A Real-World Evaluation of Clinical Prognostic Scores in Advanced Melanoma Treated with Immune Checkpoint Inhibitors
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Patient Selection
2.2. Laboratory and Score Calculations
2.3. Statistical Analysis
3. Results
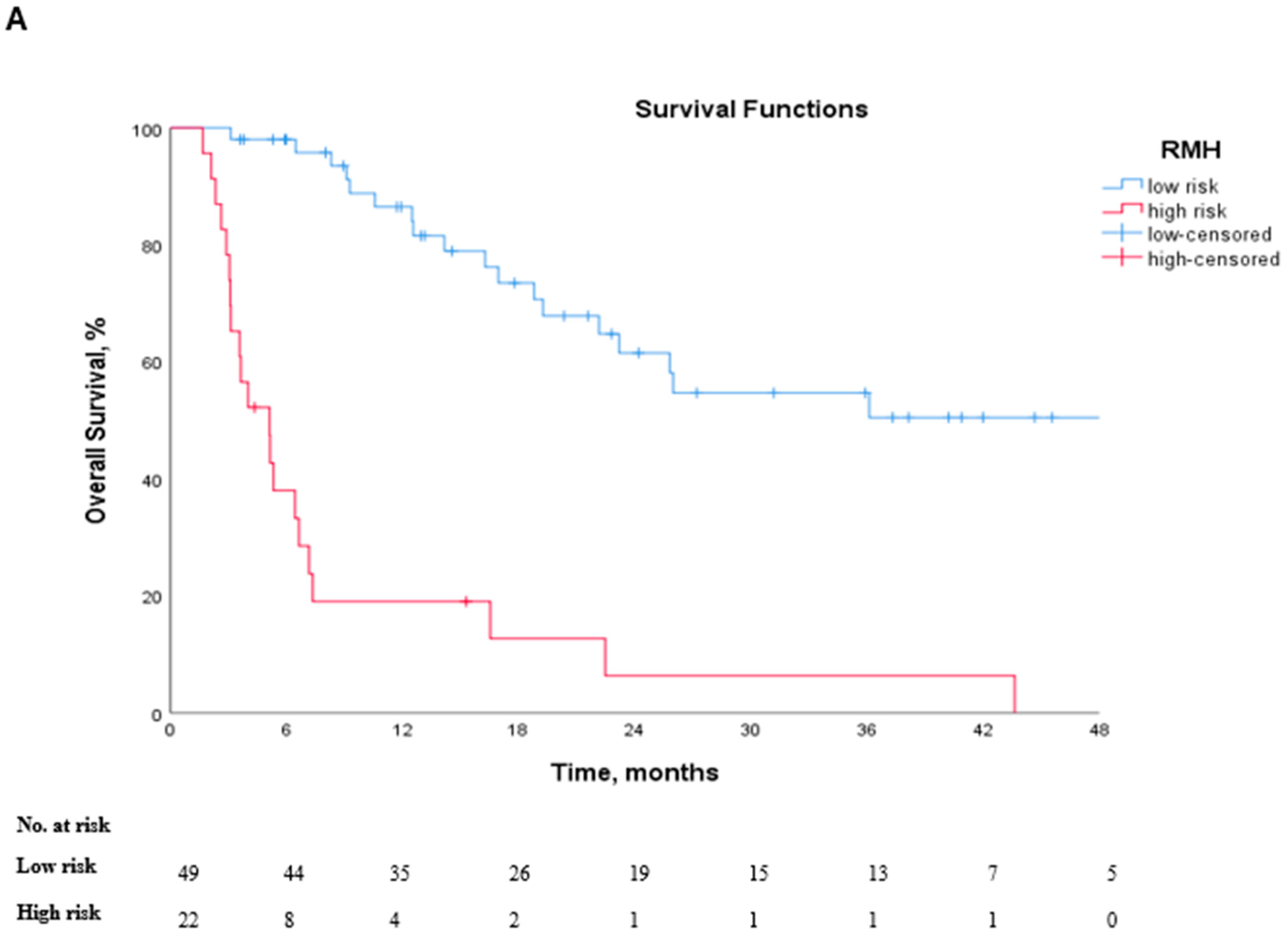
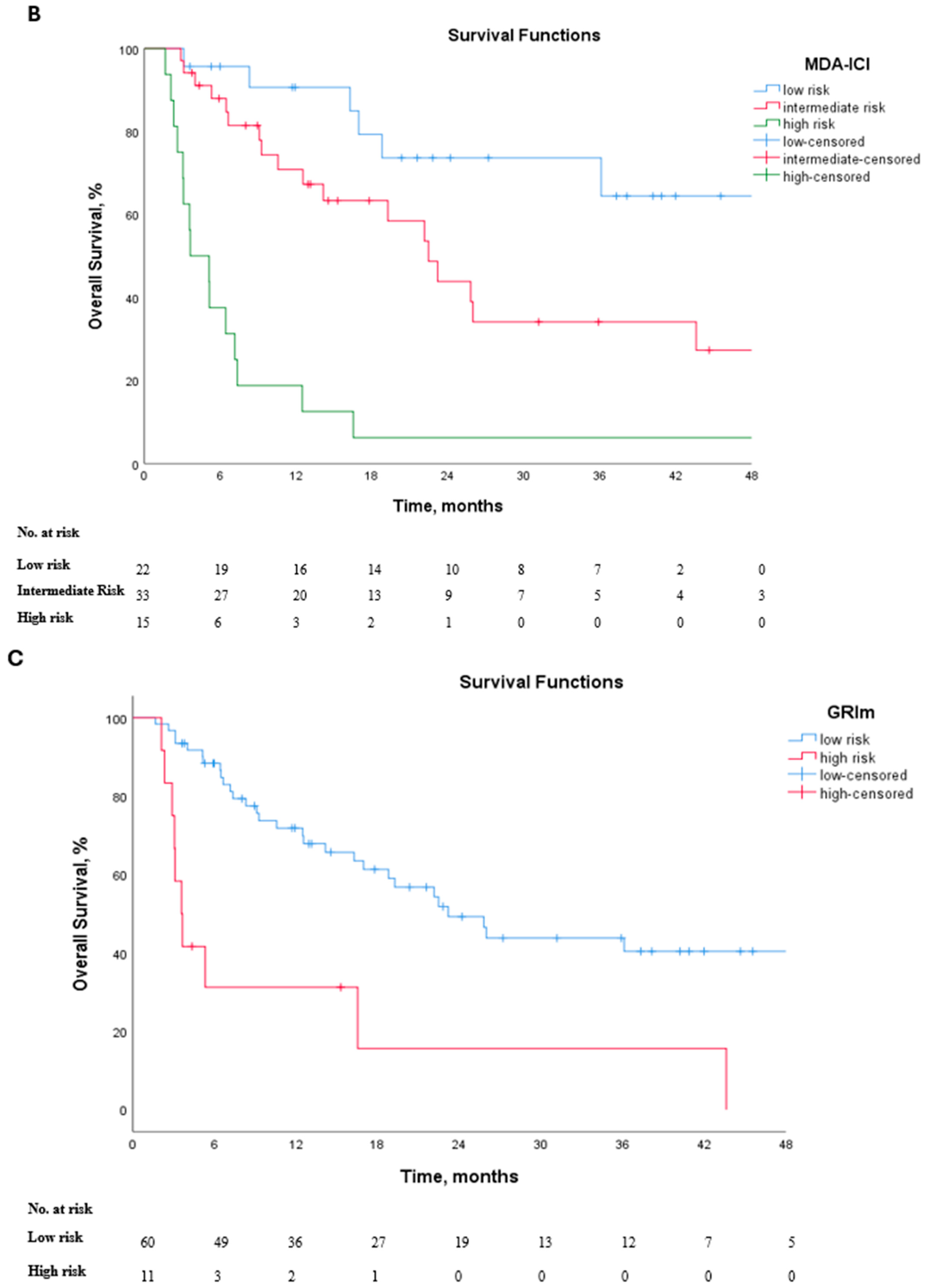
3.1. Survival Outcomes
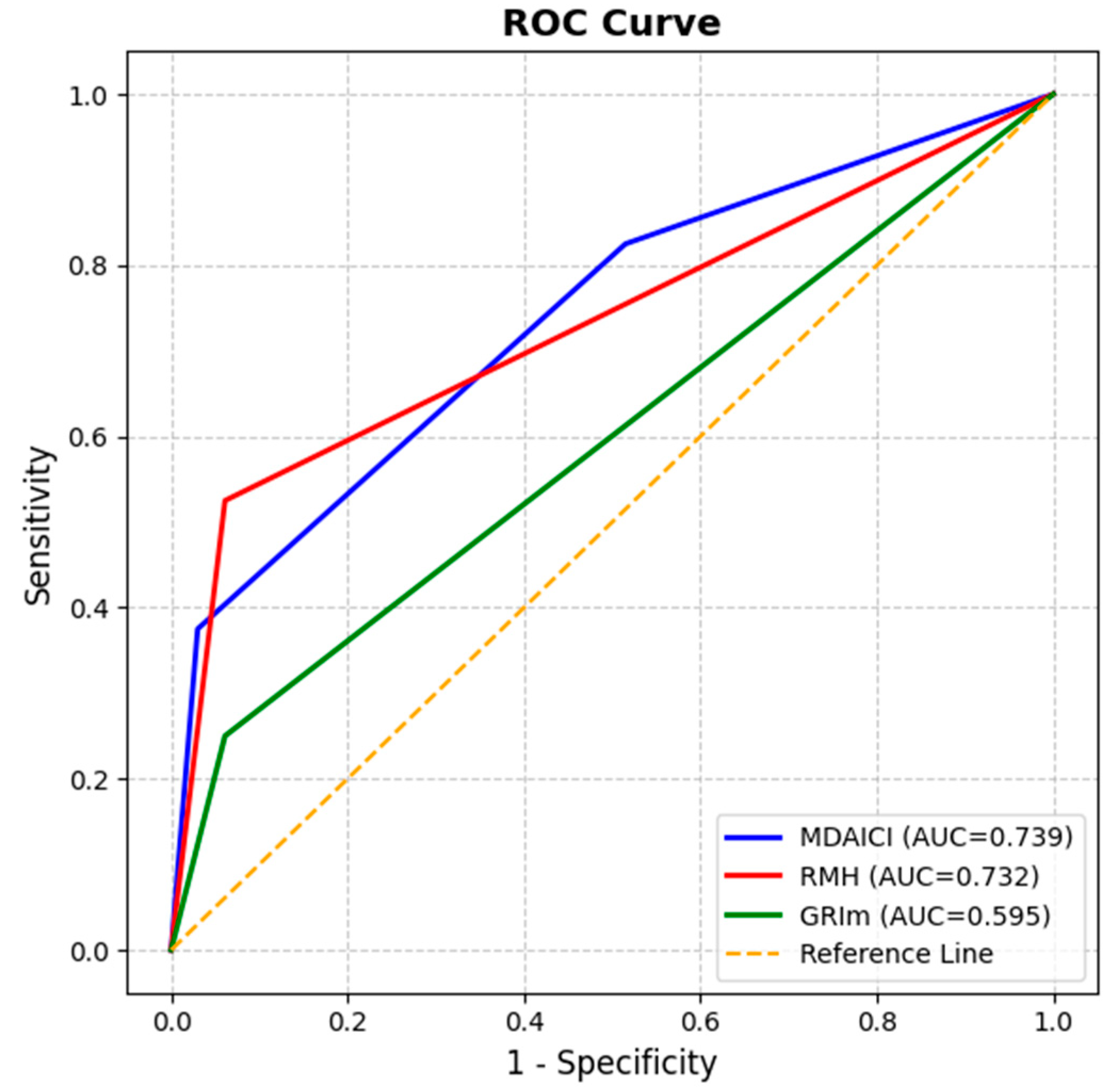
3.2. Prognostic Performance of Clinical Risk Scores
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Seth, R.; Agarwala, S.S.; Messersmith, H.; Alluri, K.C.; Ascierto, P.A.; Atkins, M.B.; Bollin, K.; Chacon, M.; Davis, N.; Faries, M.B.; et al. Systemic Therapy for Melanoma: ASCO Guideline Update. J. Clin. Oncol. 2023, 41, 4794–4820. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Robert, C.; Grob, J.J.; Stroyakovskiy, D.; Karaszewska, B.; Hauschild, A.; Levchenko, E.; Chiarion Sileni, V.; Schachter, J.; Garbe, C.; Bondarenko, I.; et al. Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. N. Engl. J. Med. 2019, 381, 626–636. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef]
- Robert, C.; Carlino, M.S.; McNeil, C.; Ribas, A.; Grob, J.J.; Schachter, J.; Nyakas, M.; Kee, D.; Petrella, T.M.; Blaustein, A.; et al. Seven-Year Follow-Up of the Phase III KEYNOTE-006 Study: Pembrolizumab Versus Ipilimumab in Advanced Melanoma. J. Clin. Oncol. 2023, 41, 3998–4003. [Google Scholar] [CrossRef]
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Chew, V.; Toh, H.C.; Abastado, J.-P. Immune Microenvironment in Tumor Progression: Characteristics and Challenges for Therapy. J. Oncol. 2012, 2012, 608406. [Google Scholar] [CrossRef]
