Predictors of Super-Responder Status to Anti-IL-23 Therapies in Moderate-to-Severe Plaque Psoriasis: A Real-World Monocenter Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Clinical Assessment of SR Status
2.3. Safety Assessment
2.4. Statistical Analysis
2.5. Ethical Consideration
3. Results
3.1. Study Population
3.2. “Super-Responder” Status
3.3. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PsA | Psoriatic Arthritis |
| TNF | Tumor Necrosis Factor |
| IFN | Interferon |
| IL | Interleukin |
| BSA | Body Surface Area |
| PASI | Psoriasis Area and Severity Index |
| DLQI | Dermatology Life Quality Index |
| PDE-4 | Phosphodiesterase-4 |
| AEs | Adverse Events |
| Treg | Regulatory T cells |
| TRM | Tissue-Resident Memory T cells |
| SR | Super-Responder |
| BMI | Body Mass Index |
| MAFLD | Metabolic-Associated Fatty Liver Disease |
| SD | Standard Deviation |
| OR | Odds Ratio |
| CI | Confidence Interval |
| URTIs | Upper Respiratory Tract Infections |
| TB | Tuberculosis |
| HBV | Hepatitis B Virus |
| HCV | Hepatitis C Virus |
| SDD | Short Disease Duration |
| LDD | Long Disease Duration |
| CMD | Cardiometabolic Comorbidities |
References
- Griffiths, C.E.M.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J.N.W.N. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef]
- Greb, J.E.; Goldminz, A.M.; Elder, J.T.; Lebwohl, M.G.; Gladman, D.D.; Wu, J.J.; Mehta, N.N.; Finlay, A.Y.; Gottlieb, A.B. Psoriasis. Nat. Rev. Dis. Primers 2016, 2, 16082. [Google Scholar] [CrossRef]
- Nair, R.P.; Stuart, P.E.; Nistor, I.; Hiremagalore, R.; Chia, N.V.; Jenisch, S.; Weichenthal, M.; Abecasis, G.R.; Lim, H.W.; Christophers, E.; et al. Sequence and haplotype analysis supports HLA-C as the psoriasis susceptibility 1 gene. Am. J. Hum. Genet. 2006, 78, 827–851. [Google Scholar] [CrossRef]
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef]
- Sieminska, I.; Pieniawska, M.; Grzywa, T.M. The Immunology of Psoriasis-Current Concepts in Pathogenesis. Clin. Rev. Allergy Immunol. 2024, 66, 164–191. [Google Scholar] [CrossRef] [PubMed]
- Boehncke, W.H.; Schön, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Finlay, A.Y. Current severe psoriasis and the rule of tens. Br. J. Dermatol. 2005, 152, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Fargnoli, M.C.; De Simone, C.; Gisondi, P.; Pellacani, G.; Calzavara-Pinton, P. Topical Treatment for the Management of Mild-to-Moderate Psoriasis: A Critical Appraisal of the Current Literature. Dermatol. Ther. 2023, 13, 2527–2547. [Google Scholar] [CrossRef]
- Nast, A.; Smith, C.; Spuls, P.I.; Valle, G.A.; Bata-Csörgö, Z.; Boonen, H.; De Jong, E.; Garcia-Doval, I.; Gisondi, P.; Kaur-Knudsen, D.; et al. EuroGuiDerm Guideline on the systemic treatment of Psoriasis vulgaris—Part 2: Specific clinical and comorbid situations. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 281–317. [Google Scholar] [CrossRef]
- Nast, A.; Smith, C.; Spuls, P.I.; Valle, G.A.; Bata-Csörgö, Z.; Boonen, H.; De Jong, E.; Garcia-Doval, I.; Gisondi, P.; Kaur-Knudsen, D.; et al. EuroGuiDerm Guideline on the systemic treatment of Psoriasis vulgaris—Part 1: Treatment and monitoring recommendations. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2461–2498. [Google Scholar] [CrossRef]
