Beyond the Cardio–Renal–Metabolic Axis: Emerging Therapeutic Targets and Novel Mechanisms of Action of Flozins
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Mechanism of Action and Therapeutic Effects of SGLT-2i
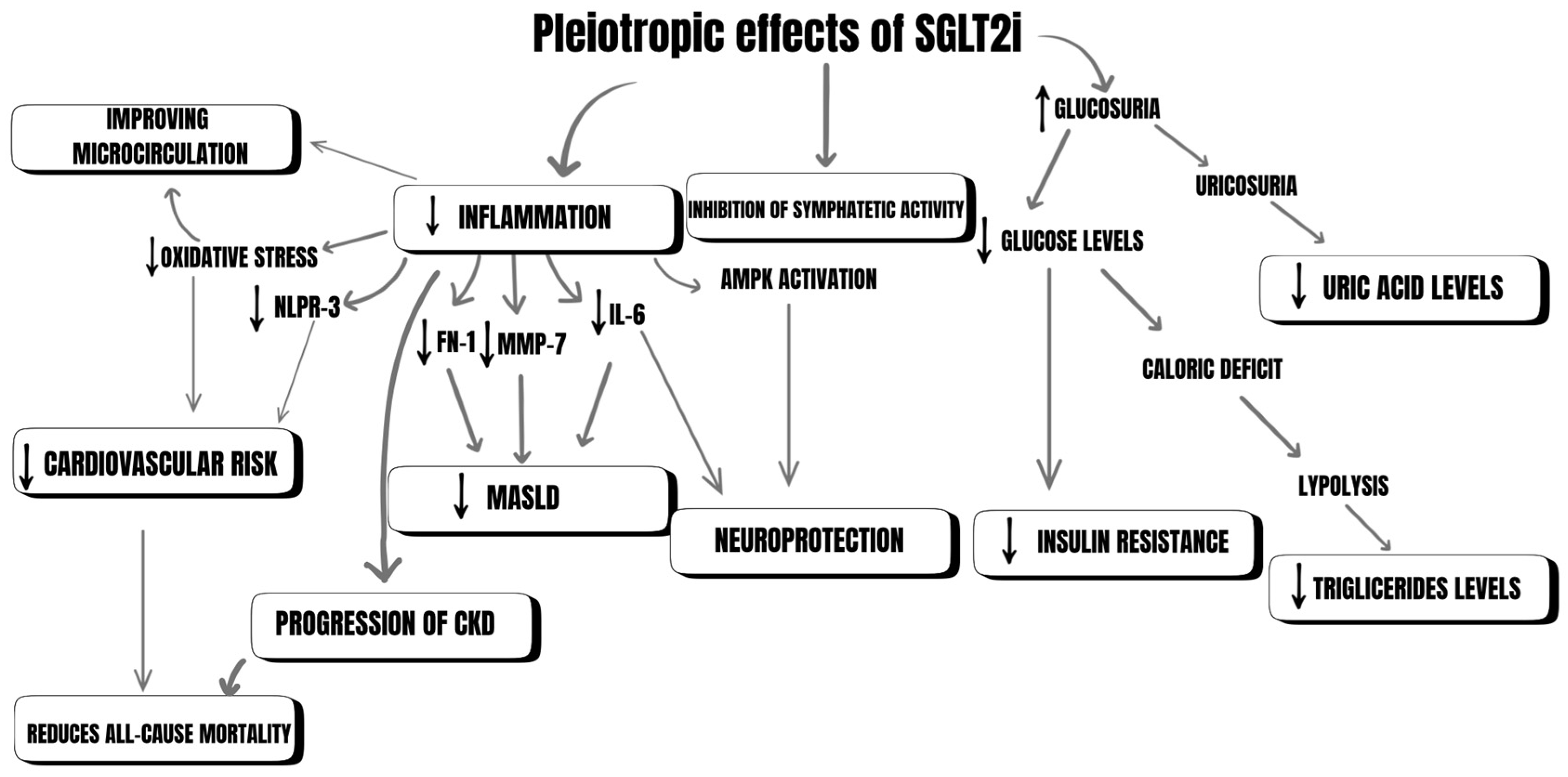
3.2. Pleiotropic Effects of SGLT-2i
3.2.1. Neuroprotection
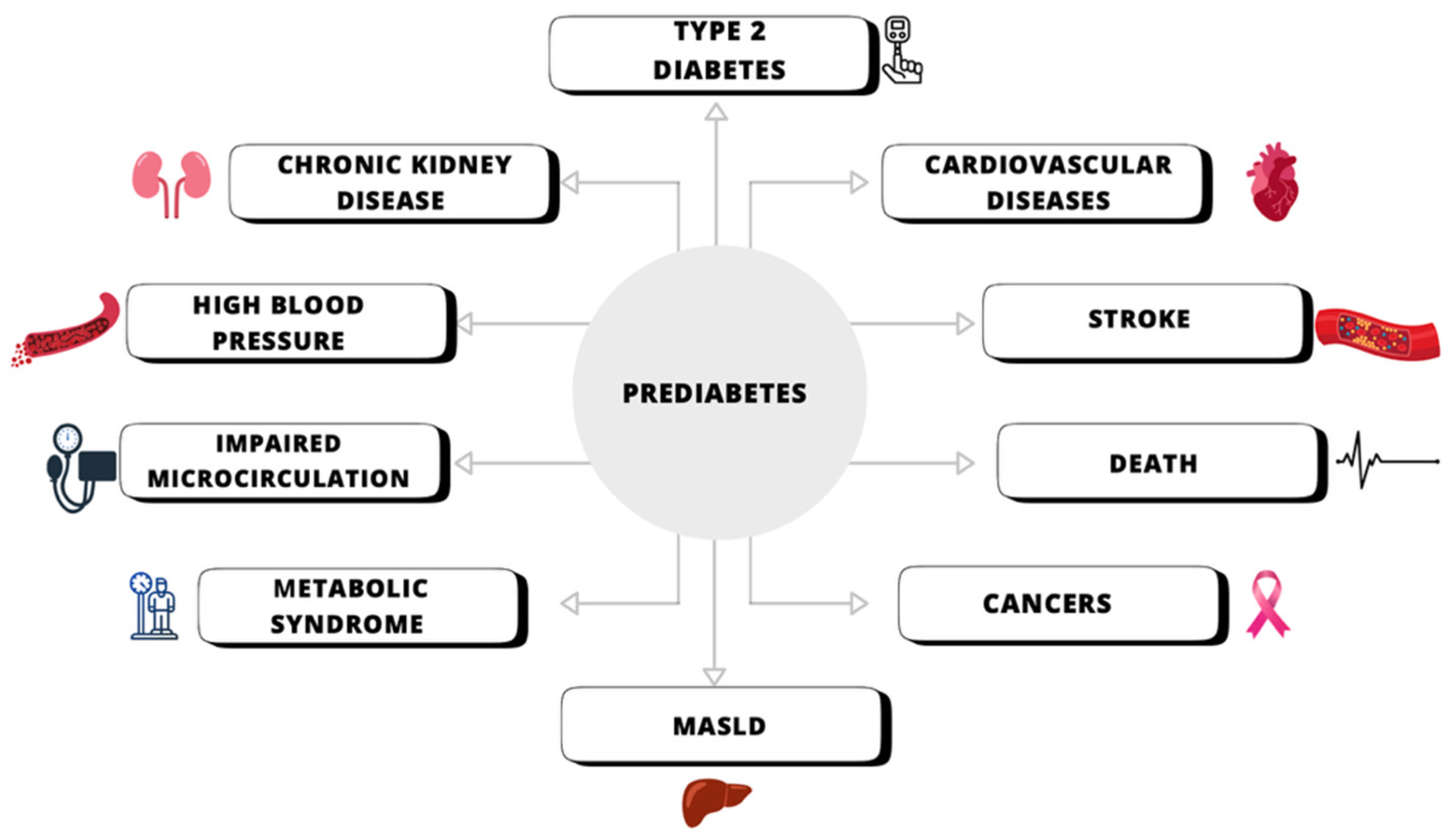
3.2.2. Decreasing the Risk of Developing Type 2 Diabetes
3.2.3. Managing MASLD
3.2.4. Reducing Uric Acid
3.2.5. SGLT2 Inhibitors and Anemia
3.2.6. Reducing Inflammation
3.2.7. Restoring Circadian Metabolic Rhythms and Enhancing Catabolic Processes in Type 2 Diabetes
3.2.8. SGLT2 Inhibitors and PCOS
3.2.9. Klotho Protein and Its Modulation by SGLT2 Inhibitors
3.2.10. Potential Role of SGLT2 Inhibitors in Oncology
Disrupting Tumor Glucose Metabolism and Cell Growth
Antitumor Activity in Animal Models
Modulation of Immune Responses and Tumor Microenvironment
Emerging Clinical Evidence
Molecular Mechanisms and Therapeutic Perspectives
3.2.11. Emerging Role of SGLT2 Inhibitors in Atrial Fibrillation Prevention
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haas, B.; Eckstein, N.; Pfeifer, V.; Mayer, P.; Hass, M.D.S. Efficacy, safety and regulatory status of SGLT2 inhibitors: Focus on canagliflozin. Nutr. Diabetes 2014, 4, e143. [Google Scholar] [CrossRef]
- Shaffner, J.; Chen, B.; Malhotra, D.K.; Dworkin, L.D.; Gong, R. Therapeutic Targeting of SGLT2: A New Era in the Treatment of Diabetes and Diabetic Kidney Disease. Front. Endocrinol. 2021, 12, 749010. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner–La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- EMPA-KIDNEY Collaborative Group. Design, recruitment, and baseline characteristics of the EMPA-KIDNEY trial. Nephrol. Dial. Transplant. 2022, 37, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.-F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- Wojciechowska, E.; Okopień, B. What Do We Know about Flozins: New, Pleiotropic Drugs. J. Health Study Med. 2023, 2023, 247–273. [Google Scholar] [CrossRef]
- Poudel, R. Renal glucose handling in diabetes and sodium glucose cotransporter 2 inhibition. Indian. J. Endocrinol. Metab. 2013, 17, 588. [Google Scholar] [CrossRef]
- Vallon, V. Glucose transporters in the kidney in health and disease. Pflug. Arch. 2020, 472, 1345–1370. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.A.; DeFronzo, R.A. Inhibition of Renal Glucose Reabsorption: A Novel Strategy for Achieving Glucose Control in Type 2 Diabetes Mellitus. Endocr. Pract. 2008, 14, 782–790. [Google Scholar] [CrossRef]
- Farber, S.J.; Berger, E.Y.; Earle, D.P. Effect of Diabetes and Insulin on the Maximum Capacity of the Renal Tubules to Reabsorb Glucose 1. J. Clin. Investig. 1951, 30, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, C.E. Maximum Tubular Reabsorption Capacity for Glucose and Renal Hemodynamics during Rapid Hypertonic Glucose Infusion in Normal and Diabetic Subjects. Scand. J. Clin. Lab. Investig. 1971, 28, 101–109. [Google Scholar] [CrossRef]
- Vallon, V. The Mechanisms and Therapeutic Potential of SGLT2 Inhibitors in Diabetes Mellitus. Annu. Rev. Med. 2015, 66, 255–270. [Google Scholar] [CrossRef]
- Nakamura, N.; Matsui, T.; Ishibashi, Y.; Yamagishi, S.I. Insulin stimulates SGLT2-mediated tubular glucose absorption via oxidative stress generation. Diabetol. Metab. Syndr. 2015, 7, 48. [Google Scholar] [CrossRef]
- Freitas, H.S.; Anhê, G.F.; Melo, K.F.S.; Okamoto, M.M.; Oliveira-Souza, M.; Bordin, S.; Machado, U.F. Na+-Glucose Transporter-2 Messenger Ribonucleic Acid Expression in Kidney of Diabetic Rats Correlates with Glycemic Levels: Involvement of Hepatocyte Nuclear Factor-1α Expression and Activity. Endocrinology 2008, 149, 717–724. [Google Scholar] [CrossRef]
- Rajeev, S.P.; Cuthbertson, D.J.; Wilding, J.P.H. Energy balance and metabolic changes with sodium-glucose co-transporter 2 inhibition. Diabetes Obes. Metab. 2016, 18, 125–134. [Google Scholar] [CrossRef]
- Brown, E.; Wilding, J.P.H.; Barber, T.M.; Alam, U.; Cuthbertson, D.J. Weight loss variability with SGLT2 inhibitors and GLP-1 receptor agonists in type 2 diabetes mellitus and obesity: Mechanistic possibilities. Obes. Rev. 2019, 20, 816–828. [Google Scholar] [CrossRef] [PubMed]
- Nespoux, J.; Vallon, V. SGLT2 inhibition and kidney protection. Clin. Sci. 2018, 132, 1329–1339. [Google Scholar] [CrossRef]
- Mori, I.; Ishizuka, T. Effects of SGLT2 Inhibitors on Renin-Aldosterone System for One Month and Six Months in Type 2 Diabetes. Diabetes 2018, 67 (Suppl. S1), 1196-P. [Google Scholar] [CrossRef]
- Schork, A.; Saynisch, J.; Vosseler, A.; Jaghutriz, B.A.; Heyne, N.; Peter, A.; Häring, H.-U.; Stefan, N.; Fritsche, A.; Artunc, F. Effect of SGLT2 inhibitors on body composition, fluid status and renin–angiotensin–aldosterone system in type 2 diabetes: A prospective study using bioimpedance spectroscopy. Cardiovasc. Diabetol. 2019, 18, 46. [Google Scholar] [CrossRef]
- Puglisi, S.; Rossini, A.; Poli, R.; Dughera, F.; Pia, A.; Terzolo, M.; Reimondo, G. Effects of SGLT2 Inhibitors and GLP-1 Receptor Agonists on Renin-Angiotensin-Aldosterone System. Front. Endocrinol. 2021, 12, 738848. [Google Scholar] [CrossRef]
- Fioretto, P.; Zambon, A.; Rossato, M.; Busetto, L.; Vettor, R. SGLT2 Inhibitors and the Diabetic Kidney. Diabetes Care 2016, 39 (Suppl. S2), S165–S171. [Google Scholar] [CrossRef]
