Mid-Term Results of a Cemented Titanium–Niobium Nitride-Coated Mobile Knee in Primary Total Knee Arthroplasty
Abstract
1. Introduction
2. Materials and Methods
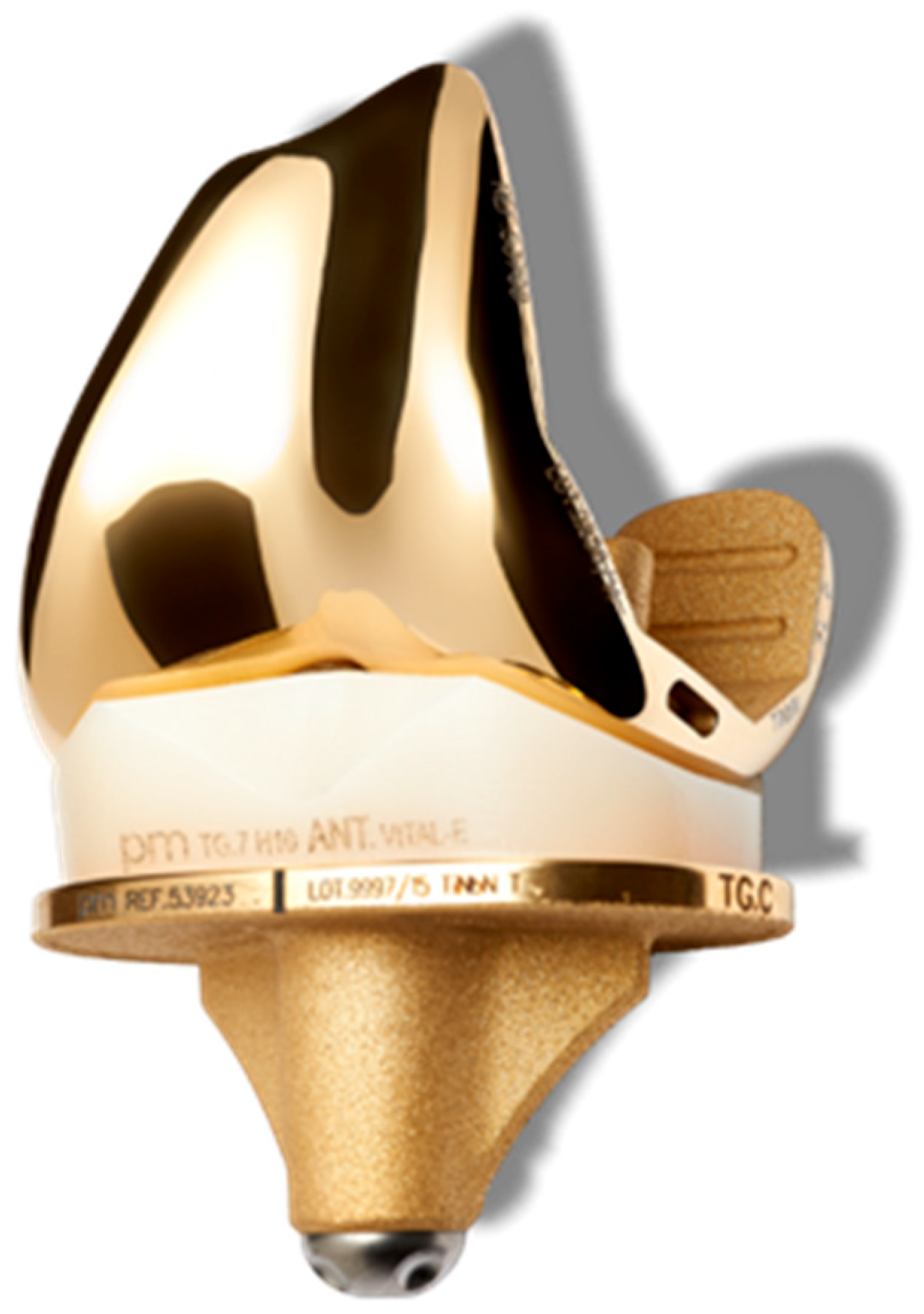
2.1. Implant Description
2.2. Surgical Procedure
2.3. Study Procedure
2.4. Clinical Assessment
2.5. Failures and Complications
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TKA | Total Knee Arthroplasty |
| TiNbN | Titanium–Niobium Nitride |
| FJS | Forgotten Joint Score |
| KOOS | Knee Injury and Osteoarthritis Outcome Score |
| CoCrMo | Cobalt–Chrome–Molybdenum |
| TiN | Titanium Nitride |
| ZrN-CN-CrCN | Zirconium Nitride multilayer coating |
| PEEK | Polyetheretherketone |
| PROMs | Patient-Reported Outcome Measures |
| BMI | Body Mass Index |
| CI | Confidence Interval |
References
- Rupp, M.; Lau, E.; Kurtz, S.M.; Alt, V. Projections of primary TKA and THA in Germany from 2016 through 2040. Clin. Orthop. Relat. Res. 2020, 478, 1622–1633. [Google Scholar] [CrossRef]
- Sadoghi, P.; Koutp, A.; Prieto, D.P.; Clauss, M.; Kayaalp, M.E.; Hirschmann, M.T. The projected economic burden and complications of revision hip and knee arthroplasties: Insights from national registry studies. Knee Surg. Sports Traumatol. Arthrosc. 2025, 33, 3211–3217. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.N.; Gill, D.R.; McAuliffe, M.J.; McDougall, C.; Stoney, J.D.; Vertullo, C.J.; Wall, C.J.; Corfield, S.; Page, R.; Cuthbert, A.R.; et al. Hip, Knee and Shoulder Arthroplasty: 2023 Annual Report; Australian Orthopaedic Association National Joint Replacement Registry, AOA: Adelaide, South Australia, 2023; Available online: https://aoanjrr.sahmri.com/annual-reports-2023 (accessed on 1 July 2025).
- Xie, F.; Sheng, S.; Ram, V.; Pandit, H. Hypoallergenic knee implant usage and clinical outcomes: Are they safe and effective? Arthroplast. Today 2024, 28, 101399. [Google Scholar] [CrossRef]
- Saccomanno, M.F.; Sircana, G.; Masci, G.; Cazzato, G.; Florio, M.; Capasso, L.; Passiatore, M.; Autore, G.; Maccauro, G.; Pola, E. Allergy in total knee replacement surgery: Is it a real problem? World J. Orthop. 2019, 10, 63–70. [Google Scholar] [CrossRef]
- Schroeder, S.; Braun, S.; Mueller, U.; Schroeder, M.; Sonntag, R.; Jaeger, S.; Kretzer, J.P. Polyethylene wear and metal release of TiNbN-coated knee implants. Wear 2020, 458–459, 203426. [Google Scholar] [CrossRef]
- Mohammed, A.; Metcalfe, A.; Woodnutt, D. Medium-term outcome of titanium nitride, mobile bearing total knee replacement. Acta Orthop. Belg. 2014, 80, 269–275. [Google Scholar]
- Banci, L.; Balato, G.; Salari, P.; Baldini, A. Systematic review and meta-analysis of ceramic coated implants in total knee arthroplasty. Comparable mid-term results to uncoated implants. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 839–851. [Google Scholar] [CrossRef]