- Gisondi, P.; Fargnoli, M.C.; Amerio, P.; Argenziano, G.; Bardazzi, F.; Bianchi, L.; Chiricozzi, A.; Conti, A.; Corazza, M.; Costanzo, A.; et al. Italian adaptation of EuroGuiDerm guideline on the systemic treatment of chronic plaque psoriasis. Ital. J. Dermatol. Venerol. 2022, 157 (Suppl. S1), 1–78. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Gooderham, M.; Warren, R.B.; Papp, K.A.; Strober, B.; Thaçi, D.; Morita, A.; Szepietowski, J.C.; Imafuku, S.; Colston, E.; et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: Efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J. Am. Acad. Dermatol. 2023, 88, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Strober, B.; Thaçi, D.; Sofen, H.; Kircik, L.; Gordon, K.B.; Foley, P.; Rich, P.; Paul, C.; Bagel, J.; Colston, E.; et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: Efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program fOr Evaluation of TYK2 inhibitor psoriasis second trial. J. Am. Acad. Dermatol. 2023, 88, 40–51. [Google Scholar] [CrossRef]
- Reich, K.; Papp, K.A.; Blauvelt, A.; Langley, R.G.; Armstrong, A.; Warren, R.B.; Gordon, K.B.; Merola, J.F.; Okubo, Y.; Madden, C.; et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): Efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet 2021, 397, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Gordon, K.B.; Foley, P.; Krueger, J.G.; Pinter, A.; Reich, K.; Vender, R.; Vanvoorden, V.; Madden, C.; White, K.; Cioffi, C.; et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): A multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet 2021, 397, 475–486. [Google Scholar] [CrossRef]
- Lebwohl, M.; Strober, B.; Menter, A.; Gordon, K.; Weglowska, J.; Puig, L.; Papp, K.; Spelman, L.; Toth, D.; Kerdel, F.; et al. Phase 3 Studies Comparing Brodalumab with Ustekinumab in Psoriasis. N. Engl. J. Med. 2015, 373, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
- Gordon, K.B.; Blauvelt, A.; Papp, K.A.; Langley, R.G.; Luger, T.; Ohtsuki, M.; Reich, K.; Amato, D.; Ball, S.G.; Braun, D.K.; et al. Phase 3 Trials of Ixekizumab in Moderate-to-Severe Plaque Psoriasis. N. Engl. J. Med. 2016, 375, 345–356. [Google Scholar] [CrossRef]
- Langley, R.G.; Elewski, B.E.; Lebwohl, M.; Reich, K.; Griffiths, C.E.; Papp, K.; Puig, L.; Nakagawa, H.; Spelman, L.; Sigurgeirsson, B.; et al. Secukinumab in plaque psoriasis--results of two phase 3 trials. N. Engl. J. Med. 2014, 371, 326–338. [Google Scholar] [CrossRef]
- Merola, J.F.; Landewé, R.; McInnes, I.B.; Mease, P.J.; Ritchlin, C.T.; Tanaka, Y.; Asahina, A.; Behrens, F.; Gladman, D.D.; Gossec, L.; et al. Bimekizumab in patients with active psoriatic arthritis and previous inadequate response or intolerance to tumour necrosis factor-α inhibitors: A randomised, double-blind, placebo-controlled, phase 3 trial (BE COMPLETE). Lancet 2023, 401, 38–48. [Google Scholar] [CrossRef]
- Gooderham, M.; Vender, R.; Crowley, J.; Hong, H.C.-H.; Feely, M.; Garrelts, A.; See, K.; Konicek, B.; Green, L. Speed and Cumulative Responses According to Body Regions in Patients with Moderate-to-Severe Plaque Psoriasis Treated with Ixekizumab (Interleukin-17A Antagonist) versus Guselkumab (Interleukin-23p19 Inhibitor). Dermatol. Ther. 2024, 14, 441–451. [Google Scholar] [CrossRef]
- Orsini, D.; Megna, M.; Assorgi, C.; Balato, A.; Balestri, R.; Bernardini, N.; Bettacchi, A.; Bianchelli, T.; Bianchi, L.; Buggiani, G.; et al. Efficacy and safety of bimekizumab in elderly patients: Real-world multicenter retrospective study—IL PSO (Italian Landscape Psoriasis). J. Dermatol. Treat. 2024, 35, 2393376. [Google Scholar] [CrossRef]
- Cheng, Y.; Ma, J.; Niu, J. Real-world safety assessment of Ixekizumab based on the FDA Adverse Event Reporting System (FAERS). PLoS ONE 2025, 20, e0323973. [Google Scholar] [CrossRef]
- Kruczek, W.; Frątczak, A.; Litwińska-Inglot, I.; Polak, K.; Pawlus, Z.; Rutecka, P.; Bergler-Czop, B.; Miziołek, B. Comparative Analysis of the Long-Term Real-World Efficacy of Interleukin-17 Inhibitors in a Cohort of Patients with Moderate-to-Severe Psoriasis Treated in Poland. J. Clin. Med. 2025, 14, 5421. [Google Scholar] [CrossRef] [PubMed]
