Evaluation of the Meet-URO Score in a Real-World Cohort of mRCC Patients Treated with First-Line TKIs
Abstract
1. Introduction
2. Material and Methods
2.1. Patient Characteristics and Study Design
2.2. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yanagisawa, T.; Mori, K.; Matsukawa, A.; Kawada, T.; Katayama, S.; Bekku, K.; Laukhtina, E.; Rajwa, P.; Quhal, F.; Pradere, B.; et al. Updated systematic review and network meta-analysis of first-line treatments for metastatic renal cell carcinoma with extended follow-up data. Cancer Immunol. Immunother. 2024, 73, 38. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.J.; Sura, S.D.; Shinde, R.; Shi, J.; Singhal, P.K.; Robert, N.J.; Vogelzang, N.J.; Perini, R.F.; Motzer, R.J. Real-world Treatment Patterns and Clinical Outcomes for Metastatic Renal Cell Carcinoma in the Current Treatment Era. Eur. Urol. Open Sci. 2023, 49, 110–118. [Google Scholar] [CrossRef]
- Goebell, P.J.; Boegemann, M.; Nusch, A.; Grünwald, V.; Mueller, L.; von der Heyde, E.; Reichert, D.; Martens, U.M.; Lennartz, C.; Koska, M.; et al. Comparison of TKI and CPI strategies as first-line treatment of patients with advanced renal cell carcinoma: Real-world outcome data from the German research platform CARAT. J. Clin. Oncol. 2024, 42 (Suppl. S16), 4528. [Google Scholar] [CrossRef]
- Castro, D.V.; Gebrael, G.; Meza, L.; Li, X.; Tripathi, N.; Tandar, C.; Sayegh, N.; Zengin, Z.B.; Chehrazi-Raffle, A.; Govindarajan, A.; et al. Impact of insurance status on survival outcomes in patients with metastatic renal cell carcinoma. JCO Oncol. Adv. 2025, 2, e2400015. [Google Scholar] [CrossRef]
- Barragan-Carrillo, R.; Saad, E.; Saliby, R.-M.; Sun, M.; Albiges, L.; Bex, A.; Heng, D.; Mejean, A.; Motzer, R.J.; Plimack, E.R.; et al. First and Second-line Treatments in Metastatic Renal Cell Carcinoma. Eur. Urol. 2025, 87, 143–154. [Google Scholar] [CrossRef]
