Effect of Visual Field Test on Intraocular Pressure in Glaucoma Patients
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Humphrey Visual Field (HVF)
2.3. Intraocular Pressure (IOP)
2.4. Optical Coherence Tomography (OCT)
2.5. Statistical Analysis
3. Results
3.1. Demographics
3.2. Relationship Between IOP Before Versus After the Visual Field Test
3.3. Relationship Between IOP Before Versus After the Visual Field Test, According to Age
3.4. Factors Influencing Changes in IOP
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agarwal, R.; Gupta, S.K.; Agarwal, P.; Saxena, R.; Agrawal, S.S. Current concepts in the pathophysiology of glaucoma. Indian J. Ophthalmol. 2009, 57, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Gabelt, B.; Kaufman, P. Aqueous humor hydrodynamics. In Adler’s Physiology of the Eye: Clinical Application; Mosby: St. Louis, MO, USA, 2003. [Google Scholar]
- Allingham, R.R.; Damji, K.F.; Freedman, S.F.; Moroi, S.E.; Rhee, D.J.; Shields, M.B. Shields Textbook of Glaucoma; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- Ha, A.; Kim, Y.K.; Park, Y.J.; Jeoung, J.W.; Park, K.H. Intraocular pressure change during reading or writing on smartphone. PLoS ONE 2018, 13, e0206061. [Google Scholar] [CrossRef]
- Ahn, J.H.; Kil, H.K.; Lee, M.V. Positional Change of Intraocular Pressure and Its Relationship to Ocular Pulse Amplitude. J. Korean Ophthalmol. Soc. 2015, 56, 234–240. [Google Scholar] [CrossRef]
- Pakravan, M.; Samaeili, A.; Esfandiari, H.; Hassanpour, K.; Hooshmandi, S.; Yazdani, S.; Sharifipour, F.; Doozandeh, A.; Einollahi, B.; Pakravan, P.; et al. The influence of near vision tasks on intraocular pressure in normal subjects and glaucoma patients. J. Ophthalmic Vis. Res. 2022, 17, 497. [Google Scholar] [CrossRef] [PubMed]
- Priluck, A.Z.; Hoie, A.B.; High, R.R.; Gulati, V.; Ghate, D.A. Effect of near work on intraocular pressure in emmetropes. J. Ophthalmol. 2020, 2020, 1352434. [Google Scholar] [CrossRef]
- He, M.; Foster, P.J.; Ge, J.; Huang, W.; Zheng, Y.; Friedman, D.S.; Lee, P.S.; Khaw, P.T. Prevalence and clinical characteristics of glaucoma in adult Chinese: A population-based study in Liwan District, Guangzhou. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2782–2788. [Google Scholar] [CrossRef]
- Khaw, P.; Elkington, A. Glaucoma—1: Diagnosis. BMJ 2004, 328, 97–99. [Google Scholar] [CrossRef]
- Orzalesi, N.; Rossetti, L.; Bottoli, A.; Fogagnolo, P. Comparison of the effects of latanoprost, travoprost, and bimatoprost on circadian intraocular pressure in patients with glaucoma or ocular hypertension. Ophthalmology 2006, 113, 239–246. [Google Scholar] [CrossRef]