- Hebert, L.E.; Weuve, J.; Scherr, P.A.; Evans, D.A. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 2013, 80, 1778–1783. [Google Scholar] [CrossRef]
- Rossi, A.; Berger, K.; Chen, H.; Leslie, D.; Mailman, R.B.; Huang, X. Projection of the prevalence of Parkinson’s disease in the coming decades: Revisited. Mov. Disord. 2018, 33, 156–159. [Google Scholar] [CrossRef]
- Gulisano, W.; Maugeri, D.; Baltrons, M.A.; Fà, M.; Amato, A.; Palmeri, A.; D’aDamio, L.; Grassi, C.; Devanand, D.; Honig, L.S.; et al. Role of Amyloid-β and Tau Proteins in Alzheimer’s Disease: Confuting the Amyloid Cascade. J. Alzheimer’s Dis. 2018, 64, S611–S631. [Google Scholar] [CrossRef]
- Frisoni, G.B.; Altomare, D.; Thal, D.R.; Ribaldi, F.; van der Kant, R.; Ossenkoppele, R.; Blennow, K.; Cummings, J.; van Duijn, C.; Nilsson, P.M.; et al. The probabilistic model of Alzheimer disease: The amyloid hypothesis revised. Nat. Rev. Neurosci. 2022, 23, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Maiti, P.; Manna, J.; Dunbar, G.L. Current understanding of the molecular mechanisms in Parkinson’s disease: Targets for potential treatments. Transl. Neurodegener. 2017, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Benito, M.; Granado, N.; García-Sanz, P.; Michel, A.; Dumoulin, M.; Moratalla, R. Modeling Parkinson’s Disease with the Alpha-Synuclein Protein. Front. Pharmacol. 2020, 11, 356. [Google Scholar] [CrossRef]
- Troncoso-Escudero, P.; Parra, A.; Nassif, M.; Vidal, R.L. Outside in: Unraveling the Role of Neuroinflammation in the Progression of Parkinson’s Disease. Front. Neurol. 2018, 9, 860. [Google Scholar] [CrossRef] [PubMed]
- Gelders, G.; Baekelandt, V.; Van der Perren, A. Linking Neuroinflammation and Neurodegeneration in Parkinson’s Disease. J. Immunol. Res. 2018, 2018, 4784268. [Google Scholar] [CrossRef]
- Sharma, N.; Nehru, B. Characterization of the lipopolysaccharide induced model of Parkinson’s disease: Role of oxidative stress and neuroinflammation. Neurochem. Int. 2015, 87, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Marttila, R.J.; Lorentz, H.; Rinne, U.K. Oxygen toxicity protecting enzymes in Parkinson’s disease. J. Neurol. Sci. 1988, 86, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Trist, B.G.; Hare, D.J.; Double, K.L. Oxidative stress in the aging substantia nigra and the etiology of Parkinson’s disease. Aging Cell 2019, 18, e13031. [Google Scholar] [CrossRef]
- Ni, A.; Ernst, C. Evidence That Substantia Nigra Pars Compacta Dopaminergic Neurons Are Selectively Vulnerable to Oxidative Stress Because They Are Highly Metabolically Active. Front. Cell. Neurosci. 2022, 16, 826193. [Google Scholar] [CrossRef]
- Pawlos, A.; Broncel, M.; Woźniak, E.; Gorzelak-Pabiś, P. Neuroprotective Effect of SGLT2 Inhibitors. Molecules 2021, 26, 7213. [Google Scholar] [CrossRef]
- Mohammed, N.N.; Tadros, M.G.; George, M.Y. Empagliflozin repurposing in Parkinson’s disease; modulation of oxidative stress, neuroinflammation, AMPK/SIRT-1/PGC-1α, and wnt/β-catenin pathways. Inflammopharmacology 2024, 32, 777–794. [Google Scholar] [CrossRef]
- Rakshe, P.S.; Dutta, B.J.; Chib, S.; Maurya, N.; Singh, S. Unveiling the interplay of AMPK/SIRT1/PGC-1α axis in brain health: Promising targets against aging and NDDs. Ageing Res. Rev. 2024, 96, 102255. [Google Scholar] [CrossRef]
- Shi, H.-J.; Xu, C.; Liu, M.-Y.; Wang, B.-K.; Liu, W.-B.; Chen, D.-H.; Zhang, L.; Xu, C.-Y.; Li, X.-F. Resveratrol Improves the Energy Sensing and Glycolipid Metabolism of Blunt Snout Bream Megalobrama amblycephala Fed High-Carbohydrate Diets by Activating the AMPK–SIRT1–PGC-1α Network. Front. Physiol. 2018, 9, 1258. [Google Scholar] [CrossRef]
- Marchetti, B. Wnt/β-Catenin Signaling Pathway Governs a Full Program for Dopaminergic Neuron Survival, Neurorescue and Regeneration in the MPTP Mouse Model of Parkinson’s Disease. Int. J. Mol. Sci. 2018, 19, 3743. [Google Scholar] [CrossRef] [PubMed]
- Heimke, M.; Lenz, F.; Rickert, U.; Lucius, R.; Cossais, F. Anti-Inflammatory Properties of the SGLT2 Inhibitor Empagliflozin in Activated Primary Microglia. Cells 2022, 11, 3107. [Google Scholar] [CrossRef] [PubMed]
- Mousa, H.H.; Sharawy, M.H.; Nader, M.A. Empagliflozin enhances neuroplasticity in rotenone-induced parkinsonism: Role of BDNF, CREB and Npas4. Life Sci. 2023, 312, 121258. [Google Scholar] [CrossRef]
- Salas, I.H.; De Strooper, B. Diabetes and Alzheimer’s Disease: A Link not as Simple as it Seems. Neurochem. Res. 2019, 44, 1271–1278. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, C.; Hua, S.; Liao, H.; Wang, M.; Xiong, Y.; Cao, F. An updated meta-analysis of cohort studies: Diabetes and risk of Alzheimer’s disease. Diabetes Res. Clin. Pract. 2017, 124, 41–47. [Google Scholar] [CrossRef]
- Hierro-Bujalance, C.; Infante-Garcia, C.; del Marco, A.; Herrera, M.; Carranza-Naval, M.J.; Suarez, J.; Alves-Martinez, P.; Lubian-Lopez, S.; Garcia-Alloza, M. Empagliflozin reduces vascular damage and cognitive impairment in a mixed murine model of Alzheimer’s disease and type 2 diabetes. Alzheimers Res. Ther. 2020, 12, 40. [Google Scholar] [CrossRef]
- Samman, W.A.; Selim, S.M.; El Fayoumi, H.M.; El-Sayed, N.M.; Mehanna, E.T.; Hazem, R.M. Dapagliflozin Ameliorates Cognitive Impairment in Aluminum-Chloride-Induced Alzheimer’s Disease via Modulation of AMPK/mTOR, Oxidative Stress and Glucose Metabolism. Pharmaceuticals 2023, 16, 753. [Google Scholar] [CrossRef]