- Heng, D.Y.C.; Xie, W.; Regan, M.M.; Warren, M.A.; Golshayan, A.R.; Sahi, C.; Eigl, B.J.; Ruether, J.D.; Cheng, T.; North, S.; et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: Results from a large, multicenter study. J. Clin. Oncol. 2009, 27, 5794–5799. [Google Scholar] [CrossRef]
- Rebuzzi, S.E.; Signori, A.; Banna, G.L.; Maruzzo, M.; De Giorgi, U.; Pedrazzoli, P.; Sbrana, A.; Zucali, P.A.; Masini, C.; Naglieri, E.; et al. Inflammatory indices and clinical factors in metastatic renal cell carcinoma patients treated with nivolumab: The development of a novel prognostic score (Meet-URO 15 study). Ther. Adv. Med. Oncol. 2021, 13, 17588359211019642. [Google Scholar] [CrossRef] [PubMed]
- Rebuzzi, S.E.; Signori, A.; Buti, S.; Banna, G.L.; Murianni, V.; Damassi, A.; Maruzzo, M.; Giannarelli, D.; Tortora, G.; Galli, L.; et al. Validation of the Meet-URO score in patients with metastatic renal cell carcinoma receiving first-line nivolumab and ipilimumab in the Italian Expanded Access Program. ESMO Open 2022, 7, 100634. [Google Scholar] [CrossRef] [PubMed]
- Damassi, A.; Cremante, M.; Signori, A.; Rebuzzi, S.E.; Fornarini, G.; Giudice, G.C.; Maruzzo, M.; Procopio, G.; Sorarù, M.; Di Napoli, M.; et al. Prognostic Stratification by the Meet-URO Score in Real-World Older Patients With Metastatic Renal Cell Carcinoma (mRCC) Receiving Cabozantinib: A Subanalysis of the Prospective ZEBRA Study (Meet-URO 9). Clin. Genitourin. Cancer 2024, 22, 126–133.e2. [Google Scholar] [CrossRef]
- Bimbatti, D.; Pierantoni, F.; Lai, E.; Ballestrin, M.; Cavasin, N.; Erbetta, E.; De Toni, C.; Basso, U.; Maruzzo, M. Advanced Non-Clear Cell Renal Cell Carcinoma Treatments and Survival: A Real-World Single-Centre Experience. Cancers 2023, 15, 4353. [Google Scholar] [CrossRef]
