Extracorporeal Life Support in a Porcine Model of Septic Endotoxemia with Acute Pulmonary Hypertension: An Experimental Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Preparation
2.2. Experimental Design
2.3. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CMA | Cranial mesenteric artery |
| ECMO | Extracorporeal membrane oxygenation |
| LV | Left ventricle |
| LVEDP | Left ventricular end-diastolic pressure |
| LVESP | Left ventricular end-systolic pressure |
| PAP | Pulmonary artery pressure |
| PAH | Pulmonary arterial hypertension |
| PaO2 | Arterial oxygen tension |
| PpaO2 | Pulmonary arterial oxygen tension |
| PCWP | Pulmonary capillary wedge pressure |
| PEEP | Positive end-expiratory pressure |
| PVR | Pulmonary vascular resistance |
| RV | Right ventricle |
| SaO2 | Arterial oxygen saturation |
| SvO2 | Mixed venous oxygen saturation |
| SVR | Systemic vascular resistance |
| V-A | Veno-arterial |
| V-VA | Veno-venoarterial |
Appendix A
- 20G peripheral intravenous line in the left auricular vein:
- -
- Induction of anesthesia, fluid administration.
- Central venous catheter in the left internal jugular vein:
- -
- Continuous infusion of anesthetic drugs, norepinephrine, lipopolysaccharide, and fluids;
- -
- Continuous measurement of central venous pressure;
- -
- Transpulmonary thermodilution.
- Pulmonary artery catheter in the right internal jugular vein:
- -
- Continuous pulmonary artery pressure measurement, pulmonary capillary wedge pressure measurement;
- -
- Pulmonary artery thermodilution measurement;
- -
- Central venous and mixed venous blood gas analysis.
- Arterial catheter in the left carotid artery:
- -
- Hemodynamic monitoring;
- -
- Arterial blood gas analysis.
- Left ventricular catheter via the right carotid artery:
- -
- Continuous left ventricular pressure monitoring.
- Venous ECMO drainage cannula in the inferior vena cava.
- Venous ECMO return cannula in the left brachiocephalic vein.
- Arterial ECMO return cannula in the distal abdominal aorta.
References
- Kovacs, G.; Bartolome, S.; Denton, C.P.; Gatzoulis, M.A.; Gu, S.; Khanna, D.; Badesch, D.; Montani, D. Definition, classification and diagnosis of pulmonary hypertension. Eur. Respir. J. 2024, 64, 2401324. [Google Scholar] [CrossRef] [PubMed]
- Spapen, H.; Vincken, W. Pulmonary Arterial Hypertension in Sepsis and the Adult Respiratory Distress Syndrome. Acta Clin. Belg. 1992, 47, 30–41. [Google Scholar] [CrossRef]
- Pischke, S.E.; Hestenes, S.; Johansen, H.T.; Fure, H.; Bugge, J.F.; Espinoza, A.; Skulstad, H.; Edvardsen, T.; Fosse, E.; Mollnes, T.E.; et al. Sepsis causes right ventricular myocardial inflammation independent of pulmonary hypertension in a porcine sepsis model. PLoS ONE 2019, 14, e0218624. [Google Scholar] [CrossRef]
- Vallabhajosyula, S.; Shankar, A.; Vojjini, R.; Cheungpasitporn, W.; Sundaragiri, P.R.; DuBrock, H.M.; Sekiguchi, H.; Frantz, R.P.; Cajigas, H.R.; Kane, G.C.; et al. Impact of Right Ventricular Dysfunction on Short-term and Long-term Mortality in Sepsis: A Meta-analysis of 1373 Patients. Chest 2021, 159, 2254–2263. [Google Scholar] [CrossRef]
- Dessap, A.M.; Boissier, F.; Charron, C.; Bégot, E.; Repessé, X.; Legras, A.; Brun-Buisson, C.; Vignon, P.; Vieillard-Baron, A. Acute cor pulmonale during protective ventilation for acute respiratory distress syndrome: Prevalence, predictors, and clinical impact. Intensive Care Med. 2016, 42, 862–870. [Google Scholar] [CrossRef]
