of encephalopathy. Resuscitation 2022, 173, 147–153. [Google Scholar] [CrossRef]
- Okazaki, T.; Hifumi, T.; Kawakita, K.; Kuroda, Y.; Japanese Association for Acute Medicine out-of-hospital cardiac arrest registry. Targeted temperature management guided by the severity of hyperlactatemia for out-of-hospital cardiac arrest patients: A post hoc analysis of a nationwide, multicenter prospective registry. Ann. Intensive Care 2019, 9, 127. [Google Scholar] [CrossRef]
- Nishikimi, M.; Ogura, T.; Nishida, K.; Hayashida, K.; Emoto, R.; Matsui, S.; Matsuda, N.; Iwami, T. Outcome Related to Level of Targeted Temperature Management in Postcardiac Arrest Syndrome of Low, Moderate, and High Severities: A Nationwide Multicenter Prospective Registry. Crit. Care Med. 2021, 49, e741–e750. [Google Scholar] [CrossRef] [PubMed]
- Belur, A.D.; Sedhai, Y.R.; Truesdell, A.G.; Khanna, A.K.; Mishkin, J.D.; Belford, P.M.; Zhao, D.X.; Vallabhajosyula, S. Targeted Temperature Management in Cardiac Arrest: An Updated Narrative Review. Cardiol. Ther. 2023, 12, 65–84. [Google Scholar] [CrossRef] [PubMed]
- Geurts, M.; Macleod, M.R.; Kollmar, R.; Kremer, P.H.; van der Worp, H.B. Therapeutic hypothermia and the risk of infection: A systematic review and meta-analysis. Crit. Care Med. 2014, 42, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Ospina-Tascón, G.A.; Teboul, J.L.; Hernandez, G.; Alvarez, I.; Sánchez-Ortiz, A.I.; Calderón-Tapia, L.E.; Manzano-Nunez, R.; Quiñones, E.; Madriñan-Navia, H.J.; Ruiz, J.E.; et al. Diastolic shock index and clinical outcomes in patients with septic shock. Ann. Intensive Care 2020, 10, 41. [Google Scholar] [CrossRef]
- Oksanen, T.; Tiainen, M.; Vaahersalo, J.; Bendel, S.; Varpula, T.; Skrifvars, M.; Pettilä, V.; Wilkman, E.; FINNRESUSCI Study Group. Lower heart rate is associated with good one-year outcome in post-resuscitation patients. Resuscitation 2018, 128, 112–118. [Google Scholar] [CrossRef]
- Stær-Jensen, H.; Sunde, K.; Olasveengen, T.M.; Jacobsen, D.; Drægni, T.; Nakstad, E.R.; Eritsland, J.; Andersen, G.Ø. Bradycardia during therapeutic hypothermia is associated with good neurologic outcome in comatose survivors of out-of-hospital cardiac arrest. Crit. Care Med. 2014, 42, 2401–2408. [Google Scholar] [CrossRef]
- Annoni, F.; Dell’Anna, A.M.; Franchi, F.; Creteur, J.; Scolletta, S.; Vincent, J.L.; Taccone, F.S. The impact of diastolic blood pressure values on the neurological outcome of cardiac arrest patients. Resuscitation 2018, 130, 167–173. [Google Scholar] [CrossRef]