- Orsini, D.; Graceffa, D.; Burlando, M.; Campanati, A.; Campione, E.; Guarneri, C.; Narcisi, A.; Pella, P.; Romita, P.; Travaglini, M.; et al. Effectiveness of Brodalumab for the Treatment of Moderate-to-Severe Psoriasis: A Retrospective, Real-World Multicenter Study with a Focus on Obese and Multi-Failure Patients—IL PSO (Italian Landscape Psoriasis). J. Clin. Med. 2025, 14, 1087. [Google Scholar] [CrossRef] [PubMed]
- Gordon, K.B.; Strober, B.; Lebwohl, M.; Augustin, M.; Blauvelt, A.; Poulin, Y.; Papp, K.A.; Sofen, H.; Puig, L.; Foley, P.; et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): Results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet 2018, 392, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Reich, K.; Papp, K.A.; Blauvelt, A.; Tyring, S.K.; Sinclair, R.; Thaçi, D.; Nograles, K.; Mehta, A.; Cichanowitz, N.; Li, Q.; et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): Results from two randomised controlled, phase 3 trials. Lancet 2017, 390, 276–288. [Google Scholar] [CrossRef]
- Gargiulo, L.; Ibba, L.; Ingurgio, R.C.; Malagoli, P.; Amoruso, F.; Balato, A.; Bardazzi, F.; Brianti, P.; Brunasso, G.; Burlando, M.; et al. Comparative effectiveness of tildrakizumab 200 mg versus tildrakizumab 100 mg in psoriatic patients with high disease burden or above 90 kg of body weight: A 16-week multicenter retrospective study—IL PSO (Italian landscape psoriasis). J. Dermatol. Treat. 2024, 35, 2350760. [Google Scholar] [CrossRef]
- Valenti, M.; Ibba, L.; Di Giulio, S.; Gargiulo, L.; Malagoli, P.; Balato, A.; Bardazzi, F.; Loconsole, F.; Burlando, M.; Cagni, A.E.; et al. Optimizing Tildrakizumab Dosing in Psoriasis: A 52-Week Multicenter Retrospective Study Comparing 100 mg and 200 mg-IL PSO (Italian Landscape Psoriasis). Dermatol. Ther. 2025, 15, 1427–1440. [Google Scholar] [CrossRef]
- Torres, T.; Puig, L.; Vender, R.; Yeung, J.; Carrascosa, J.-M.; Piaserico, S.; Gisondi, P.; Lynde, C.; Ferreira, P.; Bastos, P.M.; et al. Drug Survival of Interleukin (IL)-17 and IL-23 Inhibitors for the Treatment of Psoriasis: A Retrospective Multi-country, Multicentric Cohort Study. Am. J. Clin. Dermatol. 2022, 23, 891–904. [Google Scholar] [CrossRef]
- Gargiulo, L.; Ibba, L.; Malagoli, P.; Amoruso, F.; Argenziano, G.; Balato, A.; Bardazzi, F.; Burlando, M.; Carrera, C.G.; Damiani, G.; et al. Effectiveness, Tolerability, and Drug Survival of Risankizumab in a Real-World Setting: A Three-Year Retrospective Multicenter Study-IL PSO (ITALIAN LANDSCAPE PSORIASIS). J. Clin. Med. 2024, 13, 495. [Google Scholar] [CrossRef]
- Megna, M.; Tommasino, N.; Potestio, L.; Battista, T.; Ruggiero, A.; Noto, M.; Fabbrocini, G.; Genco, L. Real-world practice indirect comparison between guselkumab, risankizumab, and tildrakizumab: Results from an Italian 28-week retrospective study. J. Dermatol. Treat. 2022, 33, 2813–2820. [Google Scholar] [CrossRef]
- Valenti, M.; Ibba, L.; Di Giulio, S.; Dapavo, P.; Malagoli, P.; Marzano, A.V.; Loconsole, F.; Burlando, M.; Balato, A.; Dini, V.; et al. Guselkumab Retention, Effectiveness, and Safety in Psoriasis: A 260-Week Real-World Multicenter Retrospective Study Exploring the Role of Concomitant PsA-IL PSO (Italian Landscape Psoriasis). Dermatol. Ther. 2025, 15, 2423–2437. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, C.L.; Kimball, A.B.; Papp, K.A.; Yeilding, N.; Guzzo, C.; Wang, Y.; Li, S.; Dooley, L.T.; Gordon, K.B. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 2008, 371, 1665–1674. [Google Scholar] [CrossRef]
- Papp, K.A.; Langley, R.G.; Lebwohl, M.; Krueger, G.G.; Szapary, P.; Yeilding, N.; Guzzo, C.; Hsu, M.-C.; Wang, Y.; Li, S.; et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet 2008, 371, 1675–1684. [Google Scholar] [CrossRef]