- Pagnesi, M.; Baldetti, L.; Beneduce, A.; Calvo, F.; Gramegna, M.; Pazzanese, V.; Ingallina, G.; Napolano, A.; Finazzi, R.; Ruggeri, A.; et al. Pulmonary hypertension and right ventricular involvement in hospitalised patients with COVID-19. Heart 2020, 106, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Torge, D.; Bernardi, S.; Arcangeli, M.; Bianchi, S. Histopathological Features of SARS-CoV-2 in Extrapulmonary Organ Infection: A Systematic Review of Literature. Pathogens 2022, 11, 867. [Google Scholar] [CrossRef]
- Davis, A.L.M.; Carcillo, J.A.; Aneja, R.K.; Deymann, A.J.; Lin, J.C.; Nguyen, T.C.; Okhuysen-Cawley, R.S.M.; Relvas, M.S.M.; Rozenfeld, R.A.M.; Skippen, P.W.M.; et al. The American College of Critical Care Medicine Clinical Practice Parameters for Hemodynamic Support of Pediatric and Neonatal Septic Shock: Executive Summary. Pediatr. Crit. Care Med. 2017, 18, 884–890. [Google Scholar] [CrossRef]
- Vogel, D.J.; Murray, J.; Czapran, A.Z.; Camporota, L.; Ioannou, N.; Meadows, C.I.S.; Sherren, P.B.; Daly, K.; Gooby, N.; Barrett, N. Veno-arterio-venous ECMO for septic cardiomyopathy: A single-centre experience. Perfusion 2018, 33, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Stöhr, F.; Emmert, M.Y.; Lachat, M.L.; Stocker, R.; Maggiorini, M.; Falk, V.; Wilhelm, M.J. Extracorporeal membrane oxygenation for acute respiratory distress syndrome: Is the configuration mode an important predictor for the outcome? Interact. Cardiovasc. Thorac. Surg. 2011, 12, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Thiel, M.; Kreimeier, U.; Holzer, K.; Moritz, S.C.M.; Peter, K.; Messmer, K. Effects of adenosine on cardiopulmonary functions and oxygen-derived variables during endotoxemia. Crit. Care Med. 1998, 26, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Domino, K.B.; Wetstein, L.; Glasser, S.A.; Lindgren, L.; Marshall, C.; Harken, A.; Marshall, B.E. Influence of Mixed Venous Oxygen Tension (PVO2) on Blood Flow to Atelectatic Lung. Anesthesiology 1983, 59, 428–434. [Google Scholar] [CrossRef]
- Cevik, A.; Kula, S.; Olgunturk, R.; Tunaoglu, S.; Oguz, D.; Saylan, B.; Sanli, C. Evaluation of pulmonary vascular resistance and vasoreactivity testing with oxygen in children with congenital heart disease and pulmonary arterial hypertension. Anadolu Kardiyol. Dergisi/Anatol. J. Cardiol. 2014, 14, 196–198. [Google Scholar] [CrossRef]
- Price, L.C.; McAuley, D.F.; Marino, P.S.; Finney, S.J.; Griffiths, M.J.; Wort, S.J. Pathophysiology of pulmonary hypertension in acute lung injury. Am. J. Physiol. Cell. Mol. Physiol. 2012, 302, L803–L815. [Google Scholar] [CrossRef]
- Chung, M.; Shiloh, A.L.; Carlese, A. Monitoring of the Adult Patient on Venoarterial Extracorporeal Membrane Oxygenation. Sci. World J. 2014, 2014, 393258. [Google Scholar] [CrossRef] [PubMed]
- Holzgraefe, B.; Larsson, A.; Eksborg, S.; Kalzén, H. Does extracorporeal membrane oxygenation attenuate hypoxic pulmonary vasoconstriction in a porcine model of global alveolar hypoxia? Acta Anaesthesiol. Scand. 2020, 64, 992–1001. [Google Scholar] [CrossRef]
