Endurant Stents in Abdominal Aortic Aneurysm Repair: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Inclusion/Exclusion Criteria
2.2. Search Strategy
2.3. Quality Assessment
2.4. Outcomes
2.5. Statistical Analysis
2.5.1. Reconstruction of Time to Event Patient Survival Data
2.5.2. One-Stage Survival Meta-Analysis
2.5.3. Two-Stage Survival Meta-Analysis
3. Results
3.1. Study and Patient Characteristics
3.2. Study Quality and Publication Bias Assessment
3.3. Time to Event Patient Data and Kaplan–Meier Curves Reconstruction
3.4. Main Outcomes
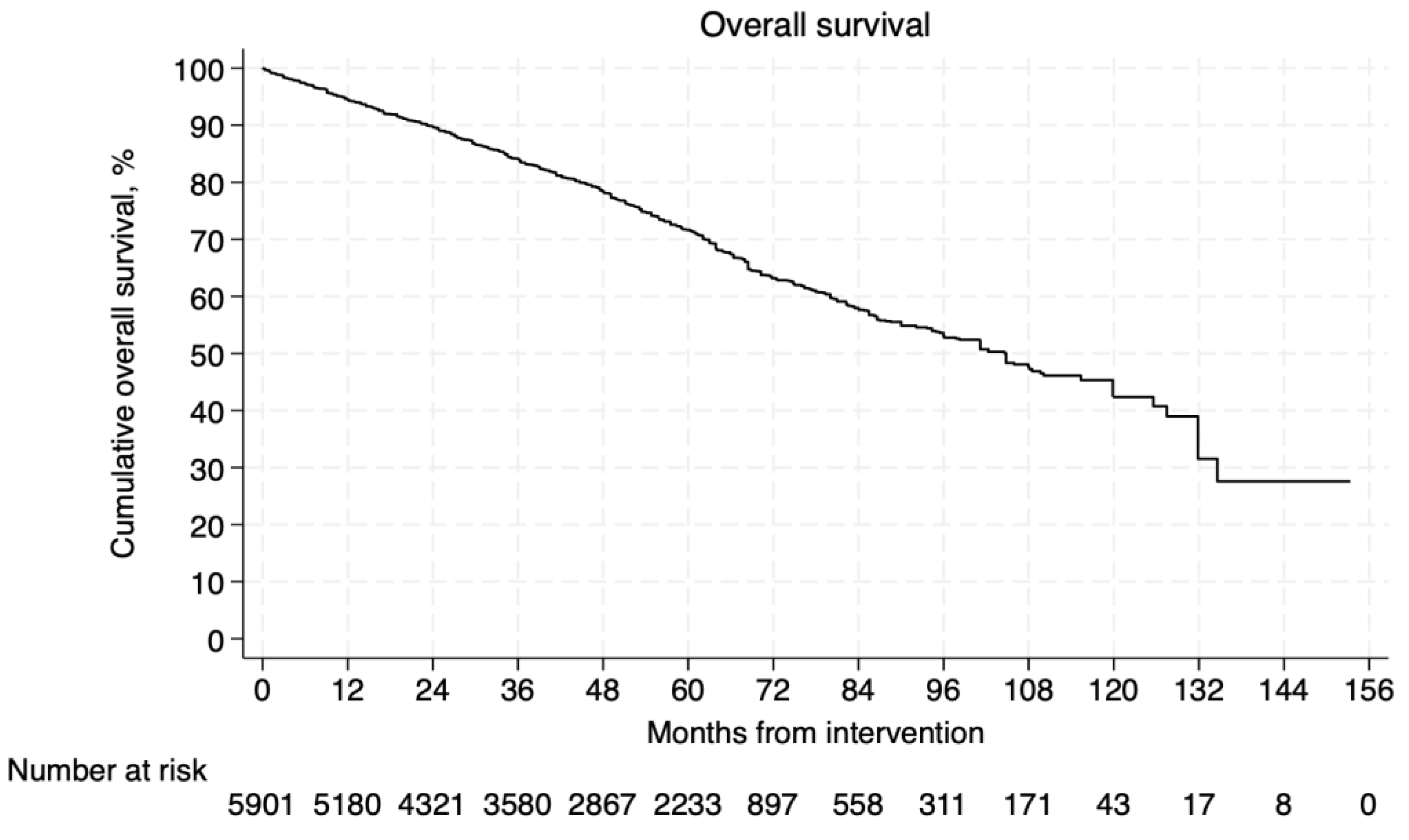
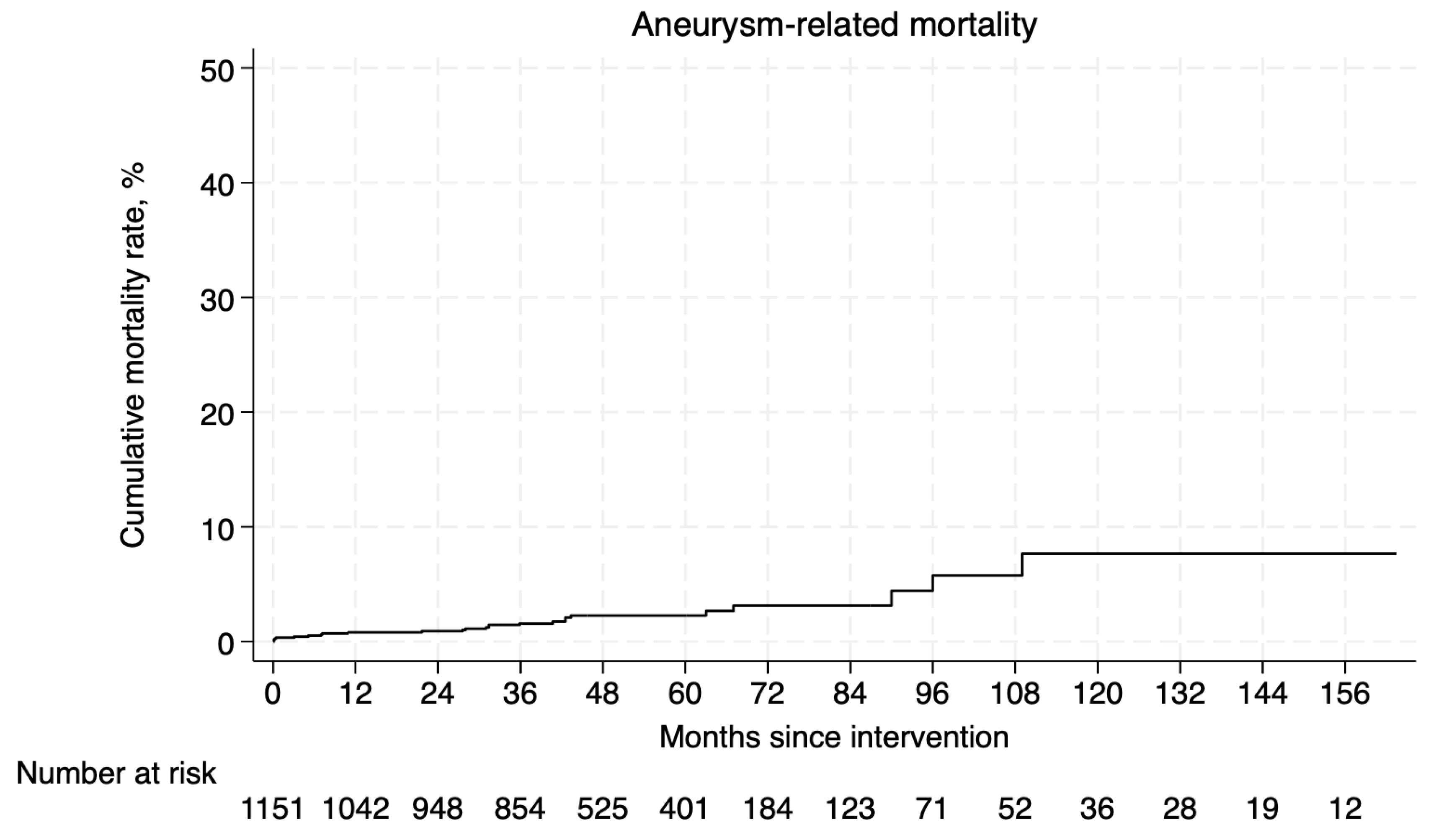
3.4.1. Survival and Aneurysm-Related Mortality
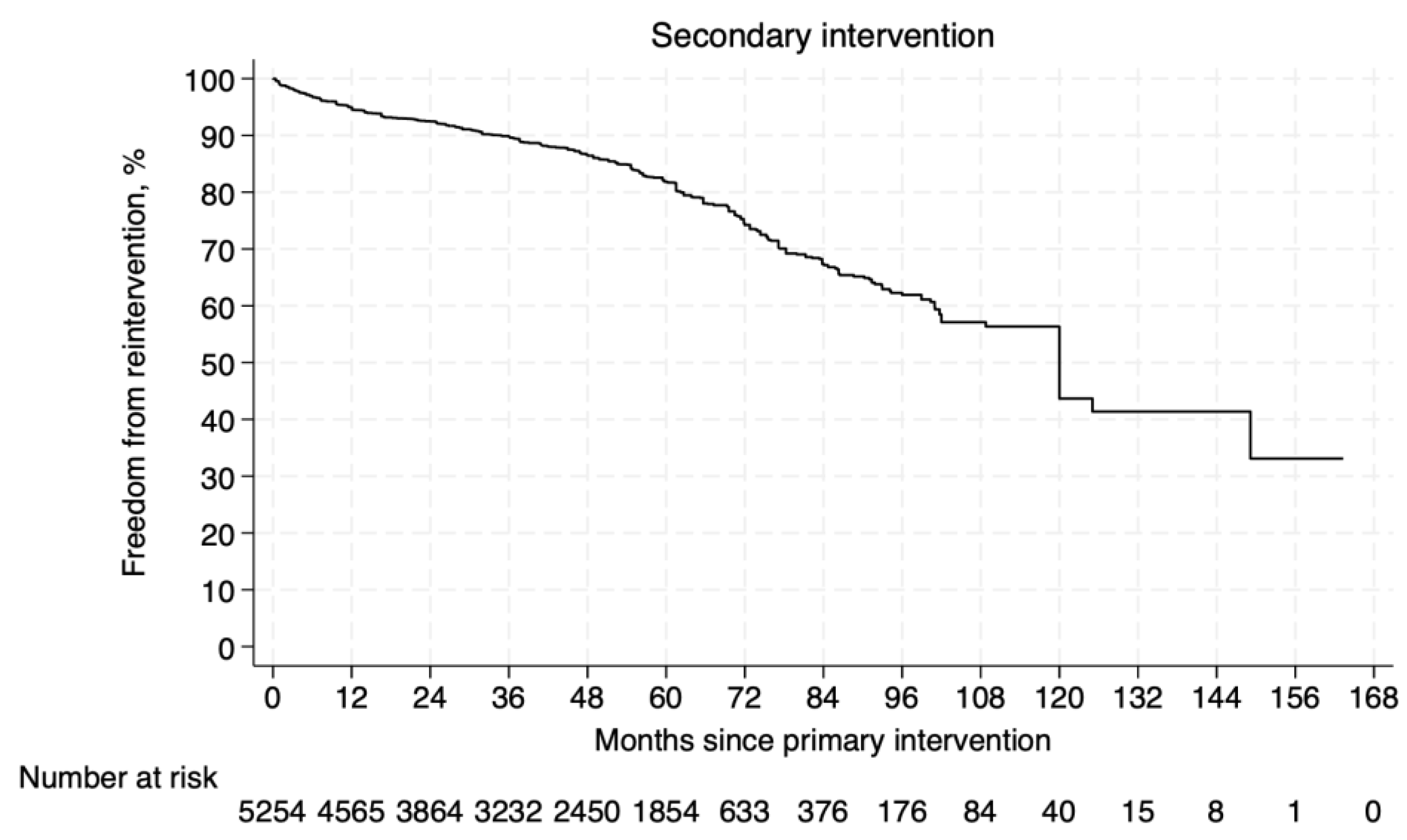
3.4.2. Freedom from Secondary Intervention
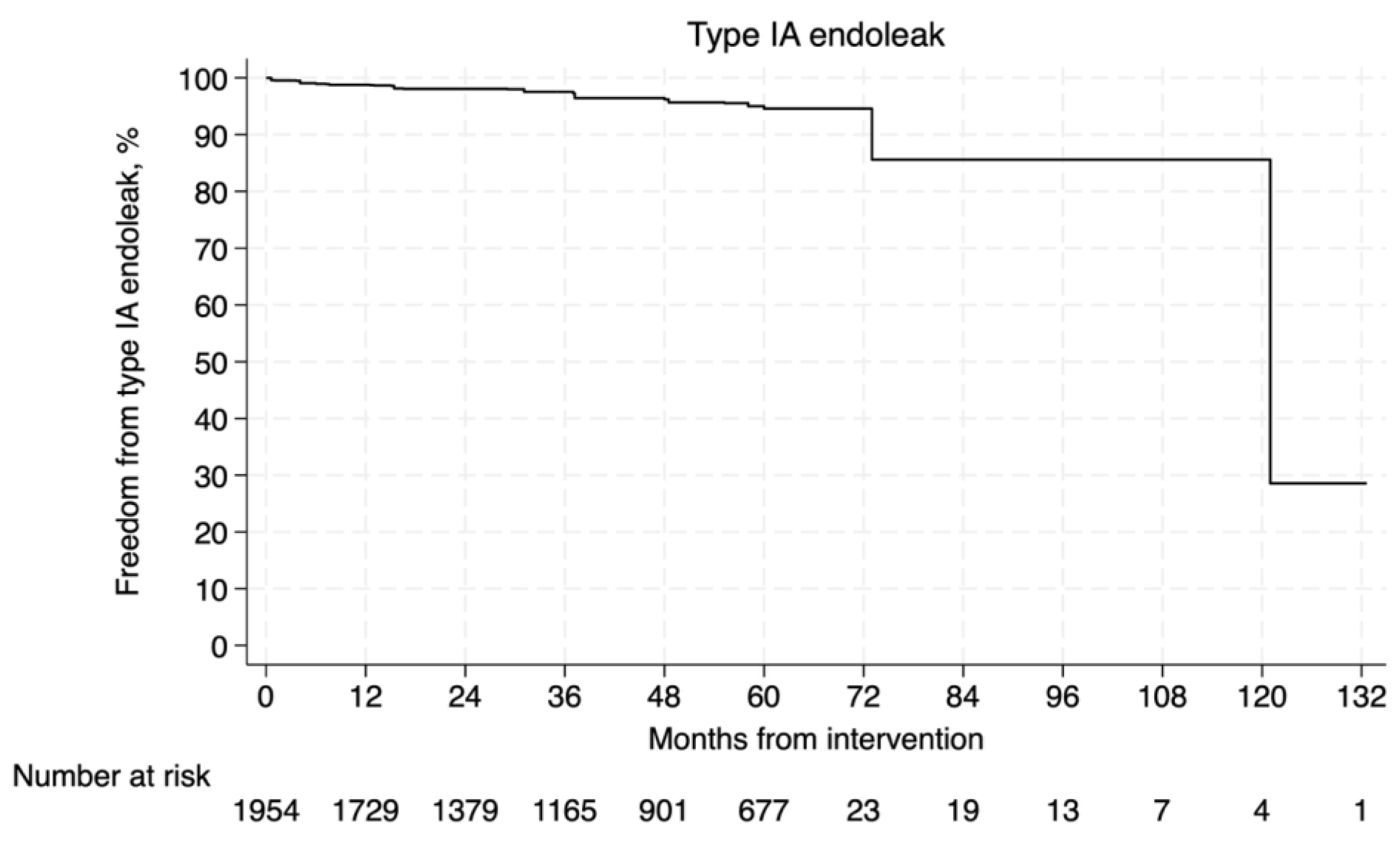
3.4.3. Type Ia Endoleak
3.5. Subgroup Analysis Within Versus Outside the Endurant’s IFU
3.5.1. Survival
3.5.2. Aneurysm-Related Mortality
3.5.3. Freedom from Secondary Intervention
3.6. Sensitivity Analysis
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wanhainen, A.; Van Herzeele, I.; Bastos Goncalves, F.; Bellmunt Montoya, S.; Berard, X.; Boyle, J.R.; D’oRia, M.; Prendes, C.F.; Karkos, C.D.; Kazimierczak, A.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2024 Clinical Practice Guidelines on the Management of Abdominal Aorto-Iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2024, 67, 192–331. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.T.; Sweeting, M.J.; Ulug, P.; Blankensteijn, J.D.; Lederle, F.A.; Becquemin, J.-P.; Greenhalgh, R.M.; Beard, J.D.; Buxton, M.J.; Brown, L.C.; et al. Meta-analysis of individual-patient data from EVAR-1, DREAM, OVER and ACE trials comparing outcomes of endovascular or open repair for abdominal aortic aneurysm over 5 years. Br. J. Surg. 2017, 104, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Colvard, B.; Georg, Y.; Chakfe, N.; Swanstrom, L. Current aortic endografts for the treatment of abdominal aortic aneurysms. Expert. Rev. Med. Devices 2016, 13, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Poublon, C.G.; Holewijn, S.; van Sterkenburg, S.M.M.; Tielliu, I.F.J.; Zeebregts, C.J.; Reijnen, M.M.P.J. Long-Term Outcome of the GORE EXCLUDER AAA Endoprosthesis for Treatment of Infrarenal Aortic Aneurysms. J. Vasc. Interv. Radiol. 2017, 28, 637–644.e1. [Google Scholar] [CrossRef]
- Mwipatayi, B.P.; Faraj, J.; Oshin, O.; Fitridge, R.; Wong, J.; Schermerhorn, M.L.; Becquemin, J.-P.; Boeckler, D.; Riambau, V.; Teijink, J.A.; et al. Endurant stent graft demonstrates promising outcomes in challenging abdominal aortic aneurysm anatomy. J. Vasc. Surg. 2021, 73, 69–80. [Google Scholar] [CrossRef]