- Thorgeirsson, G.; Thorgeirsson, G.; Sigvaldason, H.; Witteman, J. Risk factors for out-of-hospital cardiac arrest: The Reykjavik Study. Eur. Heart J. 2005, 26, 1499–1505. [Google Scholar] [CrossRef]
- Han, K.S.; Lee, S.W.; Lee, E.J.; Kwak, M.H.; Kim, S.J. Association between shockable rhythm conversion and outcomes in patients with out-of-hospital cardiac arrest and initial non-shockable rhythm, according to the cause of cardiac arrest. Resuscitation 2019, 142, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Youn, C.S.; Kim, S.H.; Lee, B.K.; Cho, I.S.; Cho, G.C.; Jeung, K.W.; Oh, S.H.; Choi, S.P.; Shin, J.H.; et al. Adverse events associated with poor neurological outcome during targeted temperature management and advanced critical care after out-of-hospital cardiac arrest. Crit. Care 2015, 19, 283. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Callaway, C.W.; Guyette, F.X.; Rittenberger, J.C.; Doshi, A.A.; Dezfulian, C.; Elmer, J.; Pittsburgh Post-Cardiac Arrest Service. Arrest etiology among patients resuscitated from cardiac arrest. Resuscitation 2018, 130, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Kürkciyan, I.; Meron, G.; Behringer, W.; Sterz, F.; Berzlanovich, A.; Domanovits, H.; Müllner, M.; Bankl, H.C.; Laggner, A.N. Accuracy and impact of presumed cause in patients with cardiac arrest. Circulation 1998, 98, 766–771. [Google Scholar] [CrossRef]
- Song, H.G.; Park, J.S.; You, Y.; Ahn, H.J.; Yoo, I.; Kim, S.W.; Lee, J.; Ryu, S.; Jeong, W.; Cho, Y.C.; et al. Using Out-of-Hospital Cardiac Arrest (OHCA) and Cardiac Arrest Hospital Prognosis (CAHP) Scores with Modified Objective Data to Improve Neurological Prognostic Performance for Out-of-Hospital Cardiac Arrest Survivors. J. Clin. Med. 2021, 10, 1825. [Google Scholar] [CrossRef]
- Kim, H.S.; Park, K.N.; Kim, S.H.; Lee, B.K.; Oh, S.H.; Jeung, K.W.; Choi, S.P.; Youn, C.S. Prognostic value of OHCA, C-GRApH and CAHP scores with initial neurologic examinations to predict neurologic outcomes in cardiac arrest patients treated with targeted temperature management. PLoS ONE 2020, 15, e0232227. [Google Scholar] [CrossRef]
- Kiss, B.; Nagy, R.; Kói, T.; Harnos, A.; Édes, I.F.; Ábrahám, P.; Mészáros, H.; Hegyi, P.; Zima, E. Prediction performance of scoring systems after out-of-hospital cardiac arrest: A systematic review and meta-analysis. PLoS ONE 2024, 19, e0293704. [Google Scholar] [CrossRef]