- Danesh-Meyer, H.V.; Boland, M.V.; Savino, P.J.; Miller, N.R.; Subramanian, P.S.; Girkin, C.A.; Quigley, H.A. Optic disc morphology in open-angle glaucoma compared with anterior ischemic optic neuropathies. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2003–2010. [Google Scholar] [CrossRef]
- Stewart, W.C.; Shields, M.B. The peripheral visual field in glaucoma: Reevaluation in the age of automated perimetry. Surv. Ophthalmol. 1991, 36, 59–69. [Google Scholar] [CrossRef]
- Odden, J.L.; Mihailovic, A.; Boland, M.V.; Friedman, D.S.; West, S.K.; Ramulu, P.Y. Evaluation of central and peripheral visual field concordance in glaucoma. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2797–2804. [Google Scholar] [CrossRef] [PubMed]
- Bertaud, S.; Skarbek Borowski, E.; Abbas, R.; Baudouin, C.; Labbé, A. Influence of automated visual field testing on intraocular pressure. BMC Ophthalmol. 2020, 20, 363. [Google Scholar] [CrossRef]
- Adhikari, P.G.; Joshi, S.N.; Poudel, A. Influence of visual field testing on intraocular pressure in patients with glaucoma suspects attending tertiary care center in Kathmandu. J. Chitwan Med. Coll. 2023, 13, 53–56. [Google Scholar] [CrossRef]
- Lee, C.M.; Yoo, Y.C. Short-Term Effect of Standard Automated Perimetry Testing on Intraocular Pressure in Patients with Open-Angle Glaucoma. Int. Sch. Res. Not. 2013, 2013, 956504. [Google Scholar] [CrossRef]
- Sawada, A.; Yamada, H.; Yamamoto, Y.; Yamamoto, T. Intraocular pressure alterations after visual field testing. Jpn. J. Ophthalmol. 2014, 58, 429–434. [Google Scholar] [CrossRef]
- Ni, N.; Tsai, J.C.; Shields, M.B.; Loewen, N.A. Elevation of intraocular pressure in glaucoma patients after automated visual field testing. J. Glaucoma 2012, 21, 590–595. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, M.; Zheng, B.; Wang, Q.; Sun, X. Impact of visual field testing on intraocular pressure change trends in healthy people and glaucoma patients. J. Ophthalmol. 2020, 2020, 7936205. [Google Scholar] [CrossRef] [PubMed]
- Recupero, S.M.; Contestabile, M.T.; Taverniti, L.; Villani, G.M.; Recupero, V. Open-angle glaucoma: Variations in the intraocular pressure after visual field examination. J. Glaucoma 2003, 12, 114–118. [Google Scholar] [CrossRef]
- Asrani, S.G.; McGlumphy, E.J.; Al-Aswad, L.A.; Chaya, C.J.; Lin, S.; Musch, D.C.; Pitha, I.; Robin, A.L.; Wirostko, B.; Johnson, T.V. The relationship between intraocular pressure and glaucoma: An evolving concept. Prog. Retin. Eye Res. 2024, 103, 101303. [Google Scholar] [CrossRef]