- Verzini, F.; Romano, L.; Parlani, G.; Isernia, G.; Simonte, G.; Loschi, D.; Lenti, M.; Cao, P. Fourteen-year outcomes of abdominal aortic endovascular repair with the Zenith stent graft. J. Vasc. Surg. 2017, 65, 318–329. [Google Scholar] [CrossRef]
- Arko, F.R.; Jordan, W.D.; Robaina, S.; Arko, M.Z.; Fogarty, T.J.; Makaroun, M.S.; Verhagen, H.J.M. Interdisciplinary and translational innovation: The Endurant Stent Graft..from bedside to benchtop and back to bedside. J. Endovasc. Ther. 2011, 18, 779–785. [Google Scholar] [CrossRef]
- Teijink, J.A.W.; Power, A.H.; Böckler, D.; Peeters, P.; van Sterkenburg, S.; Bouwman, L.H.; Verhagen, H.J.; Bosiers, M.; Riambau, V.; Becquemin, J.-P.; et al. Editor’s Choice—Five Year Outcomes of the Endurant Stent Graft for Endovascular Abdominal Aortic Aneurysm Repair in the ENGAGE Registry. Eur. J. Vasc. Endovasc. Surg. 2019, 58, 175–181. [Google Scholar] [CrossRef]
- Verhagen, H.J.M.; Torsello, G.; De Vries, J.-P.P.M.; Cuypers, P.H.; Van Herwaarden, J.A.; Florek, H.-J.; Scheinert, D.; Eckstein, H.-H.; Moll, F.L. Endurant stent-graft system: Preliminary report on an innovative treatment for challenging abdominal aortic aneurysm. J. Cardiovasc. Surg. 2009, 50, 153–158. [Google Scholar]
- Salemans, P.B.; Lind, R.C.; Van Der Linde, R.A.; Pierie, M.P.; Fritschy, W.M. Up to 10-year follow-up after EVAR with the endurant stent graft system: A single-center experience. J. Cardiovasc. Surg. 2021, 62, 242–249. [Google Scholar] [CrossRef]
- Setacci, F.; Sirignano, P.; de Donato, G.; Galzerano, G.; Messina, G.; Guerrini, S.; Mazzei, M.A.; Setacci, C. Two-year-results of Endurant stent-graft in challenging aortic neck morphologies versus standard anatomies. J. Cardiovasc. Surg. 2014, 55, 85–92. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wohlin, C. Guidelines for Snowballing in Systematic Literature Studies and a Replication in Software Engineering. In Proceedings of the 18th International Conference on Evaluation and Assessment in Software Engineering, London, UK, 13–14 May 2014; Association for Computing Machinery: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- Wells, G.A.; Wells, G.; Shea, B.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2014. Available online: https://web.archive.org/web/20210716121605id_/www3.med.unipmn.it/dispense_ebm/2009-2010/Corso%20Perfezionamento%20EBM_Faggiano/NOS_oxford.pdf (accessed on 2 April 2025).
- Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using the GRADE Approach. Updated October 2013. Available online: https://training.cochrane.org/resource/grade-handbook (accessed on 5 April 2025).
- Guyot, P.; Ades, A.E.; Ouwens, M.J.N.M.; Welton, N.J. Enhanced secondary analysis of survival data: Reconstructing the data from published Kaplan-Meier survival curves. BMC Med. Res. Methodol. 2012, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Royston, P. Reconstructing time-to-event data from published Kaplan-Meier curves. Stata J. 2017, 17, 786–802. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Zandvoort, H.J.A.; Gonçalves, F.B.; Verhagen, H.J.M.; Werson, D.A.B.; Moll, F.L.; De Vries, J.P.P.M.; van Herwaarden, J.A. Results of endovascular repair of infrarenal aortic aneurysms using the Endurant stent graft. J. Vasc. Surg. 2014, 59, 1195–1202. [Google Scholar] [CrossRef]
- Van Keulen, J.W.; De Vries, J.P.P.M.; Dekker, H.; Gonalves, F.B.; Moll, F.L.; Verhagen, H.J.; van Herwaarden, J.A. One-year multicenter results of 100 abdominal aortic aneurysm patients treated with the Endurant stent graft. J. Vasc. Surg. 2011, 54, 609–615. [Google Scholar] [CrossRef]
- Georgiadis, G.S.; Trellopoulos, G.; Antoniou, G.A.; Gallis, K.; Nikolopoulos, E.S.; Kapoulas, K.C.; Pitta, X.; Lazarides, M.K. Early results of the Endurant endograft system in patients with friendly and hostile infrarenal abdominal aortic aneurysm anatomy. J. Vasc. Surg. 2011, 54, 616–627.e4. [Google Scholar] [CrossRef]
- Antoniou, G.A.; Georgiadis, G.S.; Glancz, L.; Delbridge, M.; Murray, D.; Smyth, J.V.; Lazarides, M.K.; Serracino-Inglott, F. Outcomes of endovascular aneurysm repair with 2 different endograft systems with suprarenal fixation in patients with hostile infrarenal aortic anatomy. Vasc. Endovasc. Surg. 2013, 47, 9–18. [Google Scholar] [CrossRef]
- Stokmans, R.A.; Teijink, J.A.W.; Forbes, T.L.; Böckler, D.; Peeters, P.J.; Riambau, V.; Hayes, P.; van Sambeek, M. Early results from the ENGAGE registry: Real-world performance of the endurant stent graft for endovascular AAA repair in 1262 patients. Eur. J. Vasc. Endovasc. Surg. 2012, 44, 369–375. [Google Scholar] [CrossRef]
- Dijkstra, M.L.; Van Sterkenburg, S.M.M.; Lardenoye, J.W.; Zeebregts, C.J.; Reijnen, M.M.P.J. One-year outcomes of endovascular aneurysm repair in high-risk patients using the endurant stent-graft: Comparison of the ASA classification and SVS/AAVS medical comorbidity grading system for the prediction of mortality and adverse events. J. Endovasc. Ther. 2016, 23, 574–582. [Google Scholar] [CrossRef]