- Ghose, A.; Signori, A.; Brown, N.; Haywood, S.; Tapia, J.; Vijay, A.; Cheung, M.; Mahajan, I.; Fiala, O.; Abrol, R.; et al. External validation of the novel prognostic Meet-URO score in Metastatic Renal Cell Carcinoma on First Line Immune-combination therapy [IUC20761-50]. Int. Urol. Cancer Summit, 2024. Available online: https://urologycancersummit.org/external-validation-of-the-novel-prognostic-meet-uro-score-in-metastatic-renal-cell-carcinoma-on-first-line-immune-combination-therapy/ (accessed on 3 August 2025).
- He, S.; Liu, H.; Chen, J.; Sun, G.; Liu, Z.; Zeng, H. The prognostic value of Meet-URO score in patients with metastatic clear cell renal cell carcinoma receiving second or third-line tyrosine kinase inhibitors/immune checkpoint inhibitors combination therapy: A real-world study. J. Clin. Oncol. 2024, 42 (Suppl. S16), e16516. [Google Scholar] [CrossRef]
- Powles, T.; Albiges, L.; Bex, A.; Comperat, E.; Grünwald, V.; Kanesvaran, R.; Kitamura, H.; McKay, R.; Porta, C.; Procopio, G.; et al. Renal cell carcinoma: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2024, 35, 692–706. [Google Scholar] [CrossRef] [PubMed]
- Bex, A.; Ghanem, Y.A.; Albiges, L.; Bonn, S.; Campi, R.; Capitanio, U.; Dabestani, S.; Hora, M.; Klatte, T.; Kuusk, T.; et al. European association of urology guidelines on renal cell carcinoma: The 2025 update. Eur. Urol. 2025, 87, 683–696. [Google Scholar] [CrossRef]
- Rebuzzi, S.E.; Cerbone, L.; Signori, A.; Santoni, M.; Murianni, V.; De Giorgi, U.; Procopio, G.; Porta, C.; Milella, M.; Basso, U.; et al. Application of the Meet-URO score to metastatic renal cell carcinoma patients treated with second- and third-line cabozantinib. Ther. Adv. Med. Oncol. 2022, 14, 17588359221079580. [Google Scholar] [CrossRef]