- Tidd, J.L.; Gudapati, L.S.; Simmons, H.L.; Klika, A.K.; Pasqualini, I.; Cleveland Clinic Arthroplasty Group; Piuzzi, N.S. Do patients with hypoallergenic total knee arthroplasty implants for metal allergy do worse? An analysis of health care utilizations and patient-reported outcome measures. J. Arthroplast. 2024, 39, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Matar, H.E.; Porter, P.J.; Porter, M.L. Metal allergy in primary and revision total knee arthroplasty: A scoping review and evidence-based practical approach. Bone Jt. Open 2021, 2, 785–795. [Google Scholar] [CrossRef]
- Behrend, H.; Giesinger, K.; Giesinger, J.M.; Kuster, M.S. The “forgotten joint” as the ultimate goal in joint arthroplasty: Validation of a new patient-reported outcome measure. J. Arthroplast. 2012, 27, 430–436.e1. [Google Scholar] [CrossRef] [PubMed]
- Roos, E.M.; Roos, H.P.; Lohmander, L.S.; Ekdahl, C.; Beynnon, B.D. Knee Injury and Osteoarthritis Outcome Score (KOOS)—Development of a self-administered outcome measure. J. Orthop. Sports Phys. Ther. 1998, 28, 88–96. [Google Scholar] [CrossRef]
- Thakur, R.R.; Ast, M.P.; McGraw, M.; Bostrom, M.P.; Rodriguez, J.A.; Parks, M.L. Severe persistent synovitis after cobalt-chromium total knee arthroplasty requiring revision. Orthopedics 2013, 36, e520–e524. [Google Scholar] [CrossRef] [PubMed]
- Hörlesberger, N.; Smolle, M.A.; Leitner, L.; Hauer, G.; Leithner, A.; Sadoghi, P. Long-term clinical and radiological outcome of a cementless titanium-coated total knee arthroplasty system. Arch. Orthop. Trauma Surg. 2024, 144, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Lodge, C.J.; Matar, H.E.; Berber, R.; Radford, P.J.; Bloch, B.V. Ceramic coatings confer no survivorship advantages in total knee arthroplasty—A single-center series of 1641 knees. Arthroplast. Today 2023, 19, 101086. [Google Scholar] [CrossRef]
- Thienpont, E. Titanium niobium nitride knee implants are not inferior to chrome cobalt components for primary total knee arthroplasty at medium-term follow-up. Arch. Orthop. Trauma Surg. 2023, 143, 5269–5275. [Google Scholar] [CrossRef]
- Louwerens, J.K.G.; Hockers, N.; Achten, G.; Sierevelt, I.N.; Nolte, P.A.; van Hove, R.P. No clinical difference between TiN-coated versus uncoated cementless CoCrMo mobile-bearing total knee arthroplasty; 10-year follow-up of a randomized controlled trial. Knee Surg. Sports Traumatol. Arthrosc. 2021, 29, 750–756. [Google Scholar] [CrossRef]
- Hauer, G.; Leitner, L.; Ackerl, M.C.; Klim, S.; Vielgut, I.; Ehall, R.; Glehr, M.; Leithner, A.; Sadoghi, P. Titanium-nitride coating does not result in a better clinical outcome compared to conventional cobalt-chromium total knee arthroplasty after a long-term follow-up: A propensity score matching analysis. Coatings 2020, 10, 442. [Google Scholar] [CrossRef]