- Wang, C.H.; Huang, C.H.; Chang, W.T.; Tsai, M.S.; Yu, P.H.; Wu, Y.W.; Chen, W.J. Prognostic performance of simplified out-of-hospital cardiac arrest (OHCA) and cardiac arrest hospital prognosis (CAHP) scores in an East Asian population: A prospective cohort study. Resuscitation 2019, 137, 133–139. [Google Scholar] [CrossRef]
- Pham, V.; Laghlam, D.; Varenne, O.; Dumas, F.; Cariou, A.; Picard, F. Performance of OHCA, NULL-PLEASE and CAHP scores to predict survival in Out-of-Hospital Cardiac Arrest due to acute coronary syndrome. Resuscitation 2021, 166, 31–37. [Google Scholar] [CrossRef]
- Schmidbauer, S.; Rylander, C.; Cariou, A.; Wise, M.P.; Thomas, M.; Keeble, T.R.; Erlinge, D.; Haenggi, M.; Wendel-Garcia, P.D.; Bělohlávek, J.; et al. Comparison of four clinical risk scores in comatose patients after out-of-hospital cardiac arrest. Resuscitation 2023, 191, 109949. [Google Scholar] [CrossRef]

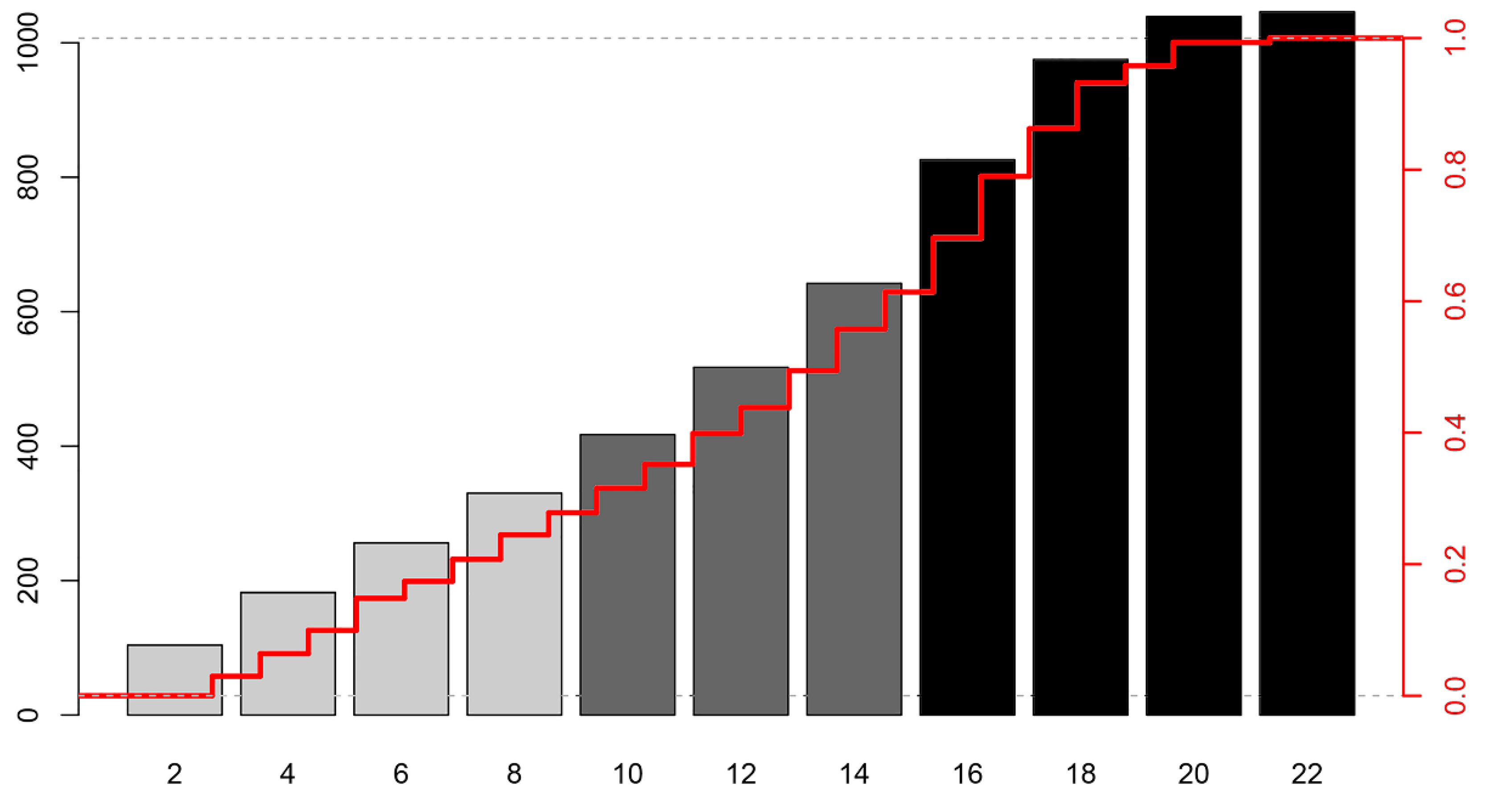

| Variable | All Patients (n = 1371) | Good Outcome (n = 428) | Poor Outcome (n = 943) | p Value 1 |
|---|---|---|---|---|
| Age, years | 62 (51–74) | 58 (48–66) | 65 (53–77) | <0.001 |
| Male, n (%) | 975 (71.1) | 333 (77.8) | 642 (68.1) | <0.001 |
| BMI, kg/m2 | 23.3 (20.9–25.7) | 23.3 (21.3–25.6) | 23.4 (20.8–25.7) | 0.823 |
| Witness arrest, n (%) | 949 (70) | 361 (84.5) | 588 (63.3) | <0.001 |
| Bystander CPR, n (%) | 843 (62.4) | 292 (69.2) | 551 (59.3) | 0.001 |
| Time from arrest to CPR start, minutes | 1 (0–7) | 1 (0–5) | 1 (0–8) | 0.005 |
| Time from CPR start to ROSC, minutes | 15 (9–22.75) | 15 (9–22.8) | 31 (20–42) | <0.001 |
| Time from ROSC to TTM start, hours | 3.4 (2.2–4.9) | 3.6 (2.5–5) | 3.3 (2–4.8) | 0.002 |
| Pre-hospital ECG rhythm | <0.001 | |||
| Asystole, n (%) | 445 (37) | 23 (6.1) | 422 (51) | |
| PEA, n (%) | 269 (22.3) | 54 (14.3) | 215 (26) | |
| Pulseless VT, n (%) | 15 (1.2) | 11 (2.9) | 4 (0.5) | |
| VF, n (%) | 448 (37.2) | 272 (72.1) | 176 (21.3) | |
| ROSC, n (%) | 27 (2.2) | 17 (4.5) | 10 (1.2) | |
| Baseline ECG findings | <0.001 | |||
| ST segment elevation, n (%) | 169 (12.5%) | 75 (17.7%) | 94 (10.2%) | |
| ST segment depression, n (%) | 250 (18.5%) | 75 (17.7%) | 175 (18.9%) | |
| Bundle branch block, n (%) | 263 (19.5%) | 59 (13.9%) | 204 (22.1%) | |
| Unspecific finding, n (%) | 666 (49.4%) | 215 (50.7%) | 451 (48.8%) | |
| Past medical history | ||||
| Cardiovascular disease 2, n (%) | 285 (20.8) | 99 (34.7) | 186 (19.7) | 0.152 |
| Neurologic disease 3, n (%) | 138 (10.1) | 27 (6.3) | 111 (11.8) | 0.002 |
| Respiratory disease, n (%) | 106 (7.7) | 13 (3) | 93 (9.9) | <0.001 |
| Cancer, n (%) | 80 (5.8) | 23 (5.4) | 57 (6) | 0.465 |
| Psychiatric disorder, n (%) | 51 (3.7) | 5 (1.2) | 46 (4.9) | <0.001 |