- Zhong, J.H.; Huang, D.H.; Chen, Z.Y. Prognostic role of systemic immune-inflammation index in solid tumors: A systematic review and meta-analysis. Oncotarget 2017, 8, 75381–75388. [Google Scholar] [CrossRef] [PubMed]
- Mallardo, D.; Fordellone, M.; White, A.; Ottaviano, M.; Sparano, F.; Bailey, M.; Facchini, A.B.; Ong, S.; Maiolino, P.; Caracò, C.; et al. CD39 and LDHA affects the prognostic role of NLR in metastatic melanoma patients treated with immunotherapy. J. Transl. Med. 2023, 21, 610. [Google Scholar] [CrossRef]
- Fucà, G.; Beninato, T.; Bini, M.; Mazzeo, L.; Di Guardo, L.; Cimminiello, C.; Randon, G.; Apollonio, G.; Bisogno, I.; Del Vecchio, M.; et al. The Pan-Immune-Inflammation Value in Patients with Metastatic Melanoma Receiving First-Line Therapy. Target Oncol. 2021, 16, 529–536. [Google Scholar] [CrossRef]
- Mesti, T.; Grašič Kuhar, C.; Ocvirk, J. Biomarkers for Outcome in Metastatic Melanoma in First Line Treatment with Immune Checkpoint Inhibitors. Biomedicines 2023, 11, 749. [Google Scholar] [CrossRef]
- Capone, M.; Giannarelli, D.; Mallardo, D.; Madonna, G.; Festino, L.; Grimaldi, A.M.; Vanella, V.; Simeone, E.; Paone, M.; Palmieri, G.; et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J. Immunother. Cancer 2018, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, P.F.; Ascierto, P.A.; Pigozzo, J.; Del Vecchio, M.; Maio, M.; Antonini Cappellini, G.C.; Guidoboni, M.; Queirolo, P.; Savoia, P.; Mandalà, M.; et al. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: Prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann. Oncol. 2016, 27, 732–738. [Google Scholar] [CrossRef]