- Langley, R.G.; Tsai, T.F.; Flavin, S.; Song, M.; Randazzo, B.; Wasfi, Y.; Jiang, J.; Li, S.; Puig, L. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: Results of the randomized, double-blind, phase III NAVIGATE trial. Br. J. Dermatol. 2018, 178, 114–123. [Google Scholar] [CrossRef]
- Krueger, J.G.; Eyerich, K.; Kuchroo, V.K.; Ritchlin, C.T.; Abreu, M.T.; Elloso, M.M.; Fourie, A.; Fakharzadeh, S.; Sherlock, J.P.; Yang, Y.-W.; et al. IL-23 past, present, and future: A roadmap to advancing IL-23 science and therapy. Front. Immunol. 2024, 15, 1331217. [Google Scholar] [CrossRef]
- Reich, K.; Armstrong, A.W.; Langley, R.G.; Flavin, S.; Randazzo, B.; Li, S.; Hsu, M.-C.; Branigan, P.; Blauvelt, A. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): Results from a phase 3, randomised controlled trial. Lancet 2019, 394, 831–839. [Google Scholar] [CrossRef]
- Eyerich, K.; Weisenseel, P.; Pinter, A.; Schäkel, K.; Asadullah, K.; Wegner, S.; Muñoz-Elias, E.J.; Bartz, H.; Taut, F.J.H.; Reich, K. IL-23 blockade with guselkumab potentially modifies psoriasis pathogenesis: Rationale and study protocol of a phase 3b, randomised, double-blind, multicentre study in participants with moderate-to-severe plaque-type psoriasis (GUIDE). BMJ Open 2021, 11, e049822. [Google Scholar] [CrossRef] [PubMed]
- Javaid, K.; Andruszka, C. Early disease intervention with guselkumab in psoriasis leads to a higher rate of stable complete skin clearance (‘clinical super response’): Week 28 results from the ongoing phase IIIb randomized, double-blind, parallel-group, GUIDE study: An analysis with considerations for future studies. J. Eur. Acad. Dermatol. Venereol. 2024, 38, e302–e303. [Google Scholar] [CrossRef] [PubMed]
- Puig, L.; Costanzo, A.; Muñoz-Elías, E.J.; Jazra, M.; Wegner, S.; Paul, C.F.; Conrad, C. The biological basis of disease recurrence in psoriasis: A historical perspective and current models. Br. J. Dermatol. 2022, 186, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Whitley, S.K.; Li, M.; Kashem, S.W.; Hirai, T.; Igyártó, B.Z.; Knizner, K.; Ho, J.; Ferris, L.K.; Weaver, C.T.; Cua, D.J.; et al. Local IL-23 is required for proliferation and retention of skin-resident memory TH17 cells. Sci. Immunol. 2022, 7, eabq3254. [Google Scholar] [CrossRef]
- Ruiz-Villaverde, R.; Rodriguez-Fernandez-Freire, L.; Armario-Hita, J.C.; Pérez-Gil, A.; Galán-Gutiérrez, M. Super responders to guselkumab treatment in moderate-to-severe psoriasis: A real clinical practice pilot series. Int. J. Dermatol. 2022, 61, 1029–1033. [Google Scholar] [CrossRef]
- Mortato, E.; Talamonti, M.; Marcelli, L.; Megna, M.; Raimondo, A.; Caldarola, G.; Bernardini, N.; Balato, A.; Campanati, A.; Esposito, M.; et al. Predictive Factors for Super Responder Status and Long-Term Effectiveness of Guselkumab in Psoriasis: A Multicenter Retrospective Study. Dermatol. Ther. 2025, 15, 1239–1250. [Google Scholar] [CrossRef]
- Gargiulo, L.; Ibba, L.; Malagoli, P.; Amoruso, F.; Argenziano, G.; Balato, A.; Bardazzi, F.; Burlando, M.; Carrera, C.G.; Damiani, G.; et al. A risankizumab super responder profile identified by long-term real-life observation-IL PSO (ITALIAN LANDSCAPE PSORIASIS). J. Eur. Acad. Dermatol. Venereol. 2024, 38, e113–e116. [Google Scholar] [CrossRef]
- European Medicines Agency. Skyrizi (Risankizumab): Summary of Product Characteristics. 2019. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/skyrizi (accessed on 26 June 2025).
- European Medicines Agency. Tremfya (Guselkumab): Summary of Product Characteristics. 2017. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/tremfya (accessed on 26 June 2025).
- European Medicines Agency. Ilumetri (Tildrakizumab): Summary of Product Characteristics. 2018. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/ilumetri (accessed on 26 June 2025).