- Torsello, G.; Troisi, N.; Tessarek, J.; Torsello, G.F.; Dorigo, W.; Pulli, R.; Pratesi, C. Endovascular Aortic Aneurysm Repair with the Endurant Stent-graft: Early and 1-year Results from a European Multicenter Experience. J. Vasc. Interv. Radiol. 2010, 21, 73–80. [Google Scholar] [CrossRef]
- Torsello, G.; Troisi, N.; Donas, K.P.; Austermann, M. Evaluation of the Endurant stent graft under instructions for use vs off-label conditions for endovascular aortic aneurysm repair. J. Vasc. Surg. 2011, 54, 300–306. [Google Scholar] [CrossRef][Green Version]
- Özdemir-van Brunschot, D.M.D.; Torsello, G.B.; Bernardini, G.; Litterscheid, S.; Torsello, G.F.; Beropoulis, E. Long-term Results of Angulated Versus Hyperangulated Neck in Endovascular Aneurysm Repair With Endurant Endoprosthesis. J. Endovasc. Ther. 2023, 30, 91–97. [Google Scholar] [CrossRef]
- Rouwet, E.V.; Torsello, G.; De Vries, J.P.P.M.; Cuypers, P.; Van Herwaarden, J.A.; Eckstein, H.H.; Beuk, R.; Florek, H.-J.; Jentjens, R.; Verhagen, H. Final results of the prospective European trial of the endurant stent graft for endovascular abdominal aortic aneurysm repair. Eur. J. Vasc. Endovasc. Surg. 2011, 42, 489–497. [Google Scholar] [CrossRef][Green Version]
- Benveniste, G.L.; Tjahjono, R.; Chen, O.; Verhagen, H.J.M.; Böckler, D.; Varcoe, R.L. Long-term Results of 180 Consecutive Patients with Abdominal Aortic Aneurysm Treated with the Endurant Stent Graft System. Ann. Vasc. Surg. 2020, 67, 265–273. [Google Scholar] [CrossRef]
- Falster, M.O.; Garland, S.K.; Jorm, L.R.; Beiles, C.B.; Freeman, A.J.; Sedrakyan, A.; Sotade, O.T.; Varcoe, R.L. Editor’s Choice—Comparison of Outcomes for Major Contemporary Endograft Devices Used for Endovascular Repair of Intact Abdominal Aortic Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2023, 65, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Kvinlaug, K.E.; Lawlor, D.K.; Forbes, T.L.; Willoughby, R.; MacKenzie, K.S.; DeRose, G.; Corriveau, M.M.; Steinmetz, O.K. Early results from a canadian multicenter prospective registry of the endurant stent graft for endovascular treatment of abdominal aortic aneurysms. J. Endovasc. Ther. 2012, 19, 58–66. [Google Scholar] [CrossRef] [PubMed]
- van Basten Batenburg, M.; ‘t Mannetje, Y.W.; van Sambeek, M.R.H.M.; Cuypers, P.W.M.; Georgiadis, G.S.; Sondakh, A.O.; Teijink, J.A. Editor’s Choice—Endurant Stent Graft in Patients with Challenging Neck Anatomy “One Step Outside Instructions for Use”: Early and Midterm Results from the EAGLE Registry. Eur. J. Vasc. Endovasc. Surg. 2022, 64, 611–619. [Google Scholar] [CrossRef]