- Beyer, F.; Lützner, C.; Kirschner, S.; Lützner, J. Midterm results after coated and uncoated TKA: A randomized controlled study. Orthopedics 2016, 39 (Suppl. 3), S13–S17. [Google Scholar] [CrossRef]
- Postler, A.; Beyer, F.; Lützner, C.; Tille, E.; Lützner, J. Similar outcome during short-term follow-up after coated and uncoated total knee arthroplasty: A randomized controlled study. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 3459–3467. [Google Scholar] [CrossRef]
- van Hove, R.P.; Brohet, R.M.; van Royen, B.J.; Nolte, P.A. No clinical benefit of titanium nitride coating in cementless mobile-bearing total knee arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Tille, E.; Beyer, F.; Lützner, C.; Postler, A.; Thomas, P.; Summer, B.; Lützner, J. No difference in patient reported outcome and inflammatory response after coated and uncoated total knee arthroplasty–A randomized controlled study. BMC Musculoskelet. Disord. 2023, 24, 968. [Google Scholar] [CrossRef]
- Breugem, S.J.M.; Linnartz, J.; Sierevelt, I.; Bruijn, J.D.; Driessen, M.J.M. Evaluation of 1031 primary titanium nitride coated mobile bearing total knee arthroplasties in an orthopedic clinic. World J. Orthop. 2017, 8, 922–928. [Google Scholar] [CrossRef]
- Grimberg, A.W.; Grupp, T.M.; Elliott, J.; Melsheimer, O.; Jansson, V.; Steinbrück, A. Ceramic coating in cemented primary total knee arthroplasty is not associated with decreased risk of revision due to early prosthetic joint infection. J. Arthroplast. 2021, 36, 991–997. [Google Scholar] [CrossRef]
- Zondervan, R.L.; Vaux, J.J.; Blackmer, M.J.; Brazier, B.G.; Taunt, C.J., Jr. Improved outcomes in patients with positive metal sensitivity following revision total knee arthroplasty. J. Orthop. Surg. Res. 2019, 14, 182. [Google Scholar] [CrossRef]
- Middleton, S.; Toms, A. Allergy in total knee arthroplasty: A review of the facts. Bone Jt. J. 2016, 98-B, 437–441. [Google Scholar] [CrossRef]
- Bravo, D.; Wagner, E.R.; Larson, D.R.; Davis, M.P.; Pagnano, M.W.; Sierra, R.J. No increased risk of knee arthroplasty failure in patients with positive skin patch testing for metal hypersensitivity: A matched cohort study. J. Arthroplast. 2016, 31, 1717–1721. [Google Scholar] [CrossRef] [PubMed]
- Münch, H.J.; Jacobsen, S.S.; Olesen, J.T.; Menné, T.; Søballe, K.; Johansen, J.D.; Thyssen, J.P. The association between metal allergy, total knee arthroplasty, and revision: Study based on the Danish Knee Arthroplasty Register. Acta Orthop. 2015, 86, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Guenther, D.; Thomas, P.; Kendoff, D.; Omar, M.; Gehrke, T.; Haasper, C. Allergic reactions in arthroplasty: Myth or serious problem? Int. Orthop. 2016, 40, 239–244. [Google Scholar] [CrossRef]