- Claps, G.; Faouzi, S.; Quidville, V.; Chehade, F.; Shen, S.; Vagner, S.; Robert, C. The multiple roles of LDH in cancer. Nat. Rev. Clin. Oncol. 2022, 19, 749–762. [Google Scholar] [CrossRef]
- Knispel, S.; Gassenmaier, M.; Menzies, A.M.; Loquai, C.; Johnson, D.B.; Franklin, C.; Gutzmer, R.; Hassel, J.C.; Weishaupt, C.; Eigentler, T.; et al. Outcome of melanoma patients with elevated LDH treated with first-line targeted therapy or PD-1-based immune checkpoint inhibition. Eur. J. Cancer 2021, 148, 61–75. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, S.; Qiao, J. Prognostic value of neutrophil-to-lymphocyte ratio in melanoma: Evidence from a PRISMA-compliant meta-analysis. Medicine 2018, 97, e11446. [Google Scholar] [CrossRef]
- McKean, M.A.; Haydu, L.E.; Ma, J.; Bassett, R.L.; Hwu, W.J.; Patel, S.P.; Diab, A.; Glitza, I.C.; Tawbi, H.A.H.; Wong, M.K.; et al. Prognostic factors for overall survival (OS) in metastatic melanoma (MM) patients (pts) treated with immune checkpoint inhibitors: A single institution study of 696 pts. J. Clin. Oncol. 2017, 35 (Suppl. 15), 9574. [Google Scholar] [CrossRef]
- Hu, H.P.; Archer, C.; Yip, D.; Peters, G. Clinical predictors of survival in real world practice in stage IV melanoma. Cancer Rep. 2023, 6, e1691. [Google Scholar] [CrossRef]
- Palmer, S.R.; Erickson, L.A.; Ichetovkin, I.; Knauer, D.J.; Markovic, S.N. Circulating serologic and molecular biomarkers in malignant melanoma. Mayo Clin. Proc. 2011, 86, 981–990. [Google Scholar] [CrossRef]
- Arkenau, H.T.; Olmos, D.; Ang, J.E.; de Bono, J.; Judson, I.; Kaye, S. Clinical outcome and prognostic factors for patients treated within the context of a phase I study: The Royal Marsden Hospital experience. Br. J. Cancer 2008, 98, 1029–1033. [Google Scholar] [CrossRef]