- Kim, H.K.; Biessels, G.J.; Yu, M.H.; Hong, N.; Lee, Y.-H.; Lee, B.-W.; Kang, E.S.; Cha, B.-S.; Lee, E.J.; Lee, M. SGLT2 Inhibitor Use and Risk of Dementia and Parkinson Disease Among Patients with Type 2 Diabetes. Neurology 2024, 103, e209805. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.B. Metformin Should Not Be Used to Treat Prediabetes. Diabetes Care 2020, 43, 1983–1987. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Duru, O.K.; Tuttle, K.R.; Fukuma, S.; Taura, D.; Harada, N.; Inagaki, N.; Inoue, K. Sodium-Glucose Cotransporter 2 Inhibitors and New-onset Type 2 Diabetes in Adults with Prediabetes: Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Endocrinol. Metab. 2023, 108, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.E.; Janson, J.; Bonner-Weir, S.; Ritzel, R.; Rizza, R.A.; Butler, P.C. β-Cell Deficit and Increased β-Cell Apoptosis in Humans with Type 2 Diabetes. Diabetes 2003, 52, 102–110. [Google Scholar] [CrossRef]
- Kou, K.; Saisho, Y.; Satoh, S.; Yamada, T.; Itoh, H. Change in β-Cell Mass in Japanese Nondiabetic Obese Individuals. J. Clin. Endocrinol. Metab. 2013, 98, 3724–3730. [Google Scholar] [CrossRef]
- Zha, J.; Chi, X.; Yu, X.; Liu, X.; Liu, D.; Zhu, J.; Ji, H.; Liu, R. Interleukin-1β-Targeted Vaccine Improves Glucose Control and β-Cell Function in a Diabetic KK-Ay Mouse Model. PLoS ONE 2016, 11, e0154298. [Google Scholar] [CrossRef]
- Asahara, S.I.; Ogawa, W. SGLT2 inhibitors and protection against pancreatic beta cell failure. Diabetol. Int. 2019, 10, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Al Jobori, H.; Daniele, G.; Adams, J.; Cersosimo, E.; Solis-Herrera, C.; Triplitt, C.; A DeFronzo, R.; Abdul-Ghani, M. Empagliflozin Treatment Is Associated with Improved β-Cell Function in Type 2 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2018, 103, 1402–1407. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef]
- Morrison, A.E.; Zaccardi, F.; Khunti, K.; Davies, M.J. Causality between non-alcoholic fatty liver disease and risk of cardiovascular disease and type 2 diabetes: A meta-analysis with bias analysis. Liver Int. 2019, 39, 557–567. [Google Scholar] [CrossRef]
- Mantovani, A.; Byrne, C.D.; Bonora, E.; Targher, G. Nonalcoholic Fatty Liver Disease and Risk of Incident Type 2 Diabetes: A Meta-analysis. Diabetes Care 2018, 41, 372–382. [Google Scholar] [CrossRef]
- Chen, S.C.C.; Tsai, S.P.; Jhao, J.Y.; Jiang, W.K.; Tsao, C.K.; Chang, L.Y. Liver Fat, Hepatic Enzymes, Alkaline Phosphatase and the Risk of Incident Type 2 Diabetes: A Prospective Study of 132,377 Adults. Sci. Rep. 2017, 7, 4649. [Google Scholar] [CrossRef]
- Calzadilla Bertot, L.; Adams, L. The Natural Course of Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2016, 17, 774. [Google Scholar] [CrossRef]
- Kontana, A.; Tziomalos, K. Role of sodium-glucose co-transporter-2 inhibitors in the management of nonalcoholic fatty liver disease. World J. Gastroenterol. 2019, 25, 3664–3668. [Google Scholar] [CrossRef]
- Meng, Z.; Liu, X.; Li, T.; Fang, T.; Cheng, Y.; Han, L.; Sun, B.; Chen, L. The SGLT2 inhibitor empagliflozin negatively regulates IL-17/IL-23 axis-mediated inflammatory responses in T2DM with NAFLD via the AMPK/mTOR/autophagy pathway. Int. Immunopharmacol. 2021, 94, 107492. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.; Lee, J.; Huang, H.; Huang, H.; Liu, H.; Huang, C. Delayed intervention with a novel SGLT2 inhibitor NGI001 suppresses diet-induced metabolic dysfunction and non-alcoholic fatty liver disease in mice. Br. J. Pharmacol. 2020, 177, 239–253. [Google Scholar] [CrossRef]
- Wu, P.; Wen, W.; Li, J.; Xu, J.; Zhao, M.; Chen, H.; Sun, J. Systematic Review and Meta-Analysis of Randomized Controlled Trials on the Effect of SGLT2 Inhibitor on Blood Leptin and Adiponectin Level in Patients with Type 2 Diabetes. Horm. Metab. Res. 2019, 51, 487–494. [Google Scholar] [CrossRef]
- Petrescu, M.; Vlaicu, S.I.; Ciumărnean, L.; Milaciu, M.V.; Mărginean, C.; Florea, M.; Vesa, Ș.C.; Popa, M. Chronic Inflammation—A Link between Nonalcoholic Fatty Liver Disease (NAFLD) and Dysfunctional Adipose Tissue. Medicina 2022, 58, 641. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Perco, P.; Mulder, S.; Leierer, J.; Hansen, M.K.; Heinzel, A.; Mayer, G. Canagliflozin reduces inflammation and fibrosis biomarkers: A potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia 2019, 62, 1154–1166. [Google Scholar] [CrossRef] [PubMed]
- Sumida, Y.; Yoneda, M.; Tokushige, K.; Kawanaka, M.; Fujii, H.; Imajo, K.; Takahashi, H.; Ono, M.; Nozaki, Y.; Hyogo, H.; et al. Hepatoprotective Effect of SGLT2 Inhibitor on Nonalcoholic Fatty Liver Disease. Diabetes Res. Open Access 2020, 2, 17–25. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Lee, M.; Lee, J.Y.; Bae, J.; Shin, E.; Lee, Y.-H.; Lee, B.-W.; Kang, E.S.; Cha, B.-S. Ipragliflozin, an SGLT2 Inhibitor, Ameliorates High-Fat Diet-Induced Metabolic Changes by Upregulating Energy Expenditure through Activation of the AMPK/SIRT1 Pathway. Diabetes Metab. J. 2021, 45, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Androutsakos, T.; Nasiri-Ansari, N.; Bakasis, A.-D.; Kyrou, I.; Efstathopoulos, E.; Randeva, H.S.; Kassi, E. SGLT-2 Inhibitors in NAFLD: Expanding Their Role beyond Diabetes and Cardioprotection. Int. J. Mol. Sci. 2022, 23, 3107. [Google Scholar] [CrossRef]