- Ibba, L.; Gargiulo, L.; Alfano, A.; Ingurgio, R.C.; Narcisi, A.; Costanzo, A.; Valenti, M. Anti-IL-23 and anti-IL-17 drugs for the treatment of non-pustular palmoplantar psoriasis: A real-life retrospective study. J. Dermatol. Treat. 2023, 34, 2199108. [Google Scholar] [CrossRef]
- Thomas, S.E.; van den Reek, J.M.P.A.; Seyger, M.M.B.; de Jong, E.M.G.J. How to define a ‘super-responder’ to biologics in psoriasis studies. Br. J. Dermatol. 2023, 189, 621–622. [Google Scholar] [CrossRef] [PubMed]
- Reich, K.; Gordon, K.B.; Strober, B.; Langley, R.; Miller, M.; Yang, Y.; Shen, Y.; You, Y.; Zhu, Y.; Foley, P.; et al. Super-response to guselkumab treatment in patients with moderate-to-severe psoriasis: Age, body weight, baseline Psoriasis Area and Severity Index, and baseline Investigator’s Global Assessment scores predict complete skin clearance. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 2393–2400. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, K.; Jian, L.; Duan, Y.; Zhang, M.; Kuang, Y. Comparison between super-responders and non-super-responders in psoriasis under adalimumab treatment: A real-life cohort study on the effectiveness and drug survival over one-year. J. Dermatol. Treat. 2024, 35, 2331782. [Google Scholar] [CrossRef]
- Feldman, S.R.; Merola, J.F.; Pariser, D.M.; Zhang, J.; Zhao, Y.; Mendelsohn, A.M.; Gottlieb, A.B. Clinical implications and predictive values of early PASI responses to tildrakizumab in patients with moderate-to-severe plaque psoriasis. J. Dermatol. Treat. 2022, 33, 1670–1675. [Google Scholar] [CrossRef] [PubMed]
- Rompoti, N.; Politou, M.; Stefanaki, I.; Vavouli, C.; Papoutsaki, M.; Neofotistou, A.; Rigopoulos, D.; Stratigos, A.; Nicolaidou, E. Brodalumab in plaque psoriasis: Real-world data on effectiveness, safety and clinical predictive factors of initial response and drug survival over a period of 104 weeks. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Mastorino, L.; Susca, S.; Cariti, C.; Verrone, A.; Stroppiana, E.; Ortoncelli, M.; Dapavo, P.; Ribero, S.; Quaglino, P. “Superresponders” at biologic treatment for psoriasis: A comparative study among IL17 and IL23 inhibitors. Exp. Dermatol. 2023, 32, 2187–2188. [Google Scholar] [CrossRef]