- Murianni, V.; Signori, A.; Buti, S.; Rebuzzi, S.E.; Bimbatti, D.; De Giorgi, U.; Chiellino, S.; Galli, L.; Zucali, P.A.; Masini, C.; et al. Time to strategy failure and treatment beyond progression in pretreated metastatic renal cell carcinoma patients receiving nivolumab: Post-hoc analysis of the Meet-URO 15 study. Front. Oncol. 2024, 14, 1307635. [Google Scholar] [CrossRef]
- Brown, J.; Santini, D.; Charnley, N.; Ogareva, A.; Chisholm, A.; Jones, R. Implications of bone metastasis on response to systemic therapy in patients with advanced renal cell carcinoma: A systematic literature review. Cancer Treat. Rev. 2024, 129, 102792. [Google Scholar] [CrossRef] [PubMed]
- Roviello, G.; Molina-Cerrillo, J.; Massari, F.; Cerbone, L.; Fiala, O.; Fornarini, G.; Monteiro, F.S.M.; Cattrini, C.; Landmesser, J.; Messina, C.; et al. First-line immune-based combinations or sunitinib in favorable-risk metastatic renal cell carcinoma: A real-world retrospective comparison from the ARON-1 study. Cancer Immunol. Immunother. 2025, 74, 65. [Google Scholar] [CrossRef] [PubMed]
- Bersanelli, M.; Buti, S.; Ghidini, A.; Tiseo, M.; Petrelli, F. A metanalysis on cabozantinib and bone metastases: True story or commercial gimmick? Anti-Cancer Drugs 2020, 31, 211–215. [Google Scholar] [CrossRef]
- Kang, M.; Choi, J.; Kim, J.; Sung, H.H.; Jeon, H.G.; Jeong, B.C.; Jeon, S.S.; Lee, H.M.; Park, S.H.; Song, C.; et al. Prognostic Impact of Bone Metastasis on Survival Outcomes in Patients with Metastatic Renal Cell Carcinoma Treated by First Line Tyrosine Kinase Inhibitors: A Propensity-Score Matching Analysis. J. Cancer 2020, 11, 7202–7208. [Google Scholar] [CrossRef] [PubMed]