- Shimizu, M.; Suzuki, K.; Kato, K.; Jojima, T.; Iijima, T.; Murohisa, T.; Iijima, M.; Takekawa, H.; Usui, I.; Hiraishi, H.; et al. Evaluation of the effects of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes. Metab. 2019, 21, 285–292. [Google Scholar] [CrossRef]
- Bajaj, H.S.; Brown, R.E.; Bhullar, L.; Sohi, N.; Kalra, S.; Aronson, R. SGLT2 inhibitors and incretin agents: Associations with alanine aminotransferase activity in type 2 diabetes. Diabetes Metab. 2018, 44, 493–499. [Google Scholar] [CrossRef]
- Kuchay, M.S.; Krishan, S.; Mishra, S.K.; Farooqui, K.J.; Singh, M.K.; Wasir, J.S.; Bansal, B.; Kaur, P.; Jevalikar, G.; Gill, H.K.; et al. Effect of Empagliflozin on Liver Fat in Patients with Type 2 Diabetes and Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial (E-LIFT Trial). Diabetes Care 2018, 41, 1801–1808. [Google Scholar] [CrossRef]
- Kahl, S.; Gancheva, S.; Straßburger, K.; Herder, C.; Machann, J.; Katsuyama, H.; Kabisch, S.; Henkel, E.; Kopf, S.; Lagerpusch, M.; et al. Empagliflozin Effectively Lowers Liver Fat Content in Well-Controlled Type 2 Diabetes: A Randomized, Double-Blind, Phase 4, Placebo-Controlled Trial. Diabetes Care 2020, 43, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Mirarchi, L.; Amodeo, S.; Citarrella, R.; Licata, A.; Soresi, M.; Giannitrapani, L. SGLT2 Inhibitors as the Most Promising Influencers on the Outcome of Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2022, 23, 3668. [Google Scholar] [CrossRef]
- Shibuya, T.; Fushimi, N.; Kawai, M.; Yoshida, Y.; Hachiya, H.; Ito, S.; Kawai, H.; Ohashi, N.; Mori, A. Luseogliflozin improves liver fat deposition compared to metformin in type 2 diabetes patients with non-alcoholic fatty liver disease: A prospective randomized controlled pilot study. Diabetes Obes. Metab. 2018, 20, 438–442. [Google Scholar] [CrossRef]
- Becker, B.F. Towards the physiological function of uric acid. Free Radic. Biol. Med. 1993, 14, 615–631. [Google Scholar] [CrossRef]
- Schmidt, J.A.; Crowe, F.L.; Appleby, P.N.; Key, T.J.; Travis, R.C. Serum Uric Acid Concentrations in Meat Eaters, Fish Eaters, Vegetarians and Vegans: A Cross-Sectional Analysis in the EPIC-Oxford Cohort. PLoS ONE 2013, 8, e56339. [Google Scholar] [CrossRef]
- Prasad Sah, O.S.; Qing, Y.X. Associations between Hyperuricemia and Chronic Kidney Disease: A Review. Nephro-Urol. Mon. 2015, 7, e27233. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, E.; Pandya, B.J.; Chung, L.; Hariri, A.; Dabbous, O. Hyperuricemia in Young Adults and Risk of Insulin Resistance, Prediabetes, and Diabetes: A 15-Year Follow-up Study. Am. J. Epidemiol. 2012, 176, 108–116. [Google Scholar] [CrossRef]
- Ahmadieh, H.; Azar, S. Effects of Sodium Glucose Cotransporter-2 Inhibitors on Serum Uric Acid in Type 2 Diabetes Mellitus. Diabetes Technol. Ther. 2017, 19, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Huang, X.; Shao, H.; Tian, F. Effects of dapagliflozin on serum uric acid levels in hospitalized type 2 diabetic patients with inadequate glycemic control: A randomized controlled trial. Ther. Clin. Risk Manag. 2018, 14, 2407–2413. [Google Scholar] [CrossRef]
- Yuan, T.; Liu, S.; Dong, Y.; Fu, Y.; Tang, Y.; Zhao, W.G. Effects of Dapagliflozin on Serum and Urinary Uric Acid Levels in Type 2 Diabetic Patients: A Prospective Pilot Trial. Res. Sq. 2020. preprint. [Google Scholar] [CrossRef]
- Aziz, H.; Noor, M.; Ahmad, B.; Ali, S.; Farhat, K.; Siddiqi, F.A. Comparison of Extraglycemic effects of Dapagliflozin and Empagliflozin. Pak. Armed Forces Med. J. 2023, 73, 938–941. [Google Scholar] [CrossRef]
- Chino, Y.; Samukawa, Y.; Sakai, S.; Nakai, Y.; Yamaguchi, J.; Nakanishi, T.; Tamai, I. SGLT2 inhibitor lowers serum uric acid through alteration of uric acid transport activity in renal tubule by increased glycosuria. Biopharm. Drug Dispos. 2014, 35, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Novikov, A.; Fu, Y.; Huang, W.; Freeman, B.; Patel, R.; van Ginkel, C.; Koepsell, H.; Busslinger, M.; Onishi, A.; Nespoux, J.; et al. SGLT2 inhibition and renal urate excretion: Role of luminal glucose, GLUT9, and URAT1. Am. J. Physiol.-Ren. Physiol. 2019, 316, F173–F185. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Chen, H.; Wen, S.; Yuan, Y.; Yang, L.; Xu, D.; Zhou, L. The Mechanism of Sodium-Glucose Cotransporter-2 Inhibitors in Reducing Uric Acid in Type 2 Diabetes Mellitus. Diabetes Metab. Syndr. Obes. 2023, 16, 437–445. [Google Scholar] [CrossRef]
- Yan, W.; Wen, S.; Zhou, L. Effect of Intestinal Flora on Hyperuricemia-Induced Chronic Kidney Injury in Type 2 Diabetic Patients and the Therapeutic Mechanism of New Anti-Diabetic Prescription Medications. Diabetes Metab. Syndr. Obes. 2023, 16, 3029–3044. [Google Scholar] [CrossRef]
- Prattichizzo, F.; De Nigris, V.; Micheloni, S.; La Sala, L.; Ceriello, A. Increases in circulating levels of ketone bodies and cardiovascular protection with SGLT2 inhibitors: Is low-grade inflammation the neglected component? Diabetes Obes. Metab. 2018, 20, 2515–2522. [Google Scholar] [CrossRef]
- Zelniker, T.A.; Wiviott, S.D.; Raz, I.; Im, K.; Goodrich, E.; Bonaca, M.P.; Mosenzon, O.; Kato, E.; Cahn, A.; Furtado, R.H.M.; et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019, 393, 31–39. [Google Scholar] [CrossRef]
- Khat, D.Z.; Husain, M. Molecular Mechanisms Underlying the Cardiovascular Benefits of SGLT2i and GLP-1RA. Curr. Diab. Rep. 2018, 18, 45. [Google Scholar] [CrossRef]