- Wheler, J.; Tsimberidou, A.M.; Hong, D.; Naing, A.; Falchook, G.; Piha-Paul, S.; Fu, S.; Moulder, S.; Stephen, B.; Wen, S.; et al. Survival of 1,181 Patients in a Phase I Clinic: The MD Anderson Clinical Center for Targeted Therapy Experience. Clin. Cancer Res. 2012, 18, 2922–2929. [Google Scholar] [CrossRef]
- Bigot, F.; Castanon, E.; Baldini, C.; Hollebecque, A.; Carmona, A.; Postel-Vinay, S.; Angevin, E.; Armand, J.P.; Ribrag, V.; Aspeslagh, S.; et al. Prospective validation of a prognostic score for patients in immunotherapy phase I trials: The Gustave Roussy Immune Score (GRIm-Score). Eur. J. Cancer 2017, 84, 212–218. [Google Scholar] [CrossRef]
- Sen, S.; Hess, K.; Hong, D.S.; Naing, A.; Piha-Paul, S.; Janku, F.; Fu, S.; Subbiah, I.M.; Liu, H.; Khanji, R.; et al. Development of a prognostic scoring system for patients with advanced cancer enrolled in immune checkpoint inhibitor phase 1 clinical trials. Br. J. Cancer 2018, 118, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Acar, C.; Yüksel, H.Ç.; Şahin, G.; Açar, F.P.; Tünbekici, S.; Çelebi, G.; Karaca, B. Efficacy and prognostic factors of anti-PD1 and nivolumab-ipilimumab therapy in advanced melanoma patients resistant to prior ICI treatment. Discov. Oncol. 2024, 15, 813. [Google Scholar] [CrossRef] [PubMed]
- Minami, S.; Ihara, S.; Ikuta, S.; Komuta, K. Gustave Roussy Immune Score and Royal Marsden Hospital Prognostic Score Are Biomarkers of Immune-Checkpoint Inhibitor for Non-Small Cell Lung Cancer. World J. Oncol. 2019, 10, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Sahin, T.K.; Rizzo, A.; Aksoy, S.; Guven, D.C. Prognostic Significance of the Royal Marsden Hospital (RMH) Score in Patients with Cancer: A Systematic Review and Meta-Analysis. Cancers 2024, 16, 1835. [Google Scholar] [CrossRef]