- Liu, T.; Cai, Y.; Hu, M.; Wang, Z.; Liu, X.; Chen, M.; Wang, K. The impact of intraocular pressure fluctuations on the progression of glaucoma and associated factors. Adv. Ophthalmol. Pract. Res. 2025, 5, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Soliman, T.T.; Abd Abd Gad, E.; Selim, S.M. Ganglion cell analysis versus retinal nerve fiber layer thickness in glaucoma diagnosis. J. Egypt. Ophthalmol. Soc. 2019, 112, 122–129. [Google Scholar] [CrossRef]
- Armaly, M.F.; Rubin, M.L. Accommodation and applanation tonometry. Arch. Ophthalmol. 1961, 65, 415–423. [Google Scholar] [CrossRef]
- Gherghel, D.; Orgül, S.; Gugleta, K.; Gekkieva, M.; Flammer, J. Relationship between ocular perfusion pressure and retrobulbar blood flow in patients with glaucoma with progressive damage. Am. J. Ophthalmol. 2000, 130, 597–605. [Google Scholar] [CrossRef]
- Flammer, J.; Orgül, S.; Costa, V.P.; Orzalesi, N.; Krieglstein, G.K.; Serra, L.M.; Renard, J.-P.; Stefánsson, E. The impact of ocular blood flow in glaucoma. Prog. Retin. Eye Res. 2002, 21, 359–393. [Google Scholar] [CrossRef] [PubMed]
- Schmidl, D.; Garhofer, G.; Schmetterer, L. The complex interaction between ocular perfusion pressure and ocular blood flow–relevance for glaucoma. Exp. Eye Res. 2011, 93, 141–155. [Google Scholar] [CrossRef]
- Mauger, R.R.; Likens, C.P.; Applebaum, M. Effects of accommodation and repeated applanation tonometry on intraocular pressure. Optom. Vis. Sci. 1984, 61, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, L.; Delaney, Y.; Fitzpatrick, P.; Blake, J. Effect of accommodation on intraocular pressure in glaucomatous eyes. Ir. J. Med. Sci. 1998, 167, 17–18. [Google Scholar] [CrossRef]
- Caprioli, J. Glaucoma: A disease of early cellular senescence. Investig. Ophthalmol. Vis. Sci. 2013, 54, ORSF60–ORSF67. [Google Scholar] [CrossRef]
- Erb, C.; Brody, S.; Rau, H. Effect of mental and physical stress on intraocular pressure—A pilot study. Klin. Monatsblatter Fur Augenheilkd. 1998, 212, 270–274. [Google Scholar] [CrossRef]
- Sator, M.O.; Joura, E.A.; Frigo, P.; Kurz, C.; Metka, M.; Hommer, A.; Huber, J.C. Hormone replacement therapy and intraocular pressure. Maturitas 1997, 28, 55–58. [Google Scholar] [CrossRef]
- Sator, M.O.; Akramian, J.; Joura, E.A.; Nessmann, A.; Wedrich, A.; Gruber, D.; Metka, M.; Huber, J.C. Reduction of intraocular pressure in a glaucoma patient undergoing hormone replacement therapy. Maturitas 1998, 29, 93–95. [Google Scholar] [CrossRef]