- Bulaïd, Y.; Djebara, A.-E.; Belhaouane, R.; Havet, E.; Dehl, M.; Mertl, P. Beneficial effect of a zirconium-nitride-coated implant in total knee arthroplasty revision for suspected metal hypersensitivity. Orthop. Traumatol. Surg. Res. 2022, 108, 103320. [Google Scholar] [CrossRef]
- Herbster, M.; Döring, J.; Nohava, J.; Lohmann, C.H.; Halle, T.; Bertrand, J. Retrieval study of commercially available knee implant coatings TiN, TiNbN and ZrN on TiAl6V4 and CoCr28Mo6. J. Mech. Behav. Biomed. Mater. 2020, 112, 104034. [Google Scholar] [CrossRef] [PubMed]
- Łapaj, Ł.; Rozwalka, J. Retrieval analysis of TiN (titanium nitride) coated knee replacements: Coating wear and degradation in vivo. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Bormann, T.; Kraenzler, S.; Jaeger, S.; Kluess, D.; Mittelmeier, W.; Renkawitz, T.; Kretzer, J.P. Stability of ceramic coatings on retrieved knee prostheses. J. Mech. Behav. Biomed. Mater. 2023, 144, 105997. [Google Scholar] [CrossRef] [PubMed]

| KOOS Section | Mean | Range |
|---|---|---|
| Symptoms | 75.7 | 36–100 |
| Pain | 86.6 | 36–100 |
| Function of daily living | 83.0 | 62–100 |
| Sport | 37.8 | 0–100 |
| Quality of life | 70.5 | 0–100 |
| Parameter | Male Group | Female Group | p-Value |
|---|---|---|---|
| Number | 24 | 78 | - |
| Mean age (SD, range) | 62.2 (8.1, 36–75) | 64.6 (8.1, 35–80) | 0.2426 |
| Mean BMI (SD, range) | 26 (1.1, 25–31) | 25 (1.3, 24–30) | 0.3562 |
| Mean follow-up | 7.5 | 7.9 | 0.1626 |
| Number of reinterventions | 0 | 2 | 1.0000 |
| Number of reinterventions without implant removal | 0 | 1 | 1.0000 |
| Number of revisions | 0 | 1 | 1.0000 |
| Survival rate (CI, 95%) | 100% | 96.2% | 0.4702 |
| Mean FJS (SD) | 77.5 (19.8) | 67.7 (21.4) | 0.0596 |
| Mean KOOS | 76.3 | 68.9 | 0.0568 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jure, S.; Čimić, M.; Delimar, D. Mid-Term Results of a Cemented Titanium–Niobium Nitride-Coated Mobile Knee in Primary Total Knee Arthroplasty. J. Clin. Med. 2025, 14, 6357. https://doi.org/10.3390/jcm14186357
Jure S, Čimić M, Delimar D. Mid-Term Results of a Cemented Titanium–Niobium Nitride-Coated Mobile Knee in Primary Total Knee Arthroplasty. Journal of Clinical Medicine. 2025; 14(18):6357. https://doi.org/10.3390/jcm14186357
Chicago/Turabian StyleJure, Serdar, Mislav Čimić, and Domagoj Delimar. 2025. "Mid-Term Results of a Cemented Titanium–Niobium Nitride-Coated Mobile Knee in Primary Total Knee Arthroplasty" Journal of Clinical Medicine 14, no. 18: 6357. https://doi.org/10.3390/jcm14186357
APA StyleJure, S., Čimić, M., & Delimar, D. (2025). Mid-Term Results of a Cemented Titanium–Niobium Nitride-Coated Mobile Knee in Primary Total Knee Arthroplasty. Journal of Clinical Medicine, 14(18), 6357. https://doi.org/10.3390/jcm14186357