- Sridhar, V.S.; Davies, M.J.; Banks, P.; Girard, M.; Carroll, A.K.; Cherney, D.Z.I. Effects of sotagliflozin on anaemia in patients with type 2 diabetes and chronic kidney disease stages 3 and 4. Diabetes Obes. Metab. 2025, 27, 1010–1013. [Google Scholar] [CrossRef]
- Shih, H.M.; Wu, C.J.; Lin, S.L. Physiology and pathophysiology of renal erythropoietin-producing cells. J. Formos. Med. Assoc. 2018, 117, 955–963. [Google Scholar] [CrossRef]
- Packer, M. Alleviation of Anemia by SGLT2 Inhibitors in Patients with CKD: Mechanisms and Results of Long-Term Placebo-Controlled Trials. Clin. J. Am. Soc. Nephrol. 2024, 19, 531–534. [Google Scholar] [CrossRef]
- Docherty, K.F.; Curtain, J.P.; Anand, I.S.; Bengtsson, O.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Langkilde, A.M.; Martinez, F.A.; Ponikowski, P.; et al. Effect of dapagliflozin on anaemia in DAPA-HF. Eur. J. Heart Fail. 2021, 23, 617–628. [Google Scholar] [CrossRef]
- Sano, M. A Role of Sodium-Glucose Co-Transporter 2 in Cardiorenal Anemia Iron Deficiency Syndrome. Int. J. Mol. Sci. 2023, 24, 5983. [Google Scholar] [CrossRef]
- Packer, M. How can sodium–glucose cotransporter 2 inhibitors stimulate erythrocytosis in patients who are iron-deficient? Implications for understanding iron homeostasis in heart failure. Eur. J. Heart Fail. 2022, 24, 2287–2296. [Google Scholar] [CrossRef]
- Oshima, M.; Neuen, B.L.; Jardine, M.J.; Bakris, G.; Edwards, R.; Levin, A.; Mahaffey, K.W.; Neal, B.; Pollock, C.; Rosenthal, N.; et al. Effects of canagliflozin on anaemia in patients with type 2 diabetes and chronic kidney disease: A post-hoc analysis from the CREDENCE trial. Lancet Diabetes Endocrinol. 2020, 8, 903–914. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Anker, S.D.; Butler, J.; Filippatos, G.; Iwata, T.; Salsali, A.; Butler, J.; Zeller, C.; Pocock, S.J.; Zannad, F.; et al. Impact of anaemia and the effect of empagliflozin in heart failure with reduced ejection fraction: Findings from EMPEROR-Reduced. Eur. J. Heart Fail. 2022, 24, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Koshino, A.; Schechter, M.; Chertow, G.M.; Vart, P.; Jongs, N.; Toto, R.D.; Rossing, P.; Correa-Rotter, R.; McMurray, J.J.; Górriz, J.L.; et al. Dapagliflozin and Anemia in Patients with Chronic Kidney Disease. NEJM Evid. 2023, 2, EVIDoa2300049. [Google Scholar] [CrossRef] [PubMed]
- Feijóo-Bandín, S.; Aragón-Herrera, A.; Otero-Santiago, M.; Anido-Varela, L.; Moraña-Fernández, S.; Tarazón, E.; Roselló-Lletí, E.; Portolés, M.; Gualillo, O.; González-Juanatey, J.R.; et al. Role of Sodium-Glucose Co-Transporter 2 Inhibitors in the Regulation of Inflammatory Processes in Animal Models. Int. J. Mol. Sci. 2022, 23, 5634. [Google Scholar] [CrossRef] [PubMed]
- Pirklbauer, M.; Sallaberger, S.; Staudinger, P.; Corazza, U.; Leierer, J.; Mayer, G.; Schramek, H. Empagliflozin Inhibits IL-1β-Mediated Inflammatory Response in Human Proximal Tubular Cells. Int. J. Mol. Sci. 2021, 22, 5089. [Google Scholar] [CrossRef]
- Lee, S.-G.; Lee, S.-J.; Lee, J.-J.; Kim, J.-S.; Lee, O.-H.; Kim, C.-K.; Kim, D.; Lee, Y.-H.; Oh, J.; Park, S.; et al. Anti-Inflammatory Effect for Atherosclerosis Progression by Sodium-Glucose Cotransporter 2 (SGLT-2) Inhibitor in a Normoglycemic Rabbit Model. Korean Circ. J. 2020, 50, 443. [Google Scholar] [CrossRef]
- Lee, N.; Heo, Y.J.; Choi, S.-E.; Jeon, J.Y.; Han, S.J.; Kim, D.J.; Kang, Y.; Lee, K.W.; Kim, H.J.; Hautz, T. Anti-inflammatory Effects of Empagliflozin and Gemigliptin on LPS-Stimulated Macrophage via the IKK/NF-κB, MKK7/JNK, and JAK2/STAT1 Signalling Pathways. J. Immunol. Res. 2021, 2021, 9944880. [Google Scholar] [CrossRef]
- Maayah, Z.H.; Ferdaoussi, M.; Takahara, S.; Soni, S.; Dyck, J.R.B. Empagliflozin suppresses inflammation and protects against acute septic renal injury. Inflammopharmacology 2021, 29, 269–279. [Google Scholar] [CrossRef]
- Schönberger, E.; Mihaljević, V.; Steiner, K.; Šarić, S.; Kurevija, T.; Majnarić, L.T.; Ćurčić, I.B.; Canecki-Varžić, S. Immunomodulatory Effects of SGLT2 Inhibitors—Targeting Inflammation and Oxidative Stress in Aging. Int. J. Environ. Res. Public Health 2023, 20, 6671. [Google Scholar] [CrossRef]
- Packer, M. Role of Impaired Nutrient and Oxygen Deprivation Signaling and Deficient Autophagic Flux in Diabetic CKD Development: Implications for Understanding the Effects of Sodium-Glucose Cotransporter 2-Inhibitors. J. Am. Soc. Nephrol. 2020, 31, 907–919. [Google Scholar] [CrossRef]
- Tuttle, K.R. Digging deep into cells to find mechanisms of kidney protection by SGLT2 inhibitors. J. Clin. Investig. 2023, 133, e167700. [Google Scholar] [CrossRef]
- Li, X.; Lu, Q.; Qiu, Y.; Carmo, J.M.D.; Wang, Z.; da Silva, A.A.; Mouton, A.; Omoto, A.C.M.; Hall, M.E.; Li, J.; et al. Direct Cardiac Actions of the Sodium Glucose Co-Transporter 2 Inhibitor Empagliflozin Improve Myocardial Oxidative Phosphorylation and Attenuate Pressure-Overload Heart Failure. J. Am. Heart Assoc. 2021, 10, e018298. [Google Scholar] [CrossRef]
