Long-Term Cardiorenal Benefits of Sodium-Glucose Cotransporter-2 Inhibitors in Patients with Type 2 Diabetes Mellitus: A Real-World Single-Center Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
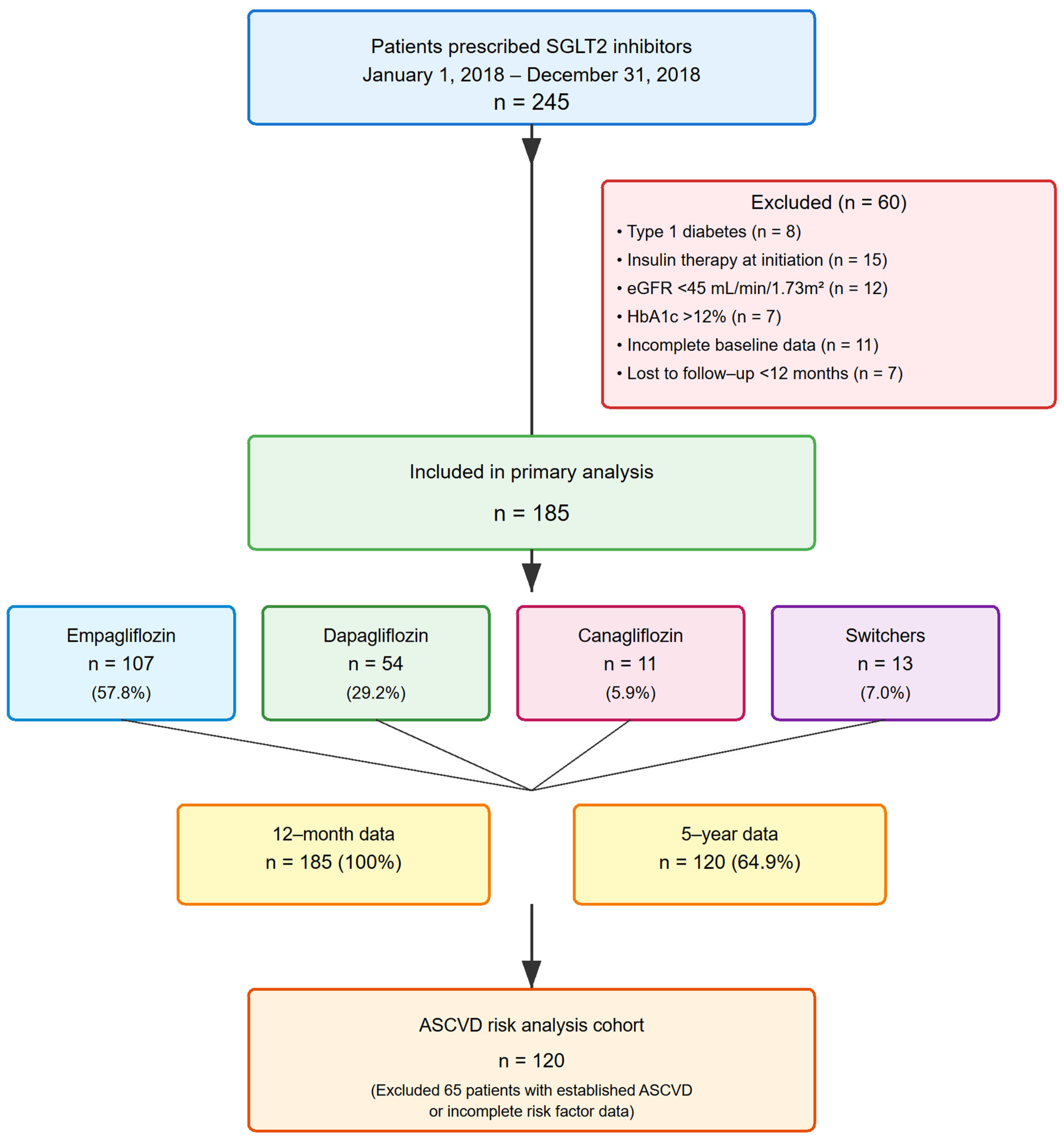
2.2. Study Population
2.3. Selection Criteria
2.4. Data Collection
2.5. Adverse Event Assessment
2.6. Cardiovascular Risk Assessment
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
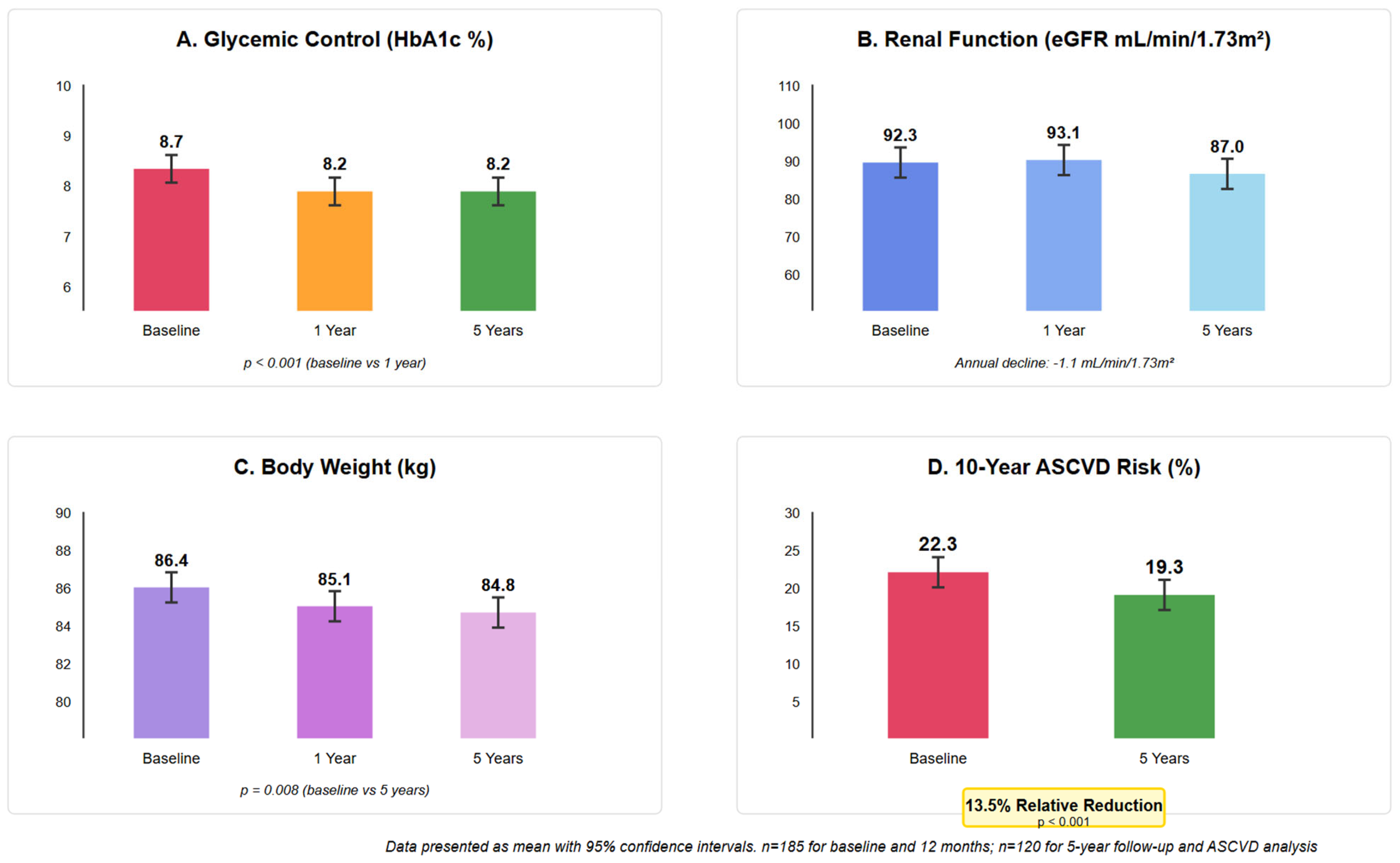
3.2. Glycemic Control
3.3. Renal Function
3.4. Weight and BMI Changes
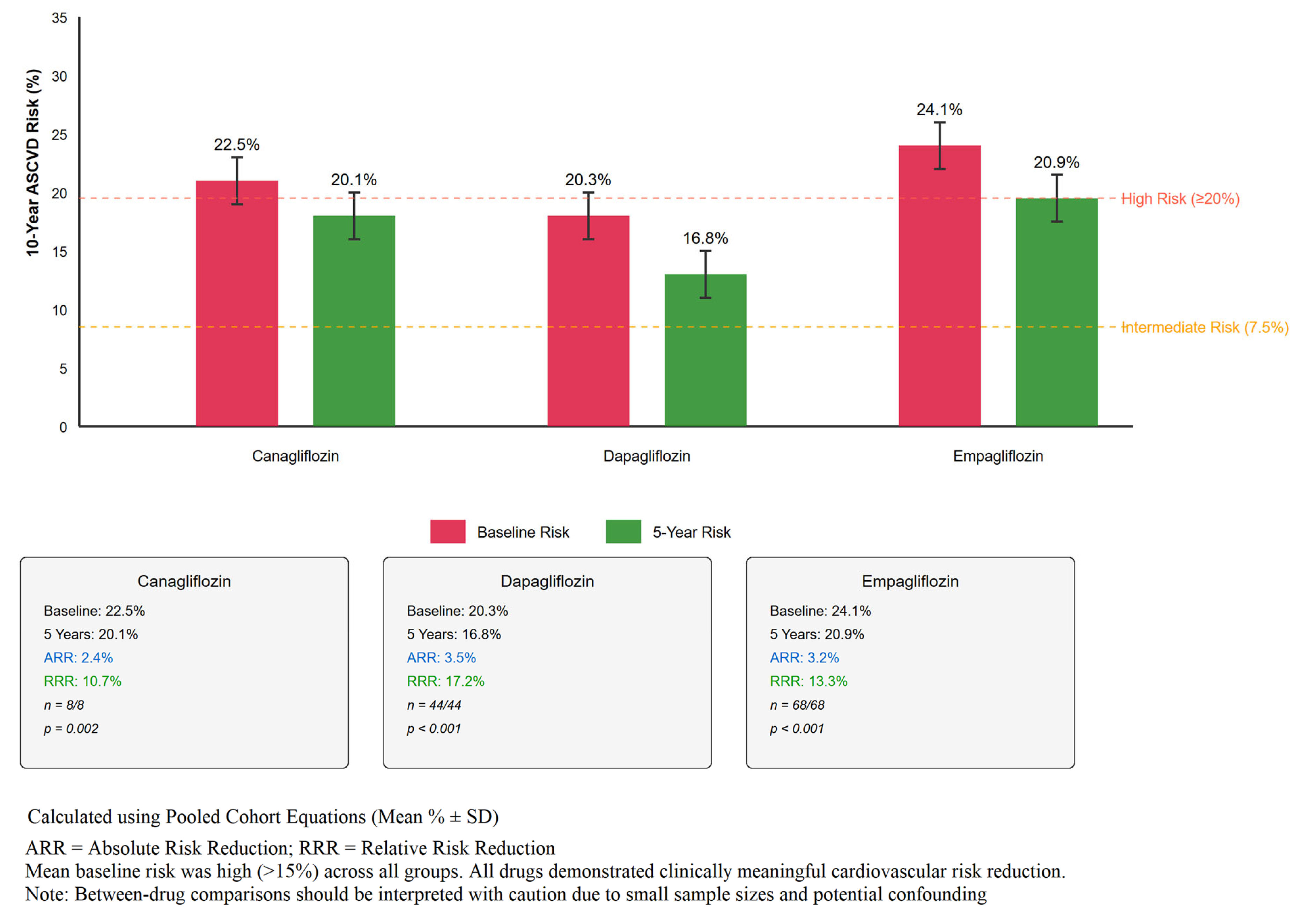
3.5. Cardiovascular Risk Assessment
3.6. Concomitant Medication Changes
3.7. Safety Outcomes
3.8. Outcomes in Patients Who Switched Between SGLT2 Inhibitors
4. Discussion
4.1. Glycemic Efficacy in Real-World Settings
4.2. Cardiovascular Risk Assessment: Interpretation and Limitations
4.3. Renal Outcomes in Context
4.4. Population-Specific Considerations
4.5. Clinical Implications
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Sulaiman, N.; Albadawi, S.; Abusnana, S.; Mairghani, M.; Hussein, A.; Al Awadi, F.; Madani, A.; Zimmet, P.; Shaw, J. High prevalence of diabetes among migrants in the United Arab Emirates using a cross-sectional survey. Sci. Rep. 2018, 8, 6862. [Google Scholar] [CrossRef] [PubMed]
- Bennet, L.; Groop, L.; Lindblad, U.; Agardh, C.-D.; Franks, P. Ethnicity is an independent risk indicator when estimating diabetes risk with FINDRISC scores: A cross sectional study comparing immigrants from the Middle East and native Swedes. Prim. Care Diabetes 2014, 8, 231–238. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Primers 2015, 1, 15019. [Google Scholar] [CrossRef]
- Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef]
- Hassanien, A.A.; Al-Shaikh, F.; Vamos, E.P.; Yadegarfar, G.; Majeed, A. Epidemiology of end-stage renal disease in the countries of the Gulf Cooperation Council: A systematic review. JRSM Short Rep. 2012, 3, 38. [Google Scholar] [CrossRef]
- Vallon, V.; Thomson, S.C. Targeting renal glucose reabsorption to treat hyperglycaemia: The pleiotropic effects of SGLT2 inhibition. Diabetologia 2017, 60, 215–225. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef]
- Verma, S.; McMurray, J.J.V. SGLT2 inhibitors and mechanisms of cardiovascular benefit: A state-of-the-art review. Diabetologia 2018, 61, 2108–2117. [Google Scholar] [CrossRef]
- Zelniker, T.A.; Braunwald, E. Mechanisms of cardiorenal effects of sodium-glucose cotransporter 2 inhibitors: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020, 75, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Kang, B.; Zhou, J. Sodium glucose cotransporter 2 inhibitors with cardiac arrhythmias in patients with type 2 diabetes mellitus: A systematic review and meta-analysis of randomized placebo-controlled trials. Clin. Res. Cardiol. 2024, 113, 910–923. [Google Scholar] [CrossRef]
- American Diabetes Association. Standards of Medical Care in Diabetes-2023. Diabetes Care 2023, 46 (Suppl. S1), S1–S267. [Google Scholar]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. European Society of Cardiology (ESC) Scientific Document Group. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef] [PubMed]