- Becquemin, J.P.; Haupert, S.; Issam, F.; Dubar, A.; Martelloni, Y.; Jousset, Y.; Sauguet, A. Five Year Patient Outcomes of Endovascular Abdominal Aortic Aneurysm Repair in the ENDURANT France Registry. Eur. J. Vasc. Endovasc. Surg. 2021, 61, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Omran, S.; Müller, V.; Schawe, L.; Bürger, M.; Kapahnke, S.; Bruder, L.; Haidar, H.; Konietschke, F.; Greiner, A. Outcomes of Endurant II Stent Graft According to Anatomic Severity Grade Score. J. Endovasc. Ther. 2023, 30, 600–608. [Google Scholar] [CrossRef]
- Özdemir-van Brunschot, D.M.D.; Holzhey, D.; Botsios, S. Mid-term results of “off-label” use of the Endurant stentgraft in patients with infrarenal abdominal aortic aneurysms. Vascular 2024, 33, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Bisdas, T.; Weiss, K.; Eisenack, M.; Austermann, M.; Torsello, G.; Donas, K.P. Durability of the Endurant stent graft in patients undergoing endovascular abdominal aortic aneurysm repair. J. Vasc. Surg. 2014, 60, 1125–1131. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Troisi, N.; Torsello, G.; Weiss, K.; Donas, K.P.; Michelagnoli, S.; Austermann, M. Midterm results of endovascular aneurysm repair using the Endurant Stent-graft according to the instructions for use vs. Off-Label conditions. J. Endovasc. Ther. 2014, 21, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Deery, S.E.; Shean, K.E.; Pothof, A.B.; O’Donnell, T.F.X.; Dalebout, B.A.; Darling, J.D.; Bodewes, T.C.; Schermerhorn, M.L. Three-Year Results of the Endurant Stent Graft System Post Approval Study. Ann. Vasc. Surg. 2018, 50, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Sekimoto, Y.; Fujimura, N.; Matsubara, K.; Uchida, N.; Asami, A.; Harada, H.; Shintani, T.; Watada, S.; Ono, S.; Fujii, T.; et al. Long-term Outcomes of the Endurant and Excluder Stent Grafts for Endovascular Aneurysm Repair in a Japanese Cohort. J. Endovasc. Ther. 2023, 30, 571–579. [Google Scholar] [CrossRef]
- Vedani, S.M.; Petitprez, S.; Weinz, E.; Corpataux, J.M.; Déglise, S.; Deslarzes-Dubuis, C.; Côté, E.; Ricco, J.-B.; Saucy, F. Predictors and Consequences of Sac Shrinkage after Endovascular Infrarenal Aortic Aneurysm Repair. J. Clin. Med. 2022, 11, 3232. [Google Scholar] [CrossRef]
- Matsagkas, M.; Kouvelos, G.; Peroulis, M.; Avgos, S.; Arnaoutoglou, E.; Papa, N.; Papadopoulos, G. Standard endovascular treatment of abdominal aortic aneurysms in patients with very short proximal necks using the Endurant stent graft. J. Vasc. Surg. 2015, 61, 9–15. [Google Scholar] [CrossRef][Green Version]