- Lou, K.; Cheng, X. Prognostic value of the neutrophil-to-lymphocyte ratio in renal cell carcinoma: A systematic review and meta-analysis. Oncol. Lett. 2025, 29, 231. [Google Scholar] [CrossRef]
- Young, M.; Tapia, J.C.; Szabados, B.; Jovaisaite, A.; Jackson-Spence, F.; Nally, E.; Powles, T. NLR Outperforms Low Hemoglobin and High Platelet Count as Predictive and Prognostic Biomarker in Metastatic Renal Cell Carcinoma Treated with Immune Checkpoint Inhibitors. Clin. Genitourin. Cancer 2024, 22, 102072. [Google Scholar] [CrossRef]
- Angulo, J.C.; Larrinaga, G.; Lecumberri, D.; Iturregui, A.M.; Solano-Iturri, J.D.; Lawrie, C.H.; Armesto, M.; Dorado, J.F.; Nunes-Xavier, C.E.; Pulido, R.; et al. Predicting Survival of Metastatic Clear Cell Renal Cell Cancer Treated with VEGFR-TKI-Based Sequential Therapy. Cancers 2024, 16, 2786. [Google Scholar] [CrossRef]
- Sobu, R.; Numakura, K.; Naito, S.; Hatakeyama, S.; Kato, R.; Koguchi, T.; Kojima, T.; Kawasaki, Y.; Kandori, S.; Kawamura, S.; et al. Clinical impact of early response to first-line VEGFR-TKI in patients with metastatic renal cell carcinoma on survival: A multi-institutional retrospective study. Cancer Med. 2023, 12, 4100–4109. [Google Scholar] [CrossRef] [PubMed]
- Rebuzzi, S.E.; Fornarini, G.; Signori, A.; Buti, S.; Procopio, G.; De Giorgi, U.; Pignata, S.; Naglieri, E.; Maruzzo, M.; Banna, G.L.; et al. International multicenter real-world REGistry for patients with metastatic renAL cell carcinoma—Meet-URO 33 study (REGAL study). BMC Cancer 2024, 24, 757. [Google Scholar] [CrossRef]