- Kosiborod, M.; Lam, C.S.P.; Kohsaka, S.; Kim, D.J.; Karasik, A.; Shaw, J.; Tangri, N.; Goh, S.Y.; Thuresson, M.; Chen, H.; et al. Cardiovascular events associated with SGLT-2 inhibitors versus other glucose-lowering drugs: The CVD-REAL 2 study. J. Am. Coll. Cardiol. 2018, 71, 2628–2639. [Google Scholar] [CrossRef] [PubMed]
- Jelinek, H.F.; Osman, W.M.; Khandoker, A.H.; Khalaf, K.; Lee, S.; Almahmeed, W.; Alsafar, H.S. Clinical profiles, comorbidities and complications of type 2 diabetes mellitus in patients from United Arab Emirates. BMJ Open Diabetes Res. Care 2017, 5, e000427. [Google Scholar] [CrossRef]
- Sadiya, A.; Ahmed, S.M.; Carlsson, M.; Tesfa, Y.; George, M.; Ali, S.H.; Siddieg, H.H.; Abusnana, S. Vitamin D supplementation in obese type 2 diabetes subjects in Ajman, UAE: A randomized controlled double-blinded clinical trial. Eur. J. Clin. Nutr. 2015, 69, 707–711. [Google Scholar]
- Haq, A.; Svobodová, J.; Imran, S.; Stanford, C.; Razzaque, M.S. Vitamin D deficiency: A single centre analysis of patients from 136 countries. J. Steroid Biochem. Mol. Biol. 2016, 164, 209–213. [Google Scholar] [CrossRef]
- Garlo, K.G.; White, W.B.; Bakris, G.L.; Zannad, F.; Wilson, C.A.; Kupfer, S.; Vaduganathan, M.; Morrow, D.A.; Cannon, C.P.; Charytan, D.M. Kidney biomarkers and decline in eGFR in patients with type 2 diabetes. Clin. J. Am. Soc. Nephrol. 2018, 13, 398–405. [Google Scholar] [CrossRef]
- Edelman, S.V.; Polonsky, W.H. Type 2 diabetes in the real world: The elusive nature of glycemic control. Diabetes Care 2017, 40, 1425–1432. [Google Scholar] [CrossRef]