- Zhu, Y.; Song, B.; Yang, Z.; Peng, Y.; Cui, Z.; Chen, L.; Song, B. Integrative lactylation and tumor microenvironment signature as prognostic and therapeutic biomarkers in skin cutaneous melanoma. J. Cancer Res. Clin. Oncol. 2023, 149, 17897–17919. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, Q.; Lan, L.; Xu, X. PANoptosis-related prognostic signature predicts overall survival of cutaneous melanoma and provides insights into immune infiltration landscape. Sci. Rep. 2023, 13, 8449. [Google Scholar] [CrossRef]
- Stukalin, I.; Navani, V.; Gupta, M.; Ruan, Y.; Boyne, D.J.; O’Sullivan, D.E.; Meyers, D.E.; Goutam, S.; Sander, M.; Ewanchuk, B.W.; et al. Development and Validation of a Prognostic Risk Model for Patients with Advanced Melanoma Treated with Immune Checkpoint Inhibitors. Oncologist 2023, 28, 812–822. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Ye, T.; Dong, Y.; Zhang, W.; Wu, F.; Bo, H.; Shao, H.; Zhang, R.; Shen, H. Prognostic modeling of patients with metastatic melanoma based on tumor immune microenvironment characteristics. Math. Biosci. Eng. MBE 2022, 19, 1448–1470. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.X.; Espin-Garcia, O.; Bach, Y.; Aoyama, H.; Allen, M.J.; Wang, X.; Darling, G.E.; Yeung, J.; Swallow, C.J.; Brar, S.; et al. Comparison of Four Clinical Prognostic Scores in Patients with Advanced Gastric and Esophageal Cancer. Oncologist 2022, 28, 214–219. [Google Scholar] [CrossRef]