- Spanos, K.; Nana, P.; Volakakis, G.; Kouvelos, G.; Dakis, K.; Karathanos, C.; Arnaoutoglou, E.; Matsagkas, M.; Giannoukas, A. Long-Term Outcomes in Patients Managed with the EndurantTM Endograft under Elective Setting. J. Clin. Med. 2024, 13, 5601. [Google Scholar] [CrossRef]
- Georgiadis, G.S.; Schoretsanitis, N.; Argyriou, C.; Nikolopoulos, E.; Kapoulas, K.; Georgakarakos, E.I.; Ktenidis, K.; Lazarides, M.K. Long-term outcomes of the Endurant endograft in patients undergoing endovascular abdominal aortic aneurysm repair. J. Vasc. Surg. 2023, 78, 668–678.e14. [Google Scholar] [CrossRef]
- Singh, M.J.; Fairman, R.; Anain, P.; Jordan, W.D.; Maldonaldo, T.; Samson, R.; Makaroun, M.S. Final results of the Endurant Stent Graft System in the United States regulatory trial. J. Vasc. Surg. 2016, 64, 55–62. [Google Scholar] [CrossRef]
- Pecoraro, F.; Corte, G.; Dinoto, E.; Badalamenti, G.; Bruno, S.; Bajardi, G. Clinical outcomes of endurant II stent-graft for infrarenal aortic aneurysm repair: Comparison of on-label versus off-label use. Diagn. Interv. Radiol. 2016, 22, 450–454. [Google Scholar] [CrossRef]
- ‘t Mannetje, Y.W.; Cuypers, P.W.M.; Saleem, B.R.; Bode, A.S.; Teijink, J.A.W.; van Sambeek, M.R.H.M. Comparison of midterm results for the Talent and Endurant stent graft. J. Vasc. Surg. 2017, 66, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Pinto, J.; Oliveira, N.F.G.; Bastos-Gonçalves, F.M.; Hoeks, S.; Van Rijn, M.J.; Raa, S.T.; Mansilha, A.; Verhagen, H.J. Long-term results after standard endovascular aneurysm repair with the Endurant and Excluder stent grafts. J. Vasc. Surg. 2020, 71, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Kemmling, S.; Wiedner, M.; Stahlberg, E.; Sieren, M.; Jacob, F.; Barkhausen, J.; Goltz, J.P. Five-year outcomes of the Bi- versus Trimodular EndurantTM stent-graft in 100 patients with infrarenal abdominal aortic repair. J. Cardiovasc. Surg. 2022, 63, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, N.F.G.; Bastos Gonçalves, F.M.; De Vries, J.P.P.M.; Ultee, K.H.J.; Werson, D.A.B.; Hoeks, S.E.; Moll, F.; van Herwaarden, J.; Verhagen, H. Mid-term results of EVAR in severe proximal aneurysm neck angulation. Eur. J. Vasc. Endovasc. Surg. 2015, 49, 19–27. [Google Scholar] [CrossRef]
- MedTroniCinC. The endurantTM II/endurantTM IIs Stent-Graft System (ifu) [Internet]. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf10/P100021s063d.pdf (accessed on 15 December 2024).