- Turner, R.C.; Cull, C.A.; Frighi, V.; Holman, R.R.; for the UK Prospective Diabetes Study (UKPDS) Group. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: Progressive requirement for multiple therapies (UKPDS 49). JAMA 1999, 281, 2005–2012. [Google Scholar] [CrossRef]

| Characteristic | Total (n = 185) | Canagliflozin (n = 11) | Dapagliflozin (n = 54) | Empagliflozin (n = 107) | Switchers (n = 13) | p-Value |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age (years) | 57.0 ± 11.8 | 53.0 ± 17.0 | 56.0 ± 9.0 | 58.0 ± 12.0 | 56.5 ± 10.2 | 0.421 |
| Female, n (%) | 104 (56.2) | 7 (63.6) | 30 (55.6) | 64 (59.8) | 3 (23.1) | 0.148 |
| Diabetes duration (years) | 8.2 ± 5.3 | 7.8 ± 4.9 | 8.1 ± 5.2 | 8.4 ± 5.5 | 8.0 ± 5.1 | 0.856 |
| Clinical parameters | ||||||
| Weight (kg) | 86.4 ± 18.2 | 88.7 ± 16.3 | 85.2 ± 17.8 | 86.9 ± 18.7 | 85.1 ± 17.9 | 0.742 |
| BMI (kg/m2) | 33.1 ± 6.7 | 33.2 ± 4.4 | 32.3 ± 5.9 | 33.2 ± 7.1 | 32.8 ± 6.2 | 0.635 |
| Systolic BP (mmHg) | 132.5 ± 16.2 | 130.2 ± 14.8 | 131.8 ± 15.9 | 133.4 ± 16.7 | 132.1 ± 15.8 | 0.684 |
| Diastolic BP (mmHg) | 78.3 ± 9.8 | 77.5 ± 8.9 | 77.9 ± 9.6 | 78.7 ± 10.1 | 78.0 ± 9.5 | 0.821 |
| Laboratory values | ||||||
| HbA1c (%) | 8.7 ± 1.8 | 9.6 ± 1.9 | 8.5 ± 1.6 | 8.5 ± 1.9 | 8.8 ± 1.7 | 0.042 |
| FPG (mmol/L) | 9.8 ± 3.2 | 10.9 ± 3.5 | 9.5 ± 3.0 | 9.7 ± 3.2 | 9.9 ± 3.1 | 0.238 |
| eGFR (mL/min/1.73 m2) | 92.3 ± 22.1 | 98.5 ± 23.2 | 97.1 ± 16.1 | 90.4 ± 23.1 | 91.2 ± 21.8 | 0.156 |
| Total cholesterol (mmol/L) | 4.5 ± 1.2 | 4.4 ± 0.9 | 4.3 ± 1.1 | 4.8 ± 1.3 | 4.5 ± 1.1 | 0.089 |
| Comorbidities, n (%) | ||||||
| Hypertension | 126 (68.1) | 7 (63.6) | 35 (64.8) | 75 (70.1) | 9 (69.2) | 0.684 |