- Kounatidis, D.; Vallianou, N.; Evangelopoulos, A.; Vlahodimitris, I.; Grivakou, E.; Kotsi, E.; Dimitriou, K.; Skourtis, A.; Mourouzis, I. SGLT-2 Inhibitors and the Inflammasome: What’s Next in the 21st Century? Nutrients 2023, 15, 2294. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Huang, C.C.; Lu, Y.; Shirazi, S.; Gajendrareddy, P.; Ravindran, S.; Cooper, L.F. Bone regeneration is mediated by macrophage extracellular vesicles. Bone 2020, 141, 115627. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.-A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Markina, Y.V.; Kirichenko, T.V.; Tolstik, T.V.; Bogatyreva, A.I.; Zotova, U.S.; Cherednichenko, V.R.; Postnov, A.Y.; Markin, A.M. Target and Cell Therapy for Atherosclerosis and CVD. Int. J. Mol. Sci. 2023, 24, 10308. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Nagata, N.; Nagashimada, M.; Zhuge, F.; Ni, Y.; Chen, G.; Mayoux, E.; Kaneko, S.; Ota, T. SGLT2 Inhibition by Empagliflozin Promotes Fat Utilization and Browning and Attenuates Inflammation and Insulin Resistance by Polarizing M2 Macrophages in Diet-induced Obese Mice. EBioMedicine 2017, 20, 137–149. [Google Scholar] [CrossRef]
- Coppack, S.W. Pro-inflammatory cytokines and adipose tissue. Proc. Nutr. Soc. 2001, 60, 349–356. [Google Scholar] [CrossRef]
- Park, H.S.; Park, J.Y.; Yu, R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-α and IL-6. Diabetes Res. Clin. Pract. 2005, 69, 29–35. [Google Scholar] [CrossRef]
- Fontana, L.; Eagon, J.C.; Trujillo, M.E.; Scherer, P.E.; Klein, S. Visceral Fat Adipokine Secretion Is Associated with Systemic Inflammation in Obese Humans. Diabetes 2007, 56, 1010–1013. [Google Scholar] [CrossRef]
- Bolinder, J.; Ljunggren, Ö.; Kullberg, J.; Johansson, L.; Wilding, J.; Langkilde, A.M.; Sugg, J.; Parikh, S. Effects of Dapagliflozin on Body Weight, Total Fat Mass, and Regional Adipose Tissue Distribution in Patients with Type 2 Diabetes Mellitus with Inadequate Glycemic Control on Metformin. J. Clin. Endocrinol. Metab. 2012, 97, 1020–1031. [Google Scholar] [CrossRef]
- Esterline, R.L.; Vaag, A.; Oscarsson, J.; Vora, J. Mechanisms in Endocrinology: SGLT2 inhibitors: Clinical benefits by restoration of normal diurnal metabolism? Eur. J. Endocrinol. 2018, 178, R113–R125. [Google Scholar] [CrossRef]
- Deswal, R.; Narwal, V.; Dang, A.; Pundir, C. The Prevalence of Polycystic Ovary Syndrome: A Brief Systematic Review. J. Hum. Reprod. Sci. 2020, 13, 261. [Google Scholar] [CrossRef]
- Zhang, J.; Xing, C.; Cheng, X.; He, B. Canagliflozin combined with metformin versus metformin monotherapy for endocrine and metabolic profiles in overweight and obese women with polycystic ovary syndrome: A single-center, open-labeled prospective randomized controlled trial. Front. Endocrinol. 2022, 13, 1003238. [Google Scholar] [CrossRef] [PubMed]
- Javed, Z.; Papageorgiou, M.; Deshmukh, H.; Rigby, A.S.; Qamar, U.; Abbas, J.; Khan, A.Y.; Kilpatrick, E.S.; Atkin, S.L.; Sathyapalan, T. Effects of empagliflozin on metabolic parameters in polycystic ovary syndrome: A randomized controlled study. Clin. Endocrinol. 2019, 90, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Ignatenko, S.; Wagner, F.; Dokras, A.; Seufert, J.; Zwanziger, D.; Dunschen, K.; Zakaria, M.; Huseinovic, N.; Basson, C.T.; et al. Licogliflozin versus placebo in women with polycystic ovary syndrome: A randomized, double-blind, phase 2 trial. Diabetes Obes. Metab. 2021, 23, 2595–2599. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Shao, X.; Xing, F.; Zhang, Y.; Gao, X.; Zeng, Q.; Dilimulati, D.; Qu, S.; Zhang, M. Efficacy of canagliflozin versus metformin in women with polycystic ovary syndrome: A randomized, open-label, noninferiority trial. Diabetes Obes. Metab. 2022, 24, 312–320. [Google Scholar] [CrossRef]
- Elkind-Hirsch, K.E.; Chappell, N.; Seidemann, E.; Storment, J.; Bellanger, D. Exenatide, Dapagliflozin, or Phentermine/Topiramate Differentially Affect Metabolic Profiles in Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2021, 106, 3019–3033. [Google Scholar] [CrossRef]
- Pruett, J.E.; Fernandez, E.D.T.; Everman, S.J.; Vinson, R.M.; Davenport, K.; Logan, M.K.; Ye, S.A.; Romero, D.G.; Cardozo, L.L.Y. Impact of SGLT-2 Inhibition on Cardiometabolic Abnormalities in a Rat Model of Polycystic Ovary Syndrome. Int. J. Mol. Sci. 2021, 22, 2576. [Google Scholar] [CrossRef]
- Nevola, R.; Villani, A.; Imbriani, S.; Alfano, M.; Criscuolo, L.; Beccia, D.; Ruocco, R.; Femine, A.D.; Gragnano, F.; Cozzolino, D.; et al. Sodium-Glucose Co-Transporters Family: Current Evidence, Clinical Applications and Perspectives. Front. Biosci.-Landmark 2023, 28, 103. [Google Scholar] [CrossRef]
- Marinkovic-Radosevic, J.; Cigrovski Berkovic, M.; Kruezi, E.; Bilic-Curcic, I.; Mrzljak, A. Exploring new treatment options for polycystic ovary syndrome: Review of a novel antidiabetic agent SGLT2 inhibitor. World J. Diabetes 2021, 12, 932–938. [Google Scholar] [CrossRef]
- Rakic, D.; Jakovljevic, V.; Jovic, N.; Ilic, M.B.; Dimitrijevic, A.; Vulovic, T.; Arsenijevic, P.; Sretenovic, J.; Nikolic, M.; Fisenko, V.P.; et al. The Potential of SGLT-2 Inhibitors in the Treatment of Polycystic Ovary Syndrome: The Current Status and Future Perspectives. Biomedicines 2023, 11, 998. [Google Scholar] [CrossRef] [PubMed]
- Sinha, B.; Ghosal, S. A Meta-Analysis of the Effect of Sodium Glucose Cotransporter-2 Inhibitors on Metabolic Parameters in Patients with Polycystic Ovary Syndrome. Front. Endocrinol. 2022, 13, 830401. [Google Scholar] [CrossRef]
- Khan, A.; Ahmad, F.; Ali, N.; Shah, K.U. Effect of canagliflozin alone and in combination with metformin on hormonal derangements and estrous cycle in a polycystic ovary syndrome rat model. Khyber Med. Univ. J. 2021, 13, 94–99. [Google Scholar] [CrossRef]