- Ranzinger, D.; Eyerich, K. Disease Modification in Psoriasis: Future Prospects for Long-Term Remission. Am. J. Clin. Dermatol. 2025, 26, 477–486. [Google Scholar] [CrossRef]
- Schäkel, K.; Reich, K.; Asadullah, K.; Pinter, A.; Jullien, D.; Weisenseel, P.; Paul, C.; Gomez, M.; Wegner, S.; Personke, Y.; et al. Early disease intervention with guselkumab in psoriasis leads to a higher rate of stable complete skin clearance (‘clinical super response’): Week 28 results from the ongoing phase IIIb randomized, double-blind, parallel-group, GUIDE study. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 2016–2027. [Google Scholar] [CrossRef]
- Eyerich, K.; Asadullah, K.; Pinter, A.; Weisenseel, P.; Reich, K.; Paul, C.; Sabat, R.; Wolk, K.; Eyerich, S.; Lauffer, F.; et al. Noninferiority of 16-Week vs 8-Week Guselkumab Dosing in Super Responders for Maintaining Control of Psoriasis: The GUIDE Randomized Clinical Trial. JAMA Dermatol. 2024, 160, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Loft, N.; Egeberg, A.; Rasmussen, M.K.; Bryld, L.; Nissen, C.; Dam, T.; Ajgeiy, K.; Iversen, L.; Skov, L. Prevalence and characterization of treatment-refractory psoriasis and super-responders to biologic treatment: A nationwide study. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1284–1291. [Google Scholar] [CrossRef]
- Marcelli, L.; Belcastro, A.; Talamonti, M.; Paganini, C.; Fico, A.; Savastano, L.; Di Raimondo, C.; Vellucci, L.; Bianchi, L.; Galluzzo, M. Characterization of Super-Responder Profile in Chronic Plaque Psoriatic Patients under Guselkumab Treatment: A Long-Term Real-Life Experience. J. Clin. Med. 2024, 13, 5175. [Google Scholar] [CrossRef] [PubMed]
- Talamonti, M.; D’ADamio, S.; Galluccio, T.; Andreani, M.; Pastorino, R.; Egan, C.; Bianchi, L.; Galluzzo, M. High-resolution HLA typing identifies a new ‘super responder’ subgroup of HLA-C*06:02-positive psoriatic patients: HLA-C*06:02/HLA-C*04, in response to ustekinumab. J. Eur. Acad. Dermatol. Venereol. 2019, 33, e364–e367. [Google Scholar] [CrossRef]