- Bunge, J.J.H.; Caliskan, K.; Gommers, D.; Miranda, D.R. Right ventricular dysfunction during acute respiratory distress syndrome and veno-venous extracorporeal membrane oxygenation. J. Thorac. Dis. 2018, 10, S674–S682. [Google Scholar] [CrossRef]
- Miranda, D.R.; van Thiel, R.; Brodie, D.; Bakker, J. Right Ventricular Unloading after Initiation of Venovenous Extracorporeal Membrane Oxygenation. Am. J. Respir. Crit. Care Med. 2015, 191, 346–348. [Google Scholar] [CrossRef]
- Graf, P.T.; Boesing, C.; Brumm, I.; Biehler, J.; Müller, K.W.; Thiel, M.; Pelosi, P.; Rocco, P.R.M.; Luecke, T.; Krebs, J. Ultraprotective versus apneic ventilation in acute respiratory distress syndrome patients with extracorporeal membrane oxygenation: A physiological study. J. Intensive Care 2022, 10, 12. [Google Scholar] [CrossRef]
- Boesing, C.; Schaefer, L.; Graf, P.T.; Pelosi, P.; Rocco, P.R.; Luecke, T.; Krebs, J. Effects of different positive end-expiratory pressure titration strategies on mechanical power during ultraprotective ventilation in ARDS patients treated with veno-venous extracorporeal membrane oxygenation: A prospective interventional study. J. Crit. Care 2024, 79, 154406. [Google Scholar] [CrossRef] [PubMed]
- Mu, T.S.; Becker, A.M.; Clark, A.J.; Batts, S.G.; Murata, L.-A.M.; Uyehara, C.F.T.; Bader, M. ECMO with vasopressor use during early endotoxic shock: Can it improve circulatory support and regional microcirculatory blood flow? PLoS ONE 2019, 14, e0223604. [Google Scholar] [CrossRef] [PubMed]
- Grant, C.; Richards, J.B.; Frakes, M.; Cohen, J.; Wilcox, S.R. ECMO and Right Ventricular Failure: Review of the Literature. J. Intensive Care Med. 2021, 36, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Geri, G.; Vignon, P.; Aubry, A.; Fedou, A.-L.; Charron, C.; Silva, S.; Repessé, X.; Vieillard-Baron, A. Cardiovascular clusters in septic shock combining clinical and echocardiographic parameters: A post hoc analysis. Intensive Care Med. 2019, 45, 657–667. [Google Scholar] [CrossRef]
- Zochios, V.; Nasa, P.; Yusuff, H.; Schultz, M.J.; Antonini, M.V.; Duggal, A.; Dugar, S.; Ramanathan, K.; Shekar, K.; Schmidt, M. Definition and management of right ventricular injury in adult patients receiving extracorporeal membrane oxygenation for respiratory support using the Delphi method: A PRORVnet study. Expert position statements. Intensive Care Med. 2024, 50, 1411–1425. [Google Scholar] [CrossRef]
- Broman, L.M.; Dubrovskaja, O.; Balik, M. Extracorporeal Membrane Oxygenation for Septic Shock in Adults and Children: A Narrative Review. J. Clin. Med. 2023, 12, 6661. [Google Scholar] [CrossRef]
- Sandrio, S.; Krebs, J.; Leonardy, E.; Thiel, M.; Schoettler, J.J. Vasoactive Inotropic Score as a Prognostic Factor during (Cardio-) Respiratory ECMO. J. Clin. Med. 2022, 11, 2390. [Google Scholar] [CrossRef] [PubMed]
- Schmittinger, C.A.; Torgersen, C.; Luckner, G.; Schröder, D.C.H.; Lorenz, I.; Dünser, M.W. Adverse cardiac events during catecholamine vasopressor therapy: A prospective observational study. Intensive Care Med. 2012, 38, 950–958. [Google Scholar] [CrossRef]
- Rudiger, A.; Singer, M. Decatecholaminisation during sepsis. Crit. Care 2016, 20, 309. [Google Scholar] [CrossRef]
- Shanmukhappa, C.; Lokeshwaran, S. Venous Oxygen Saturation. 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK564395/ (accessed on 8 August 2025).