- Mora-Fernández, C.; Sánchez-Niño, M.D.; Donate-Correa, J.; Martín-Núñez, E.; Pérez-Delgado, N.; Valiño-Rivas, L.; Fernández-Fernández, B.; Ortiz, A.; Navarro-González, J.F. Sodium-glucose co-transporter-2 inhibitors increase Klotho in patients with diabetic kidney disease: A clinical and experimental study. Biomed. Pharmacother. 2022, 154, 113677. [Google Scholar] [CrossRef]
- Abbás, N.A.T.; Salem, A.E.; Awad, M.M. Empagliflozin attenuates renal fibrosis in rats exposed to unilateral ureteric obstruction: Potential role of Klotho expression. Naunyn Schmiedebergs Arch. Pharmacol. 2018, 391, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- Topchii, I.; Semenovykh, P.; Shcherban, T.; Galchinska, V.; Savicheva, K. Serum Klotho protein level in type 2 diabetic patients depending on the renal function. Ukr. J. Nephrol. Dial. 2021, 3, 60–66. [Google Scholar] [CrossRef]
- Wolf, I.; Föller, M.; Feger, M. The impact of SGLT2 inhibitors on α-Klotho in renal MDCK and HK-2 cells. Front. Endocrinol. 2023, 14, 1069715. [Google Scholar] [CrossRef]
- Castoldi, G.; Carletti, R.; Ippolito, S.; Colzani, M.; Barzaghi, F.; Stella, A.; Zerbini, G.; Perseghin, G.; Zatti, G.; di Gioia, C.R.T. Sodium-glucose cotransporter-2 inhibition prevents renal fibrosis in cyclosporine nephropathy. Acta Diabetol. 2021, 58, 1059–1070. [Google Scholar] [CrossRef]
- Karalliedde, J.; Fountoulakis, N.; Stathi, D.; Corcillo, A.; Flaquer, M.; Panagiotou, A.; Maltese, G.; Mangelis, A.; Ayis, S.; Gnudi, L. Does Dapagliflozin influence arterial stiffness and levels of circulating anti-aging hormone soluble Klotho in people with type 2 diabetes and kidney disease? Results of a randomized parallel group clinical trial. Front. Cardiovasc. Med. 2022, 9, 992327. [Google Scholar] [CrossRef]
- Frontiers in Endocrinology Editorial Team. Klotho’s impact on diabetic nephropathy and its emerging mechanisms. Front. Endocrinol. 2023, 14, 1180169. [Google Scholar] [CrossRef]
- Chen, X.; Tan, H.; Xu, J.; Tian, Y.; Yuan, Q.; Zuo, Y.; Chen, Q.; Hong, X.; Fu, H.; Hou, F.F.; et al. Klotho-derived peptide 6 ameliorates diabetic kidney disease by targeting Wnt/β-catenin signaling. Kidney Int. 2022, 102, 506–520. [Google Scholar] [CrossRef]
- Upadhyay, A. SGLT2 inhibitors and kidney protection: Mechanisms beyond glycemic control. Kidney360 2024, 5, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, L.; Mao, L.; Zhang, L.; Zhu, Y.; Xu, Y.; Cheng, Y.; Sun, R.; Zhang, Y.; Ke, J.; et al. SGLT2 inhibition restrains thyroid cancer growth via G1/S phase transition arrest and apoptosis mediated by DNA damage response signaling pathways. Cancer Cell Int. 2022, 22, 74. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, W. Advances in sodium-glucose transporter protein 2 inhibitors and tumors. Front. Oncol. 2025, 15, 1522059. [Google Scholar] [CrossRef] [PubMed]
- Anastasio, C.; Donisi, I.; Del Vecchio, V.; Colloca, A.; Mele, L.; Sardu, C.; Marfella, R.; Balestrieri, M.L.; D’oNofrio, N. SGLT2 inhibitor promotes mitochondrial dysfunction and ER-phagy in colorectal cancer cells. Cell. Mol. Biol. Lett. 2024, 29, 80. [Google Scholar] [CrossRef]
- Karzoon, A.; Yerer, M.B.; Cumaoğlu, A. Empagliflozin demonstrates cytotoxicity and synergy with tamoxifen in ER-positive breast cancer cells: Anti-proliferative and anti-survival effects. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 781–798. [Google Scholar] [CrossRef] [PubMed]
- Biziotis, O.; Tsakiridis, E.E.; Ali, A.; Ahmadi, E.; Wu, J.; Wang, S.; Mekhaeil, B.; Singh, K.; Menjolian, G.; Farrell, T.; et al. Canagliflozin mediates tumor suppression alone and in combination with radiotherapy in non-small cell lung cancer (NSCLC) through inhibition of HIF-1α. Mol. Oncol. 2023, 17, 2235–2256. [Google Scholar] [CrossRef]
- Shoda, K.; Tsuji, S.; Nakamura, S.; Egashira, Y.; Enomoto, Y.; Nakayama, N.; Shimazawa, M.; Iwama, T.; Hara, H. Canagliflozin Inhibits Glioblastoma Growth and Proliferation by Activating AMPK. Cell. Mol. Neurobiol. 2023, 43, 879–892. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, Z.; Jing, D.; Huang, X.; Ren, D.; Shao, Z.; Zhang, Z. SGLT2 inhibitor activates the STING/IRF3/IFN-β pathway and induces immune infiltration in osteosarcoma. Cell Death Dis. 2022, 13, 523. [Google Scholar] [CrossRef]
- Ding, L.; Chen, X.; Zhang, W.; Dai, X.; Guo, H.; Pan, X.; Xu, Y.; Feng, J.; Yuan, M.; Gao, X.; et al. Canagliflozin primes antitumor immunity by triggering PD-L1 degradation in endocytic recycling. J. Clin. Investig. 2023, 133, e154754. [Google Scholar] [CrossRef]
- Huang, Y.-M.; Chen, W.-M.; Jao, A.-T.; Chen, M.; Shia, B.-C.; Wu, S.-Y. Effects of SGLT2 inhibitors on clinical cancer survival in patients with type 2 diabetes. Diabetes Metab. 2024, 50, 101500. [Google Scholar] [CrossRef]