- Özalp, F.R.; Yörükoğlu, K.; Yıldırım, E.Ç.; Uzun, M.; Semiz, H.S. Prognostic value of B7-H3 expression in metastatic renal cell carcinoma and its impact on immunotherapy response. BMC Cancer 2024, 24, 1471. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Banchereau, R.; Hamidi, H.; Powles, T.; McDermott, D.; Atkins, M.B.; Escudier, B.; Liu, L.-F.; Leng, N.; Abbas, A.R.; et al. Molecular subsets in renal cancer determine outcome to checkpoint and angiogenesis blockade. Cancer Cell 2020, 38, 803–817.e4. [Google Scholar] [CrossRef]


| Meet-URO Score Prognostic Group | |||||
|---|---|---|---|---|---|
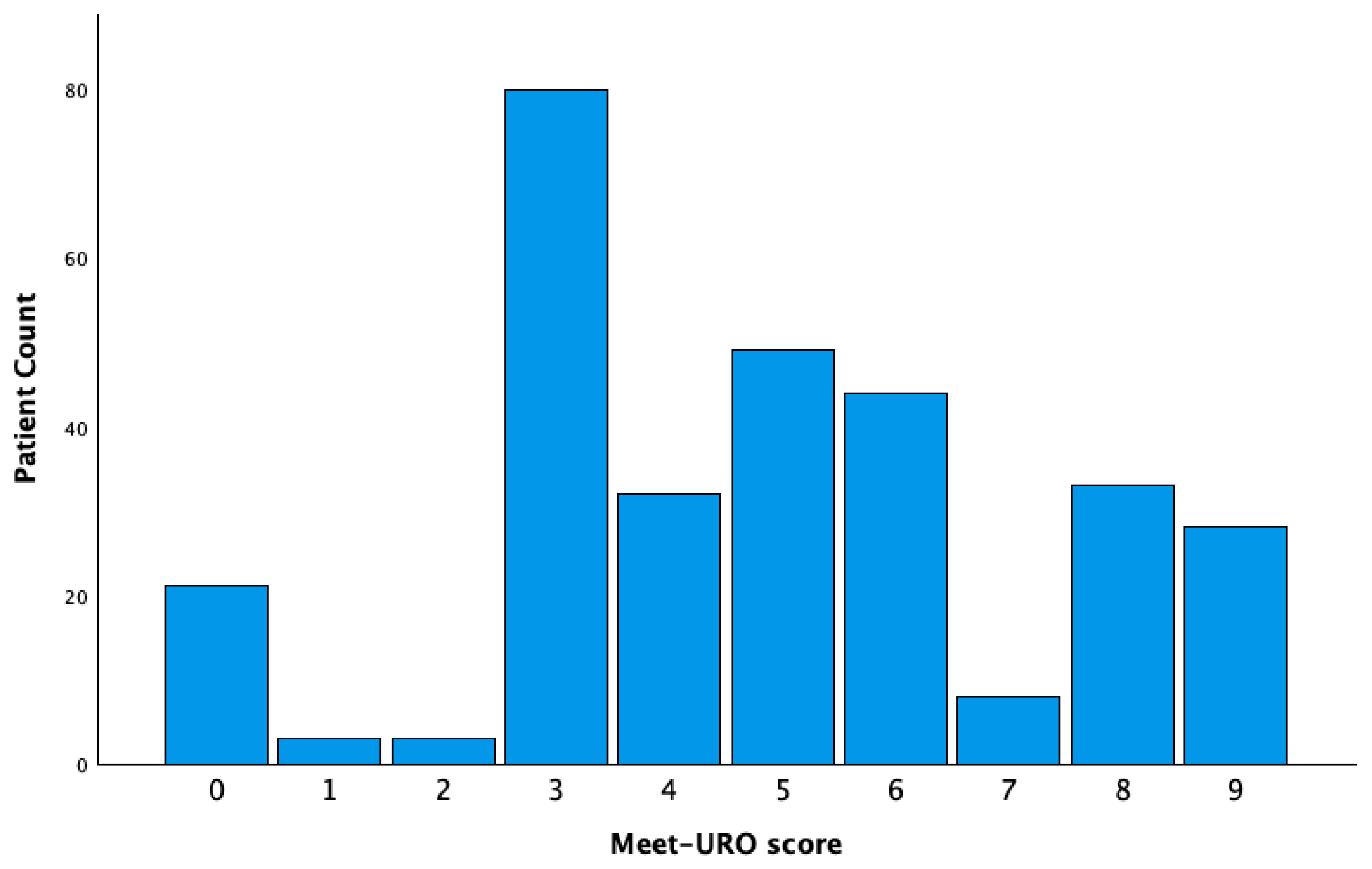
| Characteristics | All Cohort (n = 301, 100%) | 0–4 (n = 139, 46.2%) | 5–8 (n = 134, 44.5%) | 9 (n = 28, 9.3%) | p *-Value |
| Sex | |||||
| Female | 89 (29.6) | 37 (26.6) | 43 (32.1) | 9 (32.1) | |
| Male | 212 (70.4) | 102 (73.4) | 91 (67.9) | 19 (67.9) | 0.58 |
| Age group | |||||
| <70 | 236 (78.4) | 114 (82.0) | 99 (73.9) | 23 (82.1) | |
| ≥70 | 65 (21.6) | 25 (18.0) | 35 (26.1) | 5 (17.9) | 0.23 |
| Karnofsky PS | |||||
| <80% | 48 (15.9) | 13 (9.4) | 25 (18.7) | 10 (35.7) | |
| ≥80% | 253 (84.1) | 126 (90.6) | 109 (81.3) | 18 (64.3) | 0.001 |
| Histologic subtype | |||||
| Clear cell | 246 (81.7) | 117 (84.2) | 109 (81.3) | 20 (71.4) | |
| Non-clear cell | 55 (18.3) | 22 (15.8) | 25 (18.7) | 8 (28.6) | 0.28 |
| Nephrectomy | |||||
| No | 111 (36.9) | 39 (28.1) | 53 (39.6) | 19 (67.9) | |
| Yes | 190 (63.1) | 100 (71.9) | 81 (60.4) | 9 (32.1) | <0.001 |
| Metastatic at diagnosis | |||||
| Recurrent | 123 (40.9) | 70 (50.4) | 50 (37.3) | 3 (10.7) | |
| De-novo | 178 (59.1) | 69 (49.6) | 84 (62.7) | 25 (89.3) | <0.001 |
| IMDC risk group | |||||
| Good | 31 (10.3) | 31 (22.3) | 0 (0.0) | 0 (0.0) | |
| Intermediate | 185 (61.5) | 108 (77.7) | 77 (57.5) | 0 (0.0) | |
| Poor | 85 (28.2) | 0 (0.0) | 57 (42.5) | 28 (100.0) | <0.001 |
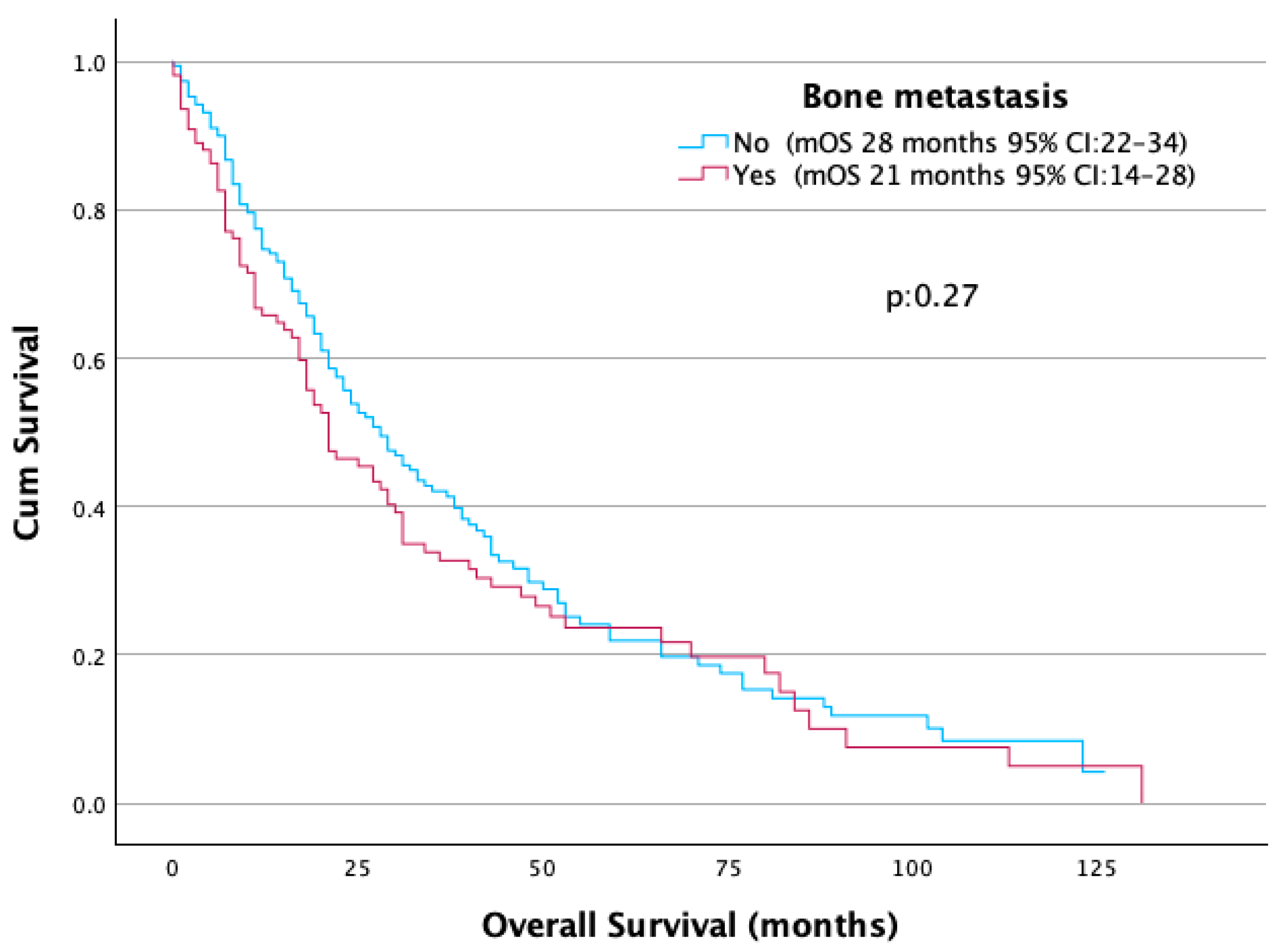
| Bone metastasis | |||||
| No | 191 (63.5) | 96 (69.1) | 95 (70.5) | 0 (0.0) | |