- Troisi, N.; Pitoulias, G.; Michelagnoli, S.; Torsello, G.; Stachmann, A.; Bisdas, T.; Li, Y.; Donas, K.P. Preliminary experience with the Endurant II short form stent-graft system. J. Cardiovasc. Surg. 2019, 60, 364–368. [Google Scholar] [CrossRef]
- Becquemin, J.-P.; Pillet, J.-C.; Lescalie, F.; Sapoval, M.; Goueffic, Y.; Lermusiaux, P.; Steinmetz, E.; Marzelle, J. A randomized controlled trial of endovascular aneurysm repair versus open surgery for abdominal aortic aneurysms in low- to moderate-risk patients. J. Vasc. Surg. 2011, 53, 1167–1173.e1. [Google Scholar] [CrossRef]
- Prinssen, M.; Verhoeven, E.L.G.; Buth, J.; Cuypers, P.W.M.; van Sambeek, M.R.H.M.; Balm, R.; Buskens, E.; Grobbee, D.E.; Blankensteijn, J.D. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N. Engl. J. Med. 2004, 351, 1607–1618. [Google Scholar] [CrossRef]
- Lederle, F.A.; Kyriakides, T.C.; Stroupe, K.T.; Freischlag, J.A.; Padberg, F.T.J.; Matsumura, J.S.; Huo, Z.; Johnson, G.R. Open versus Endovascular Repair of Abdominal Aortic Aneurysm. N. Engl. J. Med. 2019, 380, 2126–2135. [Google Scholar] [CrossRef]
- Greenhalgh, R.M.; Brown, L.C.; Powell, J.T.; Thompson, S.G.; Epstein, D.; Sculpher, M.J. Endovascular versus open repair of abdominal aortic aneurysm. N. Engl. J. Med. 2010, 362, 1863–1871. [Google Scholar] [CrossRef]
- Wanken, Z.J.; Barnes, J.A.; Trooboff, S.W.; Columbo, J.A.; Jella, T.K.; Kim, D.J.; Khoshgowari, A.; Riblet, N.B.; Goodney, P.P. A systematic review and meta-analysis of long-term reintervention after endovascular abdominal aortic aneurysm repair. J. Vasc. Surg. 2020, 72, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Columbo, J.A.; Martinez-Camblor, P.; O’Malley, A.J.; Suckow, B.D.; Hoel, A.W.; Stone, D.H.; Schanzer, A.; Schermerhorn, M.L.; Sedrakyan, A.; Goodney, P.P. Long-term Reintervention After Endovascular Abdominal Aortic Aneurysm Repair. Ann. Surg. 2021, 274, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Nana, P.; Kölbel, T.; Behrendt, C.-A.; Kouvelos, G.; Giannoukas, A.; Haulon, S.; Spanos, K. Systematic review of reintervention with fenestrated or branched devices after failed previous endovascular aortic aneurysm repair. J. Vasc. Surg. 2023, 77, 1806–1814.e2. [Google Scholar] [CrossRef] [PubMed]
- Doumenc, B.; Mesnard, T.; Patterson, B.O.; Azzaoui, R.; De Préville, A.; Haulon, S.; Sobocinski, J. Management of Type IA Endoleak After EVAR by Explantation or Custom Made Fenestrated Endovascular Aortic Aneurysm Repair. Eur. J. Vasc. Endovasc. Surg. 2021, 61, 571–578. [Google Scholar] [CrossRef]
- Bianchini Massoni, C.; Perini, P.; Tecchio, T.; Azzarone, M.; de Troia, A.; Freyrie, A. A systematic review of treatment modalities and outcomes of type Ib endoleak after endovascular abdominal aneurysm repair. Vascular 2018, 26, 90–98. [Google Scholar] [CrossRef]
- Zuccon, G.; D’Oria, M.; Gonçalves, F.B.; Fernandez-Prendes, C.; Mani, K.; Caldeira, D.; Koelemay, M.; Bissacco, D.; Trimarchi, S.; Van Herzeele, I.; et al. Incidence, Risk Factors, and Prognostic Impact of Type Ib Endoleak Following Endovascular Repair for Abdominal Aortic Aneurysm: Scoping Review. Eur. J. Vasc. Endovasc. Surg. 2023, 66, 352–361. [Google Scholar] [CrossRef]
- Özdemir-van Brunschot, D.M.D.; Harrich, F.; Tevs, M.; Holzhey, D. Risk factors of type 1A endoleak following endovascular aortic aneurysm repair. Vascular 2024, 32, 737–744. [Google Scholar] [CrossRef]
- Patelis, N.D.; Malli, A.; Mylonas, K.S.; Schizas, D.; Papa, N.; Economopoulos, K.P.; Damaskos, C.; Katsargyris, A.; Georgopoulos, S.; Klonaris, C.; et al. Suitability study of current endovascular aortic repair devices based on real-life anatomic data. Expert. Rev. Med. Devices 2019, 16, 165–171. [Google Scholar] [CrossRef]
- Schanzer, A.; Greenberg, R.K.; Hevelone, N.; Robinson, W.P.; Eslami, M.H.; Goldberg, R.J.; Messina, L. Predictors of abdominal aortic aneurysm sac enlargement after endovascular repair. Circulation 2011, 123, 2848–2855. [Google Scholar] [CrossRef]
- Antoniou, G.A.; Georgiadis, G.S.; Antoniou, S.A.; Kuhan, G.; Murray, D. A meta-analysis of outcomes of endovascular abdominal aortic aneurysm repair in patients with hostile and friendly neck anatomy. J. Vasc. Surg. 2013, 57, 527–538. [Google Scholar] [CrossRef] [PubMed]

| PICO Element | Description |
|---|---|
| Population | Adult patients undergoing EVAR for infrarenal unruptured AAA, including those with symptomatic status, if mentioned |
| Intervention | EVAR using the Endurant endograft |
| Comparison | Non-applicable |
| Outcomes | Survival, freedom from reintervention, aneurysm-related mortality, and freedom from type IA endoleak rates |
| Search Criteria | Description |
|---|---|
| Inclusion criteria | Studies reporting on the outcomes of interest concerning patients undergoing EVAR for unruptured AAA with the Endurant endograft |
| Exclusion criteria | (i) Studies involving complex or ruptured AAAs (ii) Studies using the Endurant stent with other stents, treating isolated iliac aneurysms, or using EndoAnchors (iii) Non-English language publications (iv) Meta-analyses, systematic reviews, editorials, and letters to the editor |
| Study selection | Inclusion of only studies with the largest sample size or best quality data (including Kaplan–Meier curves) |
| Algorithm | PUBMED: ((endostent*) OR (endurant*) OR (“endovascular graft”)) AND (((“AAA”) OR (“abdominal aortic aneurysm”) OR (“aortic aneurysm”)) OR (abdominal aorta aneurysm [MeSH Terms]) OR (aneurysm, abdominal aortic [MeSH Terms])). Scopus: TITLE-ABS-KEY ((“Endurant stent” OR “Endurant graft” OR “endovascular graft”) AND (“abdominal aortic aneurysm” OR “infrarenal abdominal aortic aneurysm”)) Cochrane (Title Abstract Keyword): ((endostent) OR (endurant*) OR (“endovascular graft”)) AND ((“AAA”) OR (“abdominal aortic aneurysm”) OR (“aortic aneurysm”)) |