- Minichsdorfer, C.; Gleiss, A.; Aretin, M.B.; Schmidinger, M.; Fuereder, T. Serum parameters as prognostic biomarkers in a real world cancer patient population treated with anti PD-1/PD-L1 therapy. Ann. Med. 2022, 54, 1339–1349. [Google Scholar] [CrossRef]
- Shangguan, J.; Huang, X.; Liu, X.; Zhang, Z.; Zhang, X.; Yu, J.; Chen, D. Gustave Roussy immune score is a prognostic marker in patients with small cell lung cancer undergoing immunotherapy: A real-world retrospective study. Front. Oncol. 2023, 13, 1195499. [Google Scholar] [CrossRef] [PubMed]
- Nieder, C.; Marienhagen, K.; Geinitz, H.; Grosu, A.L. Can current prognostic scores reliably guide treatment decisions in patients with brain metastases from malignant melanoma? J. Cancer Res. Ther. 2011, 7, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Al Darazi, G.; Martin, E.; Delord, J.-P.; Korakis, I.; Betrian, S.; Estrabaut, M.; Poublanc, M.; Gomez-Roca, C.; Filleron, T. Improving patient selection for immuno-oncology phase 1 trials: External validation of six prognostic scores in a French Cancer Center. Int. J. Cancer. 2021, 148, 2502–2511. [Google Scholar] [CrossRef]
- García-Corbacho, J.; Indacochea, A.; Victoria, I.; Moreno, D.; Angelats, L.; González Navarro, A.E.; Mezquita, L.; Brasó-Maristany, F.; Galván, P.; Mellado, B.; et al. Blood-based prognostic scores and early dynamics under immunotherapy to select patients with metastatic solid tumors for continuing immune check-point inhibition: A prospective longitudinal study. Cancer Immunol. Immunother. 2025, 74, 85. [Google Scholar] [CrossRef]
- Grad, R.N.; Jung, S.; Ye, F.; Sun, L.; Johnson, D.B.; Agarwal, R. Prognostic Risk Stratification and End-of-Life Care Outcomes in Patients with Metastatic Melanoma Treated with Immune Checkpoint Inhibitors. Oncologist 2023, 28, 911–916. [Google Scholar] [CrossRef]