- Ibba, L.; Di Giulio, S.; Gargiulo, L.; Facheris, P.; Perugini, C.; Costanzo, A.; Narcisi, A.; Valenti, M. Long-term effectiveness and safety of risankizumab in patients with moderate-to-severe psoriasis with and without cardiometabolic comorbidities: A single-center retrospective study. J. Dermatol. Treat. 2024, 35, 2425029. [Google Scholar] [CrossRef]
- Orsini, D.; Gargiulo, L.; Ibba, L.; Ingurgio, R.C.; Valenti, M.; Perugini, C.; Pacifico, A.; Maramao, F.S.; Frascione, P.; Costanzo, A.; et al. Effectiveness of risankizumab in plaque psoriasis with involvement of difficult-to-treat areas: A real-world experience from two referral centers. J. Dermatol. Treat. 2023, 34, 2220849. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Zhao, Z.; Zhai, Y.; Ye, X.; Xu, F. Adverse events associated with IL-23 and IL-12/23 inhibitors in the clinical management of psoriasis: A comprehensive pharmacovigilance analysis. BMC Pharmacol. Toxicol. 2025, 26, 11. [Google Scholar] [CrossRef]
- Ibba, L.; Gargiulo, L.; Vignoli, C.A.; Fiorillo, G.; Valenti, M.; Costanzo, A.; Narcisi, A. Safety of anti-IL-23 drugs in patients with moderate-to-severe plaque psoriasis and previous tuberculosis infection: A monocentric retrospective study. J. Dermatol. Treat. 2023, 34, 2241585. [Google Scholar] [CrossRef] [PubMed]
| Total Patients | 611 |
|---|---|
| N (%) | |
| Male | 391 (64) |
| PsA | 91 (14.9) |
| At least one difficult-to-treat area | 478 (78.2) |
| Cardiometabolic comorbidities | 314 (51.4) |
| Bio-Naïve | 420 (68.7) |
| Anti-IL-23 drug | |
| Risankizumab | 380 (62.2) |
| Tildrakizumab | 84 (13.8) |
| Guselkumab | 147 (24.1) |
| Mean (SD) | |
| Age, years | 53.83 (15.17) |
| BMI, kg/m2 | 26.94 (5.66) |
| Disease duration, years | 19.78 (14.30) |
| PASI at baseline | 12.17 (6.78) |
| Categorical Variables | ||
|---|---|---|
| Super-Responder Status | p-Value | |
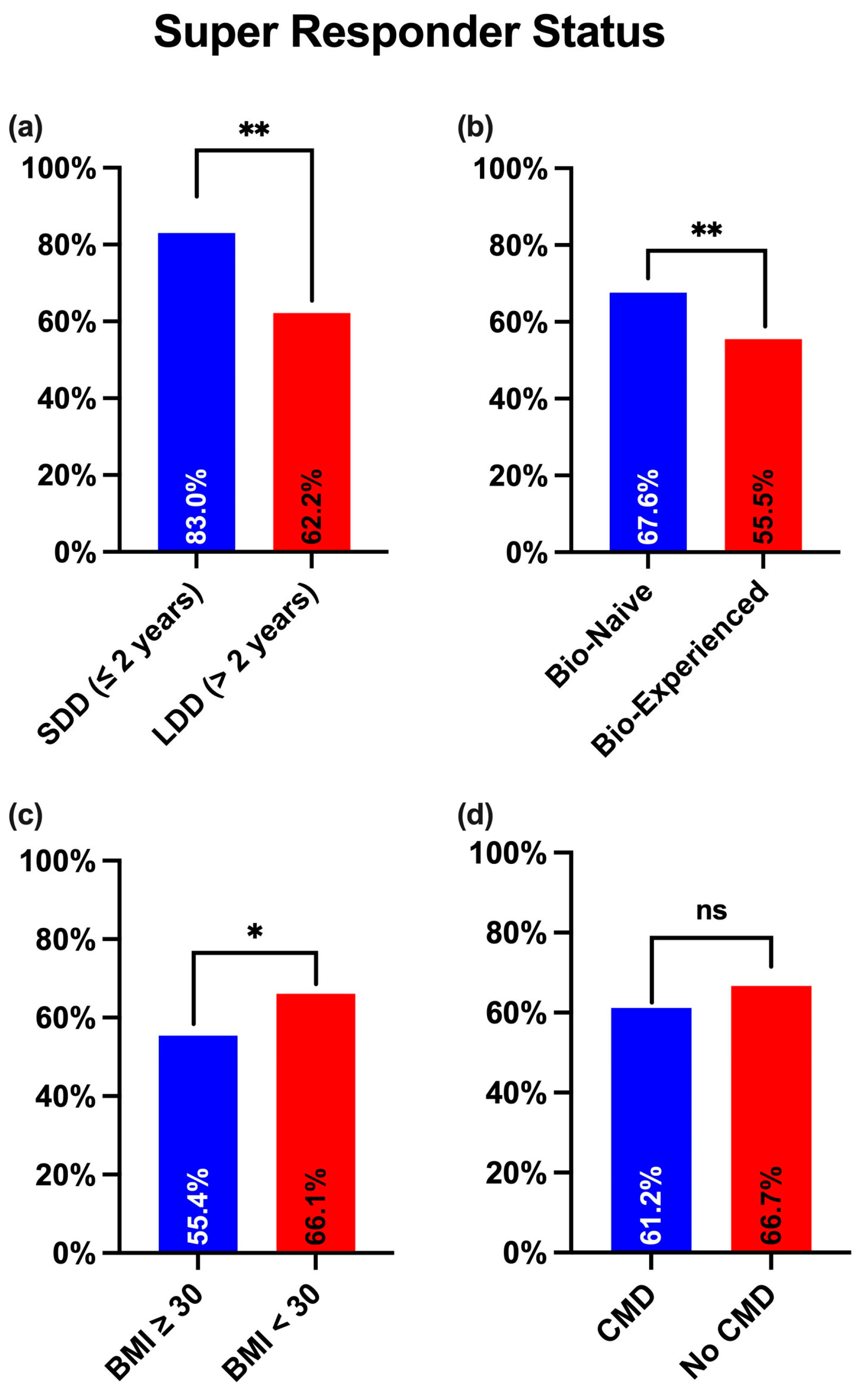
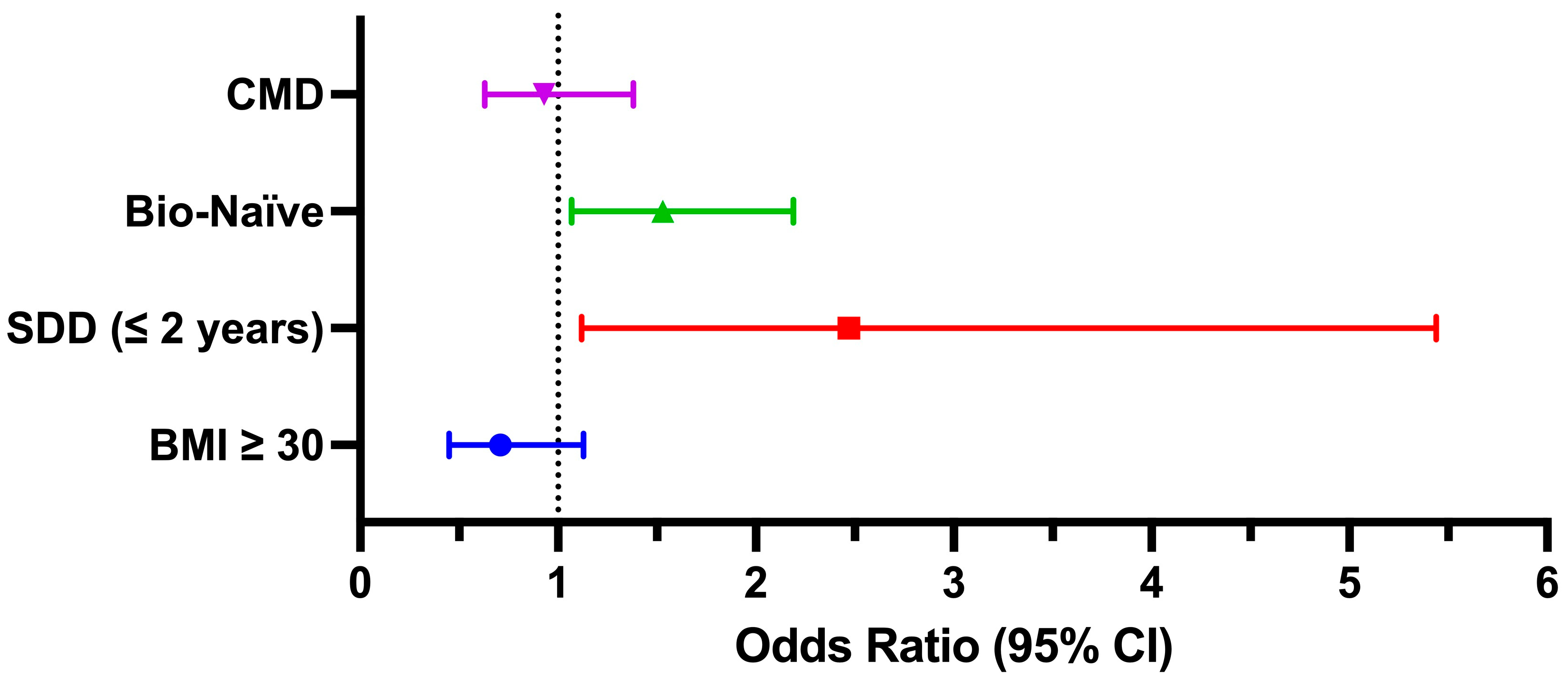
| SDD (≤2 years) | 39/47 (83%) | 0.004 |
| LDD (>2 years) | 351/564 (62.2%) | |
| Bio-Naïve | 284/420 (67.6%) | 0.004 |
| Bio-Experienced | 106/191 (55.5%) | |
| BMI ≥ 30 | 72/130 (55.4%) | 0.024 |
| BMI < 30 | 318/481 (66.1%) | |
| Tildrakizumab | 51/84 (60.7%) | 0.781 |
| Risankizumab | 243/380 (64%) | |
| Guselkumab | 96/147 (65.3%) | |
| Male | 255/391 (65.2%) | 0.341 |
| Female | 135/220 (61.4%) | |
| PASI ≥ 12 | 200/316 (63.3%) | 0.774 |