- Kang, J.; Lee, K.; Lee, H.S.; Lee, H.; Ahn, H.; Han, J.; Yang, H.; Park, K.W.; Lee, H.; Kang, H.; et al. Differential effect of left ventricular unloading according to the aetiology of cardiogenic shock. ESC Heart Fail. 2024, 11, 338–348. [Google Scholar] [CrossRef]
- Sandrio, S.; Springer, W.; Karck, M.; Gorenflo, M.; Weymann, A.; Ruhparwar, A.; Loukanov, T. Extracorporeal life support with an integrated left ventricular vent in children with a low cardiac output. Cardiol. Young 2014, 24, 654–660. [Google Scholar] [CrossRef]
- Naeije, R.; Chin, K. Differentiating Precapillary from Postcapillary Pulmonary Hypertension. Circulation 2019, 140, 712–714. [Google Scholar] [CrossRef] [PubMed]
- Baldetti, L.; Gallone, G. Left ventricular unloading and venting in veno-arterial extracorporeal membrane oxygenation: The importance of cardiogenic shock aetiology in guiding treatment strategies. ESC Heart Fail. 2024, 11, 615–618. [Google Scholar] [CrossRef]
- Silva, P.L.; Scharffenberg, M.; Rocco, P.R.M. Understanding the mechanisms of ventilator-induced lung injury using animal models. Intensive Care Med. Exp. 2023, 11, 82. [Google Scholar] [CrossRef]
- Lewis, G.D.; Bossone, E.; Naeije, R.; Grünig, E.; Saggar, R.; Lancellotti, P.; Ghio, S.; Varga, J.; Rajagopalan, S.; Oudiz, R.; et al. Pulmonary Vascular Hemodynamic Response to Exercise in Cardiopulmonary Diseases. Circulation 2013, 128, 1470–1479. [Google Scholar] [CrossRef]
- Conrad, A.M.; Duerschmied, D.; Boesing, C.; Thiel, M.; Beck, G.; Luecke, T.; Rocco, P.R.M.; Krebs, J.; Loosen, G. Impact of Veno-Venous Extracorporeal Membrane Oxygenation on Right Ventricular Impairment in Severe ARDS: A Prospective Observational Longitudinal Study. J. Intensive Care Med. 2025, 24, 8850666251352445. [Google Scholar] [CrossRef] [PubMed]