- Diem, S.; Kasenda, B.; Martin-Liberal, J.; Lee, A.; Chauhan, D.; Gore, M.; Larkin, J. Prognostic score for patients with advanced melanoma treated with ipilimumab. Eur. J. Cancer 2015, 51, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
| Prognostic Scores | Components | Risk |
|---|---|---|
| Royal Marsden Hospital score (RMH) | >2 metastatic sites = 1 point Serum albumin < 35 g/L = 1 point LDH > ULN = 1 point | 0–1: Low risk 2–3: High risk |
| Gustave Roussy Immune Score (GRIm) | Serum albumin < 35 g/L = 1 point LDH > ULN = 1 point NLR > 6 = 1 point | 0–1: Low risk 2–3: High risk |
| MD Anderson Immune Checkpoint Inhibitor Score (MDA-ICI) | Age > 52 years = 1 point ECOG > 1 = 1 point LDH > 0.75 × ULN = 1 point Platelets > 300 (103/mL) = 1 point ANC > 4.9(103/mL) = 1 point ALC < 1.8 (103/mL) = 1 point Liver metastases = 1 point | 0–2: Low risk 3–4: Intermediate risk 5–7: High risk |
| Variables | N (%) |
|---|---|
| Age, median, IQR | 64 (52.5–74) |
| <65 | 38 (52.1) |
| >65 | 35 (47.9) |
| Sex | |
| Female | 34 (46.6) |
| Male | 39 (53.4) |
| ECOG PS | |
| 0–1 | 53 (72.6) |
| 2–3 | 20 (27.4) |
| BRAF mutation | 16 (21.9) |
| Denovo metastatic disease | 32 (43.8) |
| Metastatic sites number | |
| 1–2 | 53 (72.6) |
| >2 | 20 (27.4) |
| Metastatic site | |
| Lymph node | 53 (72.6) |
| Lung | 34 (46.6) |
| Liver | 18 (24.7) |
| Brain | 17 (23.3) |
| Bone | 20 (27.4) |
| Type of immunotherapy | |
| Nivolumab | 59 (80.8) |
| Ipilumumab + Nivolumab | 14 (19.2) |
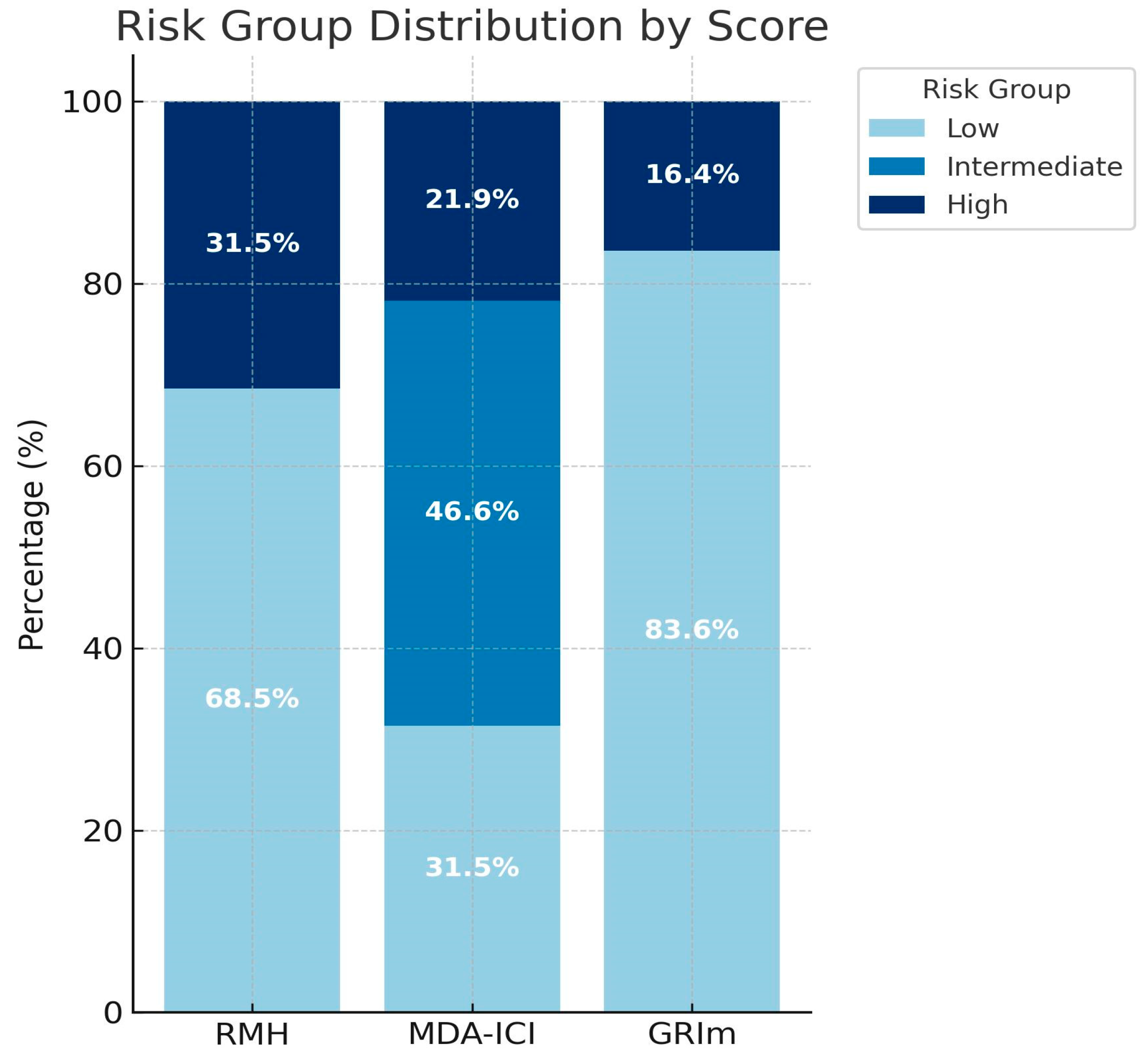
| Risk group distribution | |
| RMH score | |
| Low risk | 50 (68.8) |