| Cardiac-origin cardiac arrest, n (%) | 850 (62) | 372 (86.9) | 478 (50.7) | <0.001 |
| Cardiac arrest etiology, n (%) | <0.001 | |||
| Medical causes, n (%) | 851 (62.1) | 479 (50.8) | 372 (86.9) | |
| Traumatic causes, n (%) | 28 (2) | 2 (0.5) | 26 (2.8) | |
| Submersion injury, n (%) | 19 (1.4) | 4 (0.9) | 2 (0.2) | |
| Electrical injury, n (%) | 6 (0.4) | 3 (0.5) | 3 (0.4) | |
| Toxicological cause, n (%) | 16 (1.2) | 5 (1.2) | 11 (1.2) | |
| Hypoxic injury, n (%) | 78 (5.7) | 6 (1.4) | 72 (7.6) | |
| Strangulation, n (%) | 160 (11.7) | 12 (2.8) | 148 (15.7) | |
| Miscellaneous causes, n (%) | 213 (15.5) | 23 (5.4) | 190 (20.1) | |
| Pre-cardiac arrest CPC score | 1 (1–1) | 1 (1–1) | 1 (1–1) | <0.001 |
| Pre-cardiac arrest MRS | 0 (0–1) | 0 (0–0) | 0 (0–1) | <0.001 |
| Pupillary light reflex, n (%) | 643 (47.3) | 346 (81) | 297 (31.8) | <0.001 |
| Motor response on GCS, score | 1 (1–1) | 1 (1–3) | 1 (1–1) | <0.001 |
| Four-scale score 4 | 0 (0–3) | 4 (0–7) | 0 (0–1) | <0.001 |
| Cardiovascular SOFA 5 | 4 (2–4) | 3 (0–4) | 4 (3–4) | <0.001 |
| Diastolic shock index | 1.41 (1.1–1.9) | 1.25 (1–1.6) | 1.5 (1.1–2.1) | <0.001 |
| Epinephrine administration dose, mg | 2 (0–4) | 0 (0–1) | 2 (1–4) | <0.001 |
| pH | 7.08 (6.92–7.23) | 7.22 (7.09–7.3) | 7.02 (6.89–7.17) | <0.001 |
| PaCO2, mmHg | 108.9 (72.7–196.8) | 108.8 (73.7–192.7) | 109.4 (72–199.8) | <0.001 |
| Lactate, mg/dL | 9.7 (6.1–12.9) | 7.1 (4.3–10.9) | 10.6 (7.5–13.6) | <0.001 |
| Creatinine, mg/dL | 1.3 (1.1–1.8) | 1.2 (1–1.4) | 1.4 (1.1–2.2) | <0.001 |
| Glucose, mg/dL | 255 (190–330) | 238 (180–297) | 266 (197–347) | <0.001 |
| Variable | Β-Coefficient | Adjusted OR | p Value | 95% CI |
|---|---|---|---|---|
| Low-flow time > 21 min | 1.455 | 4.287 | <0.001 | 2.62–7.013 |
| HR/DAP > 1.7 | 1.259 | 3.523 | <0.001 | 2.037–6.095 |
| Non-cardiac-origin arrest | 1.235 | 3.438 | <0.001 | 2.097–5.49 |
| Absent pupillary light reflex | 1.222 | 3.393 | <0.001 | 1.82–6.496 |
| Non-shockable rhythm | 1.127 | 3.087 | <0.001 | 1.842–5.175 |
| Motor response on GCS ≤ 1 | 0.961 | 2.614 | <0.001 | 1.579–4.328 |
| Epinephrine administration | 0.927 | 2.526 | <0.001 | 1.539–4.144 |
| Age > 66 years old | 0.883 | 2.419 | <0.001 | 1.518–3.854 |
| Creatinine > 1.4 mg/dL | 0.814 | 2.257 | 0.001 | 1.38–3.692 |
| Unwitnessed arrest | 0.802 | 2.231 | 0.007 | 1.24–4.014 |
| Lactate > 8.2 mg/dL | 0.671 | 1.957 | 0.004 | 1.237–3.095 |
| ST elevation | 0.652 | 1.919 | 0.037 | 1.039–3.545 |
| PaCO2 > 48 mmHg | 0.628 | 1.875 | 0.008 | 1.176–2.989 |