- Araos, J.; Alegria, L.; Garcia, P.; Cruces, P.; Soto, D.; Erranz, B.; Amthauer, M.; Salomon, T.; Medina, T.; Rodriguez, F.; et al. Near-Apneic Ventilation Decreases Lung Injury and Fibroproliferation in an Acute Respiratory Distress Syndrome Model with Extracorporeal Membrane Oxygenation. Am. J. Respir. Crit. Care Med. 2019, 199, 603–612. [Google Scholar] [CrossRef]
- Price, L.C.; Wort, S.J.; Finney, S.J.; Marino, P.S.; Brett, S.J. Pulmonary vascular and right ventricular dysfunction in adult critical care: Current and emerging options for management: A systematic literature review. Crit. Care 2010, 14, R169. [Google Scholar] [CrossRef] [PubMed]



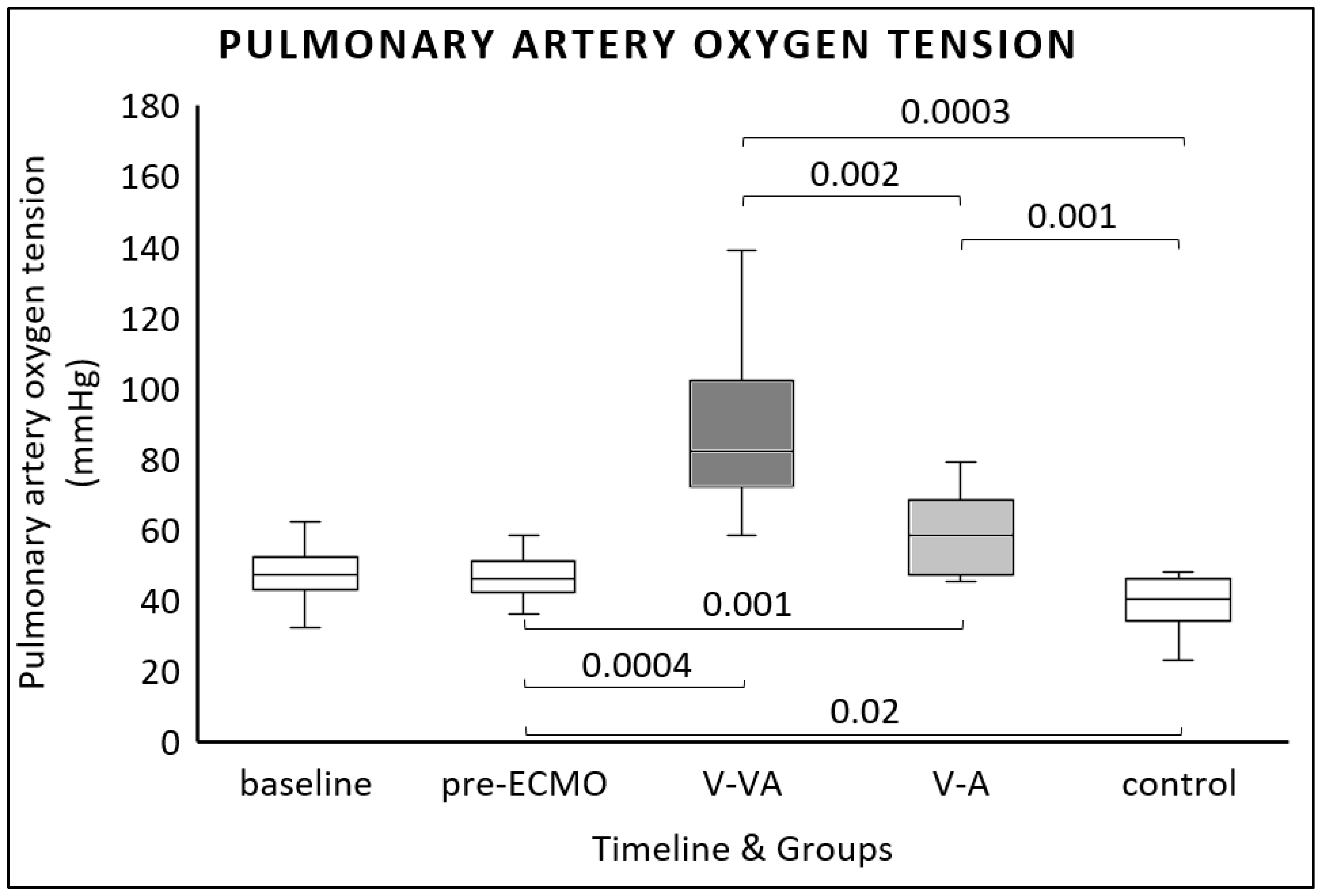
| Baseline | Pre-ECMO | Final V-VA Group | Final V-A Group | Final Control Group | V-A vs. V-VA | V-A vs. Control | V-VA vs. Control | |
|---|---|---|---|---|---|---|---|---|
| n = 34 | n = 34 | n = 12 | n = 12 | n = 10 | ||||
| ECMO total flow [L/min] | 2.3 (2.2–2.7) | 1.7 (1.5–2.2) | 0.04 | |||||
| ECMO arterial flow [L/min] | 1.3 (1.2–1.7) | 1.7 (1.5–2.2) | 0.01 | |||||
| Mean arterial pressure [mmHg] | 82 (73–99) | 100 (88–113) | 78 (61–102) | 75 (67–88) | 97 (85–102) | 0.9 | 0.2 | 0.6 |
| Norepinephrine [µg/kg/min] | 0.1 (0.01–0.3) | 0.01 (0.01–0.1) | 0.1 (0.01–1.8) | 0.1 (0.02–5.2) | 0.01 (0.01–0.01) | 0.8 | 0.02 | 0.1 |
| Fluid substitution [L] | 6.3 (4.3–7.3) | 8 (6–10.7) | 7.4 (6.2–9.6) | 4.5 (4–7.7) | 0.9 | 0.1 | 0.02 | |
| Heart rate [beat/min] | 85 (74–110) | 130 (95–152) | 129 (89–148) | 120 (102–173) | 101 (64–144) | 0.9 | 0.4 | 0.7 |
| Pulmonary circulation | ||||||||
| Central venous pressure [mmHg] | 14 (13–15) | 14 (13–15) | 15 (11–16) | 15 (13–17.5) | 17 (16–20) | 0.6 | 0.1 | 0.02 |
| Systolic PAP [mmHg] | 34 (30–38) | 53 (42–60) | 60 (39–69) | 47 (43–51) | 56 (52–70) | 0.2 | 0.04 | 1 |
| Diastolic PAP [mmHg] | 21 (19–24) | 31 (25–37) | 33 (29–39) | 29 (25–34) | 37 (31–39) | 0.5 | 0.3 | 0.9 |
| Mean PAP [mmHg] | 26 (24–30) | 40 (34–46) | 42 (30–48) | 36 (33–41) | 47 (39–52) | 0.5 | 0.07 | 0.7 |
| Transonic cardiac output [L/min] | 2.7 (2.1–3.3) | 2.6 (1.9–3) | 1.7 (0.9–2.3) | 1.7 (1–1.9) | 3 (1.9–3.9) | 1 | 0.05 | 0.07 |