| Dyslipidemia | 143 (77.3) | 8 (72.7) | 40 (74.1) | 85 (79.4) | 10 (76.9) | 0.625 |
| Current smoker | 19 (10.3) | 1 (9.1) | 5 (9.3) | 12 (11.2) | 1 (7.7) | 0.891 |
| Concomitant medications, n (%) | ||||||
| Metformin | 172 (93.0) | 10 (90.9) | 50 (92.6) | 100 (93.5) | 12 (92.3) | 0.912 |
| Sulfonylurea | 68 (36.8) | 4 (36.4) | 19 (35.2) | 40 (37.4) | 5 (38.5) | 0.954 |
| ACE-I/ARB | 118 (63.8) | 6 (54.5) | 33 (61.1) | 71 (66.4) | 8 (61.5) | 0.598 |
| Statin | 132 (71.4) | 7 (63.6) | 37 (68.5) | 79 (73.8) | 9 (69.2) | 0.612 |
| Adverse Events | Total Cohort (n = 185) | Canagliflozin (n = 11) | Dapagliflozin (n = 54) | Empagliflozin (n = 107) | p-Value |
|---|---|---|---|---|---|
| Common adverse events, n (%) | |||||
| Genital mycotic infections | 16 (8.6) | 1 (9.1) | 4 (7.4) | 10 (9.3) | 0.912 |
| 13/104 (12.5) | 1/7 (14.3) | 3/30 (10.0) | 8/64 (12.5) | 0.891 |
| 3/81 (3.7) | 0/4 (0.0) | 1/24 (4.2) | 2/43 (4.7) | 0.854 |
| Urinary tract infections | 21 (11.4) | 1 (9.1) | 6 (11.1) | 13 (12.1) | 0.932 |
| Volume depletion symptoms † | 9 (4.9) | 0 (0.0) | 2 (3.7) | 6 (5.6) | 0.651 |
| Serious adverse events, n (%) | |||||
| Diabetic ketoacidosis | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| Severe hypoglycemia ‡ | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| Acute kidney injury | 2 (1.1) | 0 (0.0) | 1 (1.9) | 1 (0.9) | 0.821 |
| Bone fracture | 3 (1.6) | 0 (0.0) | 1 (1.9) | 2 (1.9) | 0.912 |
| Treatment discontinuation, n (%) | 7 (3.8) | 0 (0.0) | 2 (3.7) | 4 (3.7) | 0.854 |
| 5 (2.7) | 0 (0.0) | 1 (1.9) | 3 (2.8) | - |