| N | Sensitivity | Specificity | PPV | NPV | AUC | |
|---|---|---|---|---|---|---|
| Performance in previous data 1 | ||||||
| MIRACLE > 2 | 373 | 97.9 | 56.7 | 77.9 | 94.4 | 0.9 |
| TTM > 10 | 933 | 92.6 | 61.4 | 78.7 | 84.3 | 0.842 |
| CAHP > 150 | 819 | 87.1 | 67.1 | 81 | 76.4 | 0.93 |
| OHCA > 2 | 210 | 96.9 | 34.7 | 71.1 | 87.2 | 0.82 |
| Performance in the present data | ||||||
| KORHN score > 9 | 1045 | 86.01 | 85.44 | 93.4 | 71.9 | 0.925 |
| MIRACLE score > 3 | 1082 | 88.82 | 77.95 | 90.5 | 74.7 | 0.902 |
| TTM score > 12 | 1054 | 93.6 | 51.32 | 82.6 | 76.5 | 0.863 |
| CAHP score > 0.57 | 1072 | 88.22 | 76.3 | 90.2 | 72.3 | 0.894 |
| OHCA score > 12.65 | 1141 | 77.09 | 80.34 | 89.8 | 60.9 | 0.853 |
| C-GRApH score > 0.98 | 1148 | 76.92 | 76.34 | 87.9 | 59.7 | 0.827 |
| Performance based on the top 5 variables using a fixed threshold | ||||||
| KORHN5 | 1139 | 80.56 | 90.2 | 94.7 | 67.9 | 0.914 |
| TTM5 | 1054 | 84.53 | 84.21 | 93 | 68.8 | 0.903 |
| MIRACLE5 | 1082 | 86.32 | 79.19 | 90.7 | 71 | 0.89 |
| OHCA5 | 1141 | 82.41 | 76.64 | 88.8 | 68.9 | 0.889 |
| CAHP5 | 1072 | 86.65 | 75.65 | 89.8 | 69.6 | 0.881 |
| C-GRApH5 | 1148 | 83.48 | 74.65 | 88 | 66.9 | 0.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, W.J.; Lee, J.H., on behalf of the Korean Hypothermia Network Investigators. The Prediction of Early Neurological Outcomes in Out-of-Hospital Cardiac Arrest Patients: A Multicenter Prospective Cohort Study by the KORHN Registry. J. Clin. Med. 2025, 14, 6466. https://doi.org/10.3390/jcm14186466
Choi WJ, Lee JH on behalf of the Korean Hypothermia Network Investigators. The Prediction of Early Neurological Outcomes in Out-of-Hospital Cardiac Arrest Patients: A Multicenter Prospective Cohort Study by the KORHN Registry. Journal of Clinical Medicine. 2025; 14(18):6466. https://doi.org/10.3390/jcm14186466
Chicago/Turabian StyleChoi, Wook Jin, and Jae Hoon Lee on behalf of the Korean Hypothermia Network Investigators. 2025. "The Prediction of Early Neurological Outcomes in Out-of-Hospital Cardiac Arrest Patients: A Multicenter Prospective Cohort Study by the KORHN Registry" Journal of Clinical Medicine 14, no. 18: 6466. https://doi.org/10.3390/jcm14186466
APA StyleChoi, W. J., & Lee, J. H., on behalf of the Korean Hypothermia Network Investigators. (2025). The Prediction of Early Neurological Outcomes in Out-of-Hospital Cardiac Arrest Patients: A Multicenter Prospective Cohort Study by the KORHN Registry. Journal of Clinical Medicine, 14(18), 6466. https://doi.org/10.3390/jcm14186466