| High risk | 23 (31.5) |
| MDA-ICI score | |
| Low risk | 23 (31.5) |
| Intermediate risk | 34 (46.6) |
| High risk | 16 (21.9) |
| GRIm score | |
| Low risk | 61 (83.6) |
| High risk | 12 (16.4) |
| Prognostic Score | HR (95% CI) | p-Value (Cox) * | Harrell’s C-Index | AUC (95% CI) | p-Value (ROC)* |
|---|---|---|---|---|---|
| RMH (high vs. low) | 5.45 (2.13–13.96) | <0.001 | 0.742 | 0.732 (0.616–0.848) | 0.001 |
| MDA-ICI (int. vs. low) (high vs. low) | 1.62 (0.60–4.40) 4.24 (1.33–13.55) | 0.339 0.015 | 0.730 | 0.739 (0.626–0.852) | <0.001 |
| GRIm (high vs. low) | 0.75 (0.30–1.84) | 0.532 | 0.615 | 0.595 (0.465–0.725) | 0.166 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yildiran Keskin, G.S.; Karadurmus, N. A Real-World Evaluation of Clinical Prognostic Scores in Advanced Melanoma Treated with Immune Checkpoint Inhibitors. J. Clin. Med. 2025, 14, 6452. https://doi.org/10.3390/jcm14186452
Yildiran Keskin GS, Karadurmus N. A Real-World Evaluation of Clinical Prognostic Scores in Advanced Melanoma Treated with Immune Checkpoint Inhibitors. Journal of Clinical Medicine. 2025; 14(18):6452. https://doi.org/10.3390/jcm14186452
Chicago/Turabian StyleYildiran Keskin, Gul Sema, and Nuri Karadurmus. 2025. "A Real-World Evaluation of Clinical Prognostic Scores in Advanced Melanoma Treated with Immune Checkpoint Inhibitors" Journal of Clinical Medicine 14, no. 18: 6452. https://doi.org/10.3390/jcm14186452
APA StyleYildiran Keskin, G. S., & Karadurmus, N. (2025). A Real-World Evaluation of Clinical Prognostic Scores in Advanced Melanoma Treated with Immune Checkpoint Inhibitors. Journal of Clinical Medicine, 14(18), 6452. https://doi.org/10.3390/jcm14186452