- Park, L.K.; Lim, K.-H.; Volkman, J.; Abdiannia, M.; Johnston, H.; Nigogosyan, Z.; Siegel, M.J.; McGill, J.B.; McKee, A.M.; Salam, M.; et al. Safety, tolerability, and effectiveness of the sodium-glucose cotransporter 2 inhibitor (SGLT2i) dapagliflozin in combination with standard chemotherapy for patients with advanced, inoperable pancreatic adenocarcinoma: A phase 1b observational study. Cancer Metab. 2023, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.A.; Khadem, R.; Haraj, A.; Farahani, M.A.; Firouzeh, P.C.A.; Azizinezhad, A.; Raki, H.K.; Belbasi, M.; Bafrani, M.A.; Deravi, N.; et al. The impact of sodium-glucose cotransporter-2 inhibitors on breast cancer and cancer-related mortality: A systematic review and meta-analysis of randomized controlled trials. BMC Cancer 2025, 25, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Sun, Y.; Zhang, D.; Li, D.; Liu, Z.; Jin, X.; Wu, H. SGLT2 promotes pancreatic cancer progression by activating the Hippo signaling pathway via the hnRNPK-YAP1 axis. Cancer Lett. 2021, 519, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-D.; Ding, L.; Mi, L.-J.; Zhang, A.-K.; Zhang, K.; Jiang, Z.-H.; Yu, F.-Y.; Yan, X.-X.; Shen, Y.-J.; Tang, M. Sodium–glucose co-transporter-2 inhibitors for the prevention of atrial fibrillation: A systemic review and meta-analysis. Eur. J. Prev. Cardiol. 2024, 31, 770–779. [Google Scholar] [CrossRef]
- Yin, Z.; Zheng, H.; Guo, Z. Effect of Sodium-Glucose Co-transporter Protein 2 Inhibitors on Arrhythmia in Heart Failure Patients With or Without Type 2 Diabetes: A Meta-Analysis of Randomized Controlled Trials. Front. Cardiovasc. Med. 2022, 9, 902923. [Google Scholar] [CrossRef] [PubMed]
- Cetin, E.H.O.; Cetin, M.S.; Könte, H.C.; Ülvan, N.; Çelik, E.M.; Arslan, K.; Kocyigit, D.; Korkmaz, A.; Ozcan, F.; Ozeke, O.; et al. The Impact of SGLT2 Inhibitors on Atrial Fibrillation Recurrence and Long-Term Clinical Outcomes in HFrEF Patients Undergoing Cryoballoon Ablation: A New Frontier Beyond Diabetes and Heart Failure. J. Cardiovasc. Electrophysiol. 2025, 36, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, A.M.; Rivera-Caravaca, J.M.; Underhill, P.; Fauchier, L.; Lip, G.Y.H. Incident heart failure, arrhythmias and cardiovascular outcomes with sodium-glucose cotransporter 2 (SGLT2) inhibitor use in patients with diabetes: Insights from a global federated electronic medical record database. Diabetes Obes. Metab. 2022, 25, 456–465. [Google Scholar] [CrossRef] [PubMed]
| Author | SGLT2i | Study Design | Outcomes |
|---|---|---|---|
| Javed et al., 2019 [119] | Empagliflozin | Randomized controlled trial, comparing effects of empagliflozin vs. metformin on women with PCOS. | Empagliflozin was more effective than metformin in reducing weight, body fat, and waist/hip circumference. |
| Sinha et al., 2022 [127] | N/A | Meta-analysis of prospective trials comparing SGLT-2i group to control group. | Reduction in body weight, fasting plasma glucose, insulin resistance, improvement in DHEAS levels. |
| Khan et al., 2021 [128] | Canagliflozin | Animal study with Sprague Dawley rats, divided into six groups, tested with canagliflozin and metformin | Canagliflozin alone and in combination shows significant hormonal improvements compared to placebo; beter cycle regularization with combination |
| Therapeutic Area | Proposed Mechanism(s) | Key Reference |
|---|---|---|
| Atrial Fibrillation Prevention | Reduced atrial fibrosis, improved mitochondrial function, ionic balance, and anti-inflammatory effects | [150,151] |
| Oncology | Inhibition of glycolysis, AMPK/mTOR modulation, autophagy induction, and immune checkpoint regulation | [138,139,140,141] |
| PCOS | Improved insulin sensitivity, reduced hyperandrogenism and weight loss | [127,128] |
| Circadian Rhythm Restoration | Glucosuria-induced catabolism, mTORC1 inhibition, autophagy activation, and metabolic rhythm normalization | [116] |
| Klotho Modulation | Upregulation of Klotho expression, oxidative stress reduction | [129,130] |
| Anti-inflammatory Effects | NLRP3 inflammasome inhibition, cytokine suppression, and AMPK pathway activation | [98,100] |
| Anemia Management | Increased erythropoietin production, improved renal oxygenation, and reduced hepcidin | [89,90] |
| Uric Acid Reduction | Uricosuric effect via inhibition of renal tubular urate reabsorption | [80,81] |
| MASLD Management | Improved hepatic insulin sensitivity, reduced steatosis, and anti-inflammatory actions | [60,61] |
| T2D Prevention | Enhanced glucose control, reduced insulin resistance, | [49] |
| Neuroprotection | Reduction in stress and inflammation, improved mitochondrial function, modulation of AMPK/SIRT1/PGC-1α and Wnt/β-catenin pathways. | [35,37,38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matuszewski, W.; Tomaszek, L.; Szklarz, M.; Górny, J.M.; Kordas, B.; Rutkowska, J.; Juranek, J. Beyond the Cardio–Renal–Metabolic Axis: Emerging Therapeutic Targets and Novel Mechanisms of Action of Flozins. J. Clin. Med. 2025, 14, 6348. https://doi.org/10.3390/jcm14186348
Matuszewski W, Tomaszek L, Szklarz M, Górny JM, Kordas B, Rutkowska J, Juranek J. Beyond the Cardio–Renal–Metabolic Axis: Emerging Therapeutic Targets and Novel Mechanisms of Action of Flozins. Journal of Clinical Medicine. 2025; 14(18):6348. https://doi.org/10.3390/jcm14186348
Chicago/Turabian StyleMatuszewski, Wojciech, Lena Tomaszek, Michał Szklarz, Jan Marek Górny, Bernard Kordas, Joanna Rutkowska, and Judyta Juranek. 2025. "Beyond the Cardio–Renal–Metabolic Axis: Emerging Therapeutic Targets and Novel Mechanisms of Action of Flozins" Journal of Clinical Medicine 14, no. 18: 6348. https://doi.org/10.3390/jcm14186348
APA StyleMatuszewski, W., Tomaszek, L., Szklarz, M., Górny, J. M., Kordas, B., Rutkowska, J., & Juranek, J. (2025). Beyond the Cardio–Renal–Metabolic Axis: Emerging Therapeutic Targets and Novel Mechanisms of Action of Flozins. Journal of Clinical Medicine, 14(18), 6348. https://doi.org/10.3390/jcm14186348