| RV diameter [cm] | 2.5 (2.2–2.9) | 2.3 (1.9–2.5) | 2.1 (1.6–2.6) | 2.2 (1.8–2.6) | 2.8 (2.6–3) | 1 | 0.02 | 0.02 |
| RV end-diastolic area [cm2] | 6.1 (4.3–8.6) | 5.8 (4.5–8.1) | 4.3 (2.5–8.3) | 4.7 (3.3–7.6) | 8.5 (7.8–9.7) | 0.9 | 0.02 | 0.04 |
| Systemic circulation | ||||||||
| PCWP [mmHg] | 16 (14–17) | 16 (14.5–17) | 15 (14–20) | 16 (14–19) | 18 (16–20) | 0.9 | 0.7 | 0.5 |
| LVEDP [mmHg] | 14 (12–15) | 13 (11–15) | 12 (6.5–14.7) | 13 (9–15) | 14.5 (13–17.5) | 0.7 | 0.5 | 0.2 |
| LVESP [mmHg] | 109 (100–123) | 130 (107–146) | 116 (110–139) | 112 (101–126) | 117 (107–120) | 0.6 | 1 | 0.9 |
| LV diameter [cm] | 3.5 (3–3.8) | 3.1 (2.8–3.6) | 2.9 (2.5–3.4) | 3 (2.6–3.1) | 3.6 (3.2–3.8) | 1 | 0.01 | 0.07 |
| E/E’ | 5.8 (4.2–7.4) | 5.9 (3.9–10) | 5.3 (4.3–6.3) | 7.1 (3.6–10.9) | 7.4 (4.1–12.1) | 0.7 | 0.9 | 0.5 |
| S’ | 7.5 (6.8–10.5) | 7.8 (6–10) | 6.4 (5.8–10.8) | 6.6 (6.1–8.3) | 8 (7.3–10.5) | 1 | 0.1 | 0.3 |
| SVR [dynes.s.cm−5] | 2167 (1728–2637) | 2821 (2162–3528) | 3294 (1625–6022) | 2449 (1803–3728) | 1924 (1402–2821) | 0.7 | 0.6 | 0.3 |
| Mechanical ventilation | ||||||||
| Respiratory rate [breath/min] | 26 (20–28) | 28 (22–28) | 28 (13–29) | 26 (16–28) | 24 (20–26) | 1 | 0.9 | 0.7 |
| Plateau pressure [mbar] | 11 (10–12) | 13 (12–15) | 18 (15–20) | 18 (15–21) | 17 (15–20) | 0.9 | 0.9 | 1 |
| PEEP [mbar] | 5 | 5 | 5 | 5 | 5 | 1 | 1 | 1 |
| Tidal volume [mL] | 184 (178–194) | 186 (173–200) | 188 (175–196) | 189 (181–203) | 179 (174–208) | 1 | 0.7 | 0.9 |
| Baseline | Pre-ECMO | Final V-VA Group | Final V-A Group | Final Control Group | V-A vs. V-VA | V-A vs. Control | V-VA vs. Control | |
|---|---|---|---|---|---|---|---|---|
| n = 34 | n = 34 | n = 12 | n = 12 | n = 10 | ||||
| Arterial pH | 7.42 (7.38–7.45) | 7.38 (7.35–7.41) | 7.42 (7.31–7.45) | 7.40 (7.28–7.47) | 7.36 (7.29–7.38) | 1 | 0.7 | 0.4 |
| PaO2 [ mmHg] | 220 (206–230) | 195 (147–223) | 208 (146–242) | 190 (148–246) | 99 (74–127) | 1 | 0.02 | 0.002 |