| Last Search | 12 November 2024 |
| Sources | Medline, Scopus, Cohrane database, Clinicaltrial.gov |
| Subgroup analysis | Comparison of outcomes between patients treated within versus outside the Endurant’s IFUs |
| Author | Publication Year | Center | Country | Design | Study Period | Number | IFU Versus Outside IFU |
|---|---|---|---|---|---|---|---|
| Rouwet EV et al. [28] | 2011 | 10 EU centers | 10 EU centers | Prospective | November 2007–August 2008 | 80 | No |
| Benveniste GL et al. [29] | 2020 | Ashford Vascular Clinic, Adelaide, South Australia | Australia | Prospective | August 2008–March 2019 | 180 | Yes |
| ΜO Falster et al. [30] | 2023 | Australasian Vascular Audit, New South Wales | Australia | Prospective | January 2010–June 2019 | 1713 | No |
| Kvinlaug KE et al. [31] | 2012 | McGill University Health Centre in Montreal, Quebec, the London Health Sciences Centre, University of Western Ontario, the Sudbury Regional Hospital in Sudbury, Ontario | Canada | Prospective | September 2008–January 2010 | 107 | No |
| van Basten Batenburg M et al. [32] | 2022 | ΕAGLE, Multicenter Europe | Europe | Prospective | February 2012–September 2017 | 150 | No |
| Becquemin JP et al. [33] | 2021 | 20 French centers | France | Prospective | March 2012–April 2013 | 180 | No |
| Omran S et al. [34] | 2023 | Universitätsmedizin Berlin, Berlin | Germany | Prospective | January 2013–May 2021 | 165 | No |
| Özdemir-van Brunschot DMD et al. [35] | 2024 | Augusta Hospital and Catholic Hospital Group Düsseldorf, Düsseldorf | Germany | Retrospective | January 2012–December 2020 | 178 | Yes |
| Bisdas T et al. [36] | 2014 | Department of Vascular Surgery, St. Franziskus Hospital and University Clinic of Muenster, Muenster | Germany | Prospective | November 2007–December 2010 | 273 | No |
| Troisi N et al. [37] | 2014 | St. Franziskus Hospital, Munster, the Center for Vascular and Endovascular Surgery, University Hospital, Munster, Department of Vascular Surgery, University of Florence | Germany, Italy | Prospective | November 2007–March 2010 | 173 | Yes |
| Deery SE et al. [38] | 2019 | 24 USA centers, ENGAGE post-approval | USA | Prospective | June 2011–August 2012 | 178 | No |
| ENGAGE [5] | 2021 | ENGAGE registry, 79 centers worldwide | International | Prospective | March 2009–April 2011 | 1263 | Yes |
| Teijink JAW et al. [8] | 2019 | ENGAGE registry, 79 centers worldwide | International | Prospective | March 2009–April 2011 | 1263 | No |
| Sekimoto Y et al. [39] | 2023 | 10 Japanese hospitals | Japan | Retrospective | January 2012–July 2019 | 332 | No |
| Vedani SM et al. [40] | 2022 | Department of Vascular Surgery, CHUVLausanne | Switzerland | Retrospective | 2014–2018 | 60 | Yes |
| Μatsagkas M et al. [41] | 2015 | Ιoannina University Hospital | Greece | Prospective | September 2008–December 2012 | 57 | Yes |
| Georgiadis S et al. [43] | 2023 | University General Hospital of Alexandroupolis | Greece | Prospective | January 2009–December 2016 | 184 | Yes |
| Singh MJ et al. [44] | 2016 | U.S. Endurant Stent Graft System regulatory trial | USA | Prospective | June 2008–April 2009 | 150 | No |
| Pecoraro F et al. [45] | 2016 | Vascular Surgery Unit, AOUP “P. Giaccone”, University of Palermo | Ιtaly | Retrospective | December 2012–March 2015 | 64 | Yes |
| Setacci F et al. [11] | 2014 | Vascular and Endovascular Surgery Unit, University of Siena | Ιtaly | Retrospective | January 2010–December 2010 | 137 | No |
| t Mannetje YW et al. [46] | 2017 | Department of Vascular Surgery, Catharina Hospital, Eindhoven | The Netherlands | Retrospective | January 2005– December 2010 | 131 | No |
| Oliveira Pinto J et al. [47] | 2019 | Erasmus University Medical Centre, Rotterdam | Τhe Netherlands | Prospective | July 2004–December 2011 | 156 | No |
| Salemans PB et al. [10] | 2021 | Department Of Vascular Surgery, Isala, Zwolle | The Netherlands | Retrospective | February 2009–December 2012 | 165 | No |
| Kemmling S et al. [48] | 2022 | University Hospital of Schleswig Holstein, Lübeck | Germany | Prospective | 2013–2018 | 100 | No |
| Spanos K et al. [42] | 2024 | Department of Vascular Surgery, Larissa University Hospital | Greece | Retrospective | 2008–2024 | 361 | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loufopoulos, G.; Nana, P.; Dakis, K.; Kouvelos, G.; Makaloski, V.; Donas, K.P.; Matsagkas, M.; Giannoukas, A.; Spanos, K. Endurant Stents in Abdominal Aortic Aneurysm Repair: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 6453. https://doi.org/10.3390/jcm14186453
Loufopoulos G, Nana P, Dakis K, Kouvelos G, Makaloski V, Donas KP, Matsagkas M, Giannoukas A, Spanos K. Endurant Stents in Abdominal Aortic Aneurysm Repair: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(18):6453. https://doi.org/10.3390/jcm14186453
Chicago/Turabian StyleLoufopoulos, Georgios, Petroula Nana, Konstantinos Dakis, George Kouvelos, Vladimir Makaloski, Konstantinos P. Donas, Miltiadis Matsagkas, Athanasios Giannoukas, and Konstantinos Spanos. 2025. "Endurant Stents in Abdominal Aortic Aneurysm Repair: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 18: 6453. https://doi.org/10.3390/jcm14186453
APA StyleLoufopoulos, G., Nana, P., Dakis, K., Kouvelos, G., Makaloski, V., Donas, K. P., Matsagkas, M., Giannoukas, A., & Spanos, K. (2025). Endurant Stents in Abdominal Aortic Aneurysm Repair: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(18), 6453. https://doi.org/10.3390/jcm14186453