| PASI < 12 | 190/295 (64.4%) | |
| CMD | 192/314 (61.2%) | 0.156 |
| No CMD | 198/297 (66.7%) | |
| Difficult areas | 309/478 (64.6%) | 0.427 |
| No difficult areas | 81/133 (60.9%) | |
| PsA | 55/91 (60.4%) | 0.466 |
| No PsA | 335/520 (64.4%) | |
| Quantitative Variables | ||
| Mean age (SD) | p-value | |
| Super-Responder | 53.41 (15.60) | 0.359 |
| Non-Super-Responder | 54.58 (14.38) | |
| AEs | Guselkumab (n = 147) | Risankizumab (n = 380) | Tildrakizumab (n = 84) |
|---|---|---|---|
| Total | 6 (4.1%) | 15 (4%) | 3 (3.6%) |
| URTIs | 4 (2.7%) | 9 (2.4%) | 2 (2.4%) |
| Headache | 1 (0.7%) | 3 (0.8%) | 1 (1.2%) |
| Diarrhea | 1 (0.7%) | 2 (0.5%) | 0 |
| Reaction at injection site | 0 | 1 (0.3%) | 0 |
| Severe AEs | 0 | 0 | 0 |
| AEs leading to discontinuation | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Giulio, S.; Falcidia, C.; Foggi, G.; Bianco, M.; Gargiulo, L.; Valenti, M.; Costanzo, A.; Narcisi, A.; Ibba, L. Predictors of Super-Responder Status to Anti-IL-23 Therapies in Moderate-to-Severe Plaque Psoriasis: A Real-World Monocenter Study. J. Clin. Med. 2025, 14, 6371. https://doi.org/10.3390/jcm14186371
Di Giulio S, Falcidia C, Foggi G, Bianco M, Gargiulo L, Valenti M, Costanzo A, Narcisi A, Ibba L. Predictors of Super-Responder Status to Anti-IL-23 Therapies in Moderate-to-Severe Plaque Psoriasis: A Real-World Monocenter Study. Journal of Clinical Medicine. 2025; 14(18):6371. https://doi.org/10.3390/jcm14186371
Chicago/Turabian StyleDi Giulio, Sara, Costanza Falcidia, Giulio Foggi, Matteo Bianco, Luigi Gargiulo, Mario Valenti, Antonio Costanzo, Alessandra Narcisi, and Luciano Ibba. 2025. "Predictors of Super-Responder Status to Anti-IL-23 Therapies in Moderate-to-Severe Plaque Psoriasis: A Real-World Monocenter Study" Journal of Clinical Medicine 14, no. 18: 6371. https://doi.org/10.3390/jcm14186371
APA StyleDi Giulio, S., Falcidia, C., Foggi, G., Bianco, M., Gargiulo, L., Valenti, M., Costanzo, A., Narcisi, A., & Ibba, L. (2025). Predictors of Super-Responder Status to Anti-IL-23 Therapies in Moderate-to-Severe Plaque Psoriasis: A Real-World Monocenter Study. Journal of Clinical Medicine, 14(18), 6371. https://doi.org/10.3390/jcm14186371