| Yes | 110 (36.5) | 43 (30.9) | 39 (29.1) | 28 (100.0) | <0.001 |
| Brain metastasis | |||||
| No | 277 (92.0) | 125 (89.9) | 125 (93.3) | 27 (96.4) | |
| Yes | 24 (8.0) | 14 (10.1) | 9 (6.7) | 1 (3.6) | 0.39 |
| Liver metastasis | |||||
| No | 236 (78.4) | 109 (78.4) | 106 (79.1) | 21 (75.0) | |
| Yes | 65 (21.6) | 30 (21.6) | 28 (20.9) | 7 (25.0) | 0.89 |
| Sarcomatoid differentiation | |||||
| No | 284 (94.4) | 135 (97.1) | 123 (91.8) | 26 (92.9) | |
| Yes | 17 (5.6) | 4 (2.9) | 11 (8.2) | 2 (7.1) | 0.15 |
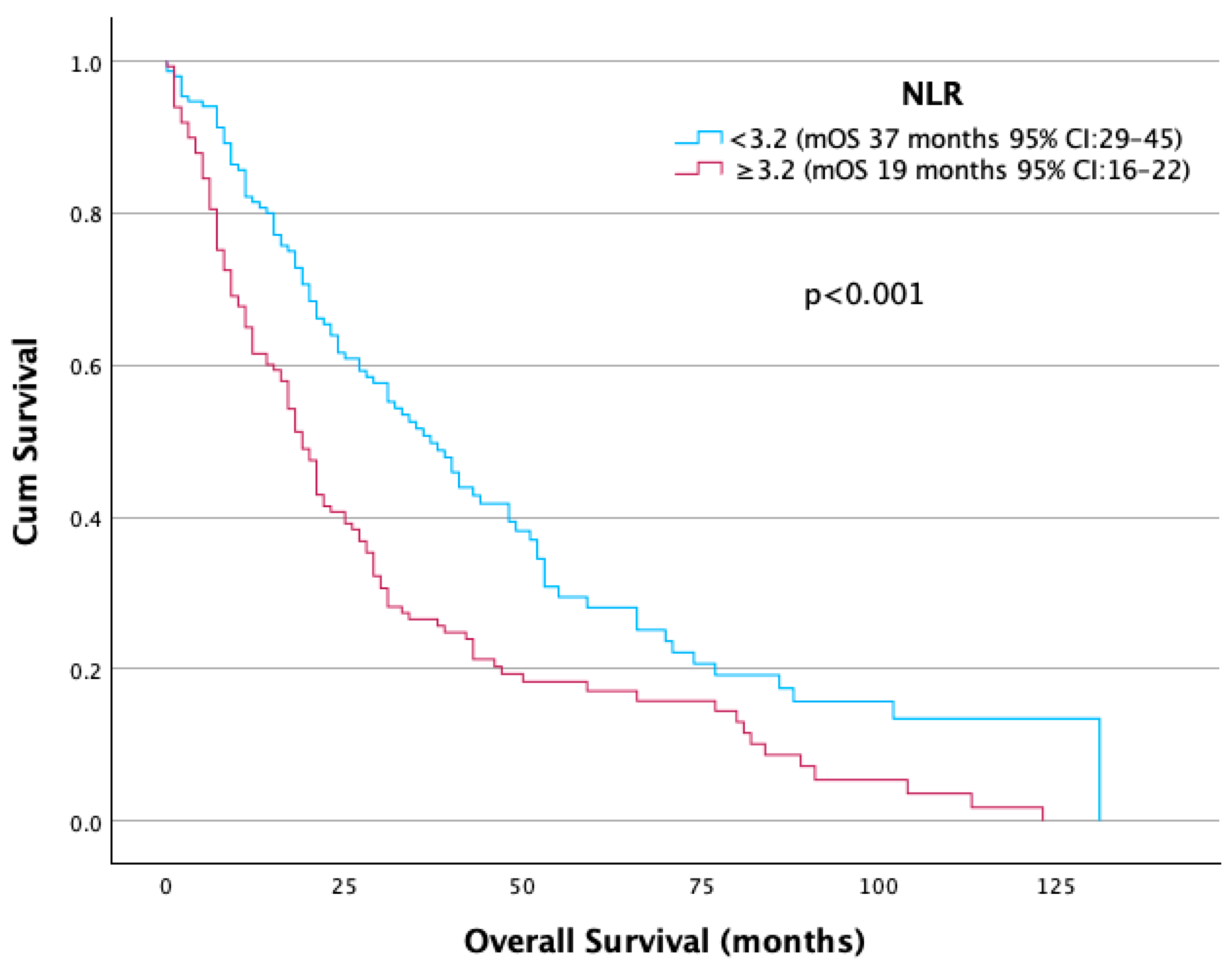
| NLR | |||||
| <3.2 | 152 (50.5) | 128 (92.1) | 24 (17.9) | 0 (0.0) | |
| ≥3.2 | 149 (49.5) | 11 (7.9) | 110 (82.1) | 28 (100) | <0.001 |
| First-Line Treatment | |||||
| Sunitinib | 145 (48.2) | 76 (54.7) | 56 (41.8) | 13 (46.4) | |
| Pazopanib | 125 (41.5) | 53 (38.1) | 60 (44.8) | 12 (42.9) | |
| Cabozantinib | 31 (10.3) | 10 (7.2) | 18 (13.4) | 3 (10.7) | 0.22 |
| Prognostic Factors | Values | mOS | HR | p-Value |
|---|---|---|---|---|
| IMDC score | Favorable | 48 | 1 | <0.001 |
| Intermediate | 30 | 1.96 (1.09–3.55) | 0.025 | |
| Poor | 11 | 4.37 (2.37–8.07) | <0.001 | |
| NLR | <3.2 | 37 | 1 | |
| ≥3.2 | 19 | 1.77 (1.35–2.31) | <0.001 | |
| Bone metastasis | Yes | 21 | 1.17 (0.89–1.53) | 0.27 |
| No | 28 | 1 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value * | HR (95% CI) | p-Value * | |
| Age (<70 vs. ≥70) | 0.99 (0.72–1.38) | 0.98 | ||
| Gender (woman vs. man) | 0.83 (0.63–1.11) | 0.21 | ||
| De-novo vs. relapse | 1.27 (0.97–1.66) | 0.08 | ||
| Meet-URO risk group | <0.001 | <0.001 | ||
| (1 vs. 2) | 1.87 (1.4–2.48) | <0.001 | 1.73 (1.29–2.32) | <0.001 |
| (1 vs. 3) | 4.0 (2.58–6.31) | <0.001 | 3.57 (2.25–5.65) | <0.001 |