| SaO2 [%] | 99.7 (99.6–99.7) | 99.7 (98.9–99.7) | 99 (98–99) | 99 (98–100) | 96 (85–98) | 0.9 | 0.05 | 0.02 |
| Baseline | Pre-ECMO | Final V-VA Group | Final V-A Group | Final Control Group | V-A vs. V-VA | V-A vs. Control | V-VA vs. Control | |
|---|---|---|---|---|---|---|---|---|
| n = 34 | n = 34 | n = 12 | n = 12 | n = 10 | ||||
| Transonic celiac trunk [L/min] | 0.15 (0.1–0.3) | 0.4 (0.1–0.6) | 0.2 (0.1–0.4) | 0.3 (0.1–0.5) | 0.2 (0.1–0.3) | 0.7 | 0.4 | 0.9 |
| Transonic CMA [L/min] | 0.6 (0.5–0.8) | 0.5 (0.3–0.7) | 0.7 (0.5–0.9) | 0.7 (0.6–1.3) | 0.6 (0.5–0.8) | 0.8 | 0.7 | 1 |
| Transonic abdominal aorta [L/min] | 1.8 (1.3–2.9) | 1.7 (1.1–2.1) | −0.1 (−0.2–0.4) | −0.2 (−0.6–0) | 1.2 (0.9–2) | 0.4 | 0.0004 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandrio, S.; Krebs, J.; Spanier, T.; Beck, G.; Thiel, M.; Graf, P.T. Extracorporeal Life Support in a Porcine Model of Septic Endotoxemia with Acute Pulmonary Hypertension: An Experimental Study. J. Clin. Med. 2025, 14, 6342. https://doi.org/10.3390/jcm14176342
Sandrio S, Krebs J, Spanier T, Beck G, Thiel M, Graf PT. Extracorporeal Life Support in a Porcine Model of Septic Endotoxemia with Acute Pulmonary Hypertension: An Experimental Study. Journal of Clinical Medicine. 2025; 14(17):6342. https://doi.org/10.3390/jcm14176342
Chicago/Turabian StyleSandrio, Stany, Joerg Krebs, Tobias Spanier, Grietje Beck, Manfred Thiel, and Peter Tobias Graf. 2025. "Extracorporeal Life Support in a Porcine Model of Septic Endotoxemia with Acute Pulmonary Hypertension: An Experimental Study" Journal of Clinical Medicine 14, no. 17: 6342. https://doi.org/10.3390/jcm14176342
APA StyleSandrio, S., Krebs, J., Spanier, T., Beck, G., Thiel, M., & Graf, P. T. (2025). Extracorporeal Life Support in a Porcine Model of Septic Endotoxemia with Acute Pulmonary Hypertension: An Experimental Study. Journal of Clinical Medicine, 14(17), 6342. https://doi.org/10.3390/jcm14176342