- Affinito, P.; Sardo, A.D.S.; Di Carlo, C.; Sammartino, A.; Tommaselli, G.A.; Bifulco, G.; Loffredo, A.; Loffredo, M.; Nappi, C. Effects of hormone replacement therapy on ocular function in postmenopause. Menopause 2003, 10, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A.; Katz, J.; Derick, R.J.; Gilbert, D.; Sommer, A. An evaluation of optic disc and nerve fiber layer examinations in monitoring progression of early glaucoma damage. Ophthalmology 1992, 99, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Sommer, A.; Quigley, H.A.; Robin, A.L.; Miller, N.R.; Katz, J.; Arkell, S. Evaluation of nerve fiber layer assessment. Arch. Ophthalmol. 1984, 102, 1766–1771. [Google Scholar] [CrossRef] [PubMed]
- Quigly, H. Optic nerve damage in human glaucoma, III Quantitative correlative correlation of nerve fiber loss and visual field defects in glaucoma, ischemic neuropathy, papilledema and toxic neuropathy. Arch. Ophthalmol. 1982, 100, 135–146. [Google Scholar] [CrossRef]
- Nishida, T.; Moghimi, S.; Chang, A.C.; Walker, E.; Liebmann, J.M.; Fazio, M.A.; Girkin, C.A.; Zangwill, L.M.; Weinreb, R.N. Association of intraocular pressure with retinal nerve fiber layer thinning in patients with glaucoma. JAMA Ophthalmol. 2022, 140, 1209–1216. [Google Scholar] [CrossRef]
- Leske, M.C.; Heijl, A.; Hyman, L.; Bengtsson, B.; Group, E.M.G.T. Early Manifest Glaucoma Trial: Design and baseline data. Ophthalmology 1999, 106, 2144–2153. [Google Scholar] [CrossRef]

| Glaucoma Patients | Control Group | p-Value * | |
|---|---|---|---|
| Number of patients | 132 | 103 | |
| Age (year, mean ±SD) | 58.66 ± 1.36 | 59.26 ± 1.85 | 0.432 |
| Sex (male, %) | 64 (48.4) | 53 (51.5) | 0.537 † |
| Hypertension (%) | 19 (14.3) | 16 (15.5) | 0.795 † |
| DM (%) | 12 (9.1) | 11 (10.7) | 0.852 † |
| IOP-lowering medication (n) | 1.52 ± 0.11 | 0 | 0.001 |
| Baseline IOP | 15.09 ± 2.24 | 15.04 ± 2.14 | 0.844 |
| CCT (mean ± SD) | 521.79 ± 5.93 | 519 ± 4.38 | 0.580 |
| BCVA (logMAR, mean ± SD) | 0.027 ± 0.01 | 0.023 ± 0.01 | 0.549 |
| Spherical equivalent (diopter, mean ± SD) | −1.14 ± 0.32 | −1.09 ± 0.34 | 0.534 |
| Axial length (mm, mean ± SD) | 23.15 ± 0.11 | 23.30 ± 0.21 | 0.155 |
| Mean RNFL thickness (µm, mean ± SD) | 80.58 ± 1.41 | 97.80 ± 7.61 | 0.001 |
| Humphrey visual field | |||
| Mean deviation (dB) | −3.02 ± 0.59 | −0.93 ± 0.36 | 0.001 |
| Pattern standard deviation (dB) | 4.56 ± 0.42 | 1.98 ± 0.93 | 0.001 |
| Visual field index (%) | 91.88 ± 1.63 | 97.69 ± 2.02 | 0.001 |
| VF testing time (min, mean ± SD) | 5.71 ± 0.13 | 4.81 ± 0.15 | 0.001 |
| Baseline IOP | 0 min | 10 min | 30 min | 60 min | p-Value * | |
|---|---|---|---|---|---|---|