| Nephrectomy (yes or no) | 0.52 (0.41–0.71) | <0.001 | 0.59 (0.45–0.8) | <0.001 |
| Histology (clear cell vs. non clear cell) | 1.12 (0.79–1.59) | 0.51 | ||
| First-line treatment | 0.057 | 0.17 | ||
| (sunitinib vs. pazopanib) | 1.24 (0.94–1.64) | 0.13 | 1.15 (0.87–1.5) | 0.33 |
| (sunitinib vs. cabozantinib) | 1.86 (1.06–3.24) | 0.03 | 1.7 (0.96–2.98) | 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özalp, F.R.; Şahin Hafızoğlu, E.; Arslan, A.M.; Çakmak, M.; Biter, S.; İzgör, R.B.; Aktepe, O.H.; Ekinci, F.; Semiz, H.S.; Kara, İ.O.; et al. Evaluation of the Meet-URO Score in a Real-World Cohort of mRCC Patients Treated with First-Line TKIs. J. Clin. Med. 2025, 14, 6385. https://doi.org/10.3390/jcm14186385
Özalp FR, Şahin Hafızoğlu E, Arslan AM, Çakmak M, Biter S, İzgör RB, Aktepe OH, Ekinci F, Semiz HS, Kara İO, et al. Evaluation of the Meet-URO Score in a Real-World Cohort of mRCC Patients Treated with First-Line TKIs. Journal of Clinical Medicine. 2025; 14(18):6385. https://doi.org/10.3390/jcm14186385
Chicago/Turabian StyleÖzalp, Faruk Recep, Ece Şahin Hafızoğlu, Ahmet Melih Arslan, Mehmet Çakmak, Sedat Biter, Rezan Berkay İzgör, Oktay Halit Aktepe, Ferhat Ekinci, Hüseyin Salih Semiz, İsmail Oğuz Kara, and et al. 2025. "Evaluation of the Meet-URO Score in a Real-World Cohort of mRCC Patients Treated with First-Line TKIs" Journal of Clinical Medicine 14, no. 18: 6385. https://doi.org/10.3390/jcm14186385
APA StyleÖzalp, F. R., Şahin Hafızoğlu, E., Arslan, A. M., Çakmak, M., Biter, S., İzgör, R. B., Aktepe, O. H., Ekinci, F., Semiz, H. S., Kara, İ. O., Erman, M., Yalçın, Ş., & Karaoğlu, A. (2025). Evaluation of the Meet-URO Score in a Real-World Cohort of mRCC Patients Treated with First-Line TKIs. Journal of Clinical Medicine, 14(18), 6385. https://doi.org/10.3390/jcm14186385