| 2 (1.1) | 0 (0.0) | 1 (1.9) | 1 (0.9) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agha, A.; Afandi, B.; AlKaabi, S.; Alshkeili, N.A.N.S.; Alshemeili, M.A.A.; Ghaithi, M.M.A.; Alsaadi, M.M.H.; Alkaabi, J. Long-Term Cardiorenal Benefits of Sodium-Glucose Cotransporter-2 Inhibitors in Patients with Type 2 Diabetes Mellitus: A Real-World Single-Center Experience. J. Clin. Med. 2025, 14, 6365. https://doi.org/10.3390/jcm14186365
Agha A, Afandi B, AlKaabi S, Alshkeili NANS, Alshemeili MAA, Ghaithi MMA, Alsaadi MMH, Alkaabi J. Long-Term Cardiorenal Benefits of Sodium-Glucose Cotransporter-2 Inhibitors in Patients with Type 2 Diabetes Mellitus: A Real-World Single-Center Experience. Journal of Clinical Medicine. 2025; 14(18):6365. https://doi.org/10.3390/jcm14186365
Chicago/Turabian StyleAgha, Adnan, Bachar Afandi, Saeed AlKaabi, Naser Abdulla Naser Salem Alshkeili, Mohammed Ali Alsharoon Alshemeili, Mohammed Mohammed Al Ghaithi, Mohammad Mohammed Hareb Alsaadi, and Juma Alkaabi. 2025. "Long-Term Cardiorenal Benefits of Sodium-Glucose Cotransporter-2 Inhibitors in Patients with Type 2 Diabetes Mellitus: A Real-World Single-Center Experience" Journal of Clinical Medicine 14, no. 18: 6365. https://doi.org/10.3390/jcm14186365
APA StyleAgha, A., Afandi, B., AlKaabi, S., Alshkeili, N. A. N. S., Alshemeili, M. A. A., Ghaithi, M. M. A., Alsaadi, M. M. H., & Alkaabi, J. (2025). Long-Term Cardiorenal Benefits of Sodium-Glucose Cotransporter-2 Inhibitors in Patients with Type 2 Diabetes Mellitus: A Real-World Single-Center Experience. Journal of Clinical Medicine, 14(18), 6365. https://doi.org/10.3390/jcm14186365