| Glaucoma patients (n = 132) | 15.09 ± 2.24 | 14.29 ± 2.25 | 13.59 ± 2.11 | 14.62 ± 2.43 | 15.01 ± 2.18 | <0.001 |
| Control group (n = 103) | 15.04 ± 2.14 | 14.79 ± 2.06 | 14.51 ± 2.01 | 14.85 ± 2.34 | 15.02 ± 2.19 | <0.001 |
| <0.001 |
| Time | Baseline IOP | 0 min | 10 min | 30 min | 60 min | p-Value * | |
|---|---|---|---|---|---|---|---|
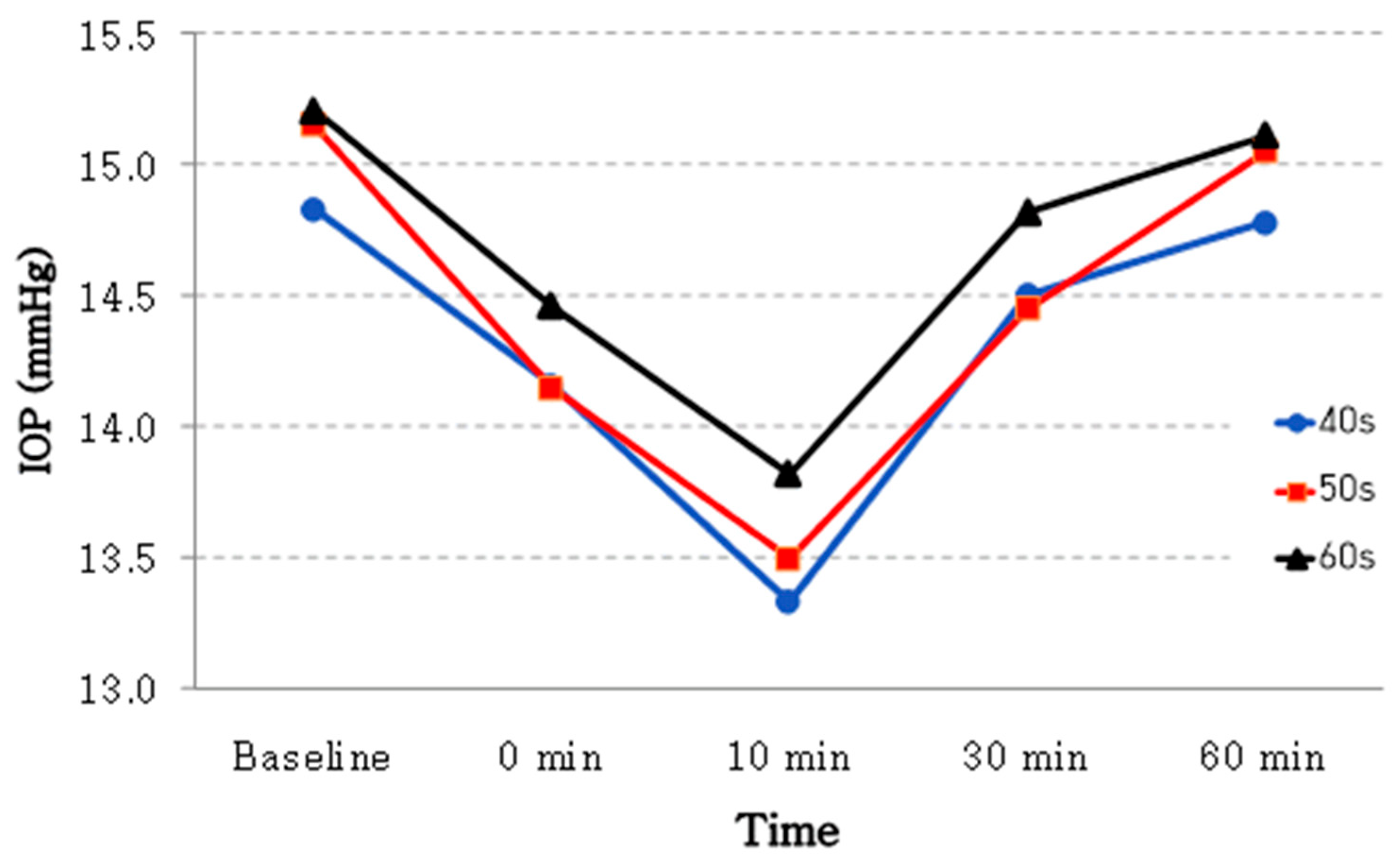
| Age groups | 40 s (n = 38) | 14.83 ± 2.28 | 14.16 ± 2.19 | 13.33 ± 2.24 | 14.50 ± 2.29 | 14.78 ± 2.28 | <0.001 |
| 50 s (n = 40) | 15.15 ± 1.81 | 14.15 ± 1.95 | 13.50 ± 1.88 | 14.45 ± 1.90 | 15.05 ± 1.85 | <0.001 | |
| 60 s (n = 54) | 15.21 ± 2.54 | 14.46 ± 2.49 | 13.82 ± 2.17 | 14.82 ± 2.54 | 15.11 ± 2.36 | <0.001 | |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Factors | Estimate (95% CI) | p-Value | Estimate (95% CI) | p-Value |
| Age | −0.003 (−0.05 to 0.06) | 0.119 | ||
| Sex (1 = male, 2 = female) | −1.375 (−2.47 to −0.29) | 0.014 | −1.415 (−2.45 to −0.38) | 0.008 |
| DM | 0.114 (−1.63 to 1.86) | 0.897 | ||
| HTN | −1.079 (−2.26 to 0.10) | 0.073 | −1.231 (−2.34 to −0.12) | 0.030 |
| IOP-lowering medication | 0.281 (−0.37 to 0.93) | 0.390 | ||
| Central cornea thickness | −0.005 (−0.02 to 0.01) | 0.415 | ||
| Axial length | −0.333 (−1.07 to 0.40) | 0.368 | ||
| BCVA(LogMAR) | −4.504 (−12.75 to 3.74) | 0.279 | ||
| Spherical equivalent | 0.069 (−0.16 to 0.29) | 0.599 | ||
| Visual field index | 0.012 (−0.03 to 0.06) | 0.583 | ||
| Mean deviation | 0.071 (−0.05 to 0.19) | 0.259 | ||
| Pattern standard deviation | −0.107 (−0.28 to 0.07) | 0.229 | ||
| Average RNFL | −0.046 (−0.09 to 0.003) | 0.069 | −0.057 (−0.10 to −0.01) | 0.017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, W.J.; Chung, H.J.; Lee, M.W.; Kim, J.T.; Lim, H.-B.; Park, K.S. Effect of Visual Field Test on Intraocular Pressure in Glaucoma Patients. J. Clin. Med. 2025, 14, 6356. https://doi.org/10.3390/jcm14186356
Jang WJ, Chung HJ, Lee MW, Kim JT, Lim H-B, Park KS. Effect of Visual Field Test on Intraocular Pressure in Glaucoma Patients. Journal of Clinical Medicine. 2025; 14(18):6356. https://doi.org/10.3390/jcm14186356
Chicago/Turabian StyleJang, Weon Jin, Han Jun Chung, Min Woo Lee, Jung Tae Kim, Hyung-Bin Lim, and Kee Sup Park. 2025. "Effect of Visual Field Test on Intraocular Pressure in Glaucoma Patients" Journal of Clinical Medicine 14, no. 18: 6356. https://doi.org/10.3390/jcm14186356
APA StyleJang, W. J., Chung, H. J., Lee, M. W., Kim, J. T., Lim, H.-B., & Park, K. S. (2025). Effect of Visual Field Test on Intraocular Pressure in Glaucoma Patients. Journal of Clinical Medicine, 14(18), 6356. https://doi.org/10.3390/jcm14186356







