Natural vs. Assisted Conception: Sleep and Emotional Health from Pregnancy to Postpartum—An Exploratory Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample and Procedure
2.2. Questionnaires
- Demographic and clinical characteristicsParticipants were asked to answer questions regarding the following:
- (I)
- Demographic characteristics: (a) Age, (b) Employment status, (c) Marital status and
- (II)
- Clinical characteristics: (a) History of depression, (b) Family history of mood disorders, (c) Current medical conditions, (d) Current use of psychotropic medication, (e) Current use of thyroid medication, (f) Current use of any other medication prescribed for medical conditions other than psychiatric or thyroid disorders.
- 2.
- Pittsburgh Sleep Quality Index (PSQI): a self-reported questionnaire consisting of 19 items evaluating sleep quality during the last month. The total score ranges from 0 to 21, where a score over 5 indicates poor sleep quality [34]. The PSQI is an internationally validated tool that has been extensively used and has been recognized in a wide range of studies conducted in Greece for both the assessment and prediction of sleep disorders in various patient populations [35,36].
- 3.
- Athens Insomnia Scale (AIS): a self-reported questionnaire consisting of eight items evaluating the presence and assessing the severity of insomnia. The answers range from 0 to 3 and the score is obtained by summing the scores on each question. The score ranges from 0 to 24 and a total score ≥ 6 indicates insufficient sleep [37].
- 4.
- Epworth Sleepiness Scale (ESS): a self-reported questionnaire for the assessment of the degree of daytime sleepiness. It includes the description of eight situations for which the participant is asked to estimate the probability of falling asleep for a reason other than tiredness. Each item corresponds to one possible answer and is scored from 0 to 3. The maximum score is 24 [38].
- 5.
- Fatigue Severity Scale (FFS): a self-reported questionnaire for the assessment of daily fatigue, consisting of nine statements that represent the severity of fatigue symptoms during the last 2 weeks. The participant is asked to choose a rating on a scale from 1 to 7, depending on the extent to which they agree with these statements, with 1 corresponding to complete disagreement and 7 to complete agreement. The total score ranges from 9 to 63, and a score above 36 indicates severe fatigue [39].
- 6.
- Edinburgh Postnatal Depression Scale (EPDS): a self-reported questionnaire consisting of 10 questions. It investigates the presence of antenatal and postnatal depressive symptomatology during the last week. The possible answers are scored from 0 to 3, with a score ranging from 0 to 30 and a cut-off point of 11/12, according to the Greek validation of the instrument [40].
2.3. Ethics
Statistical Analysis
3. Results
3.1. The Demographic and Clinical Profile of the NC and ART Groups and the Differences Between Them
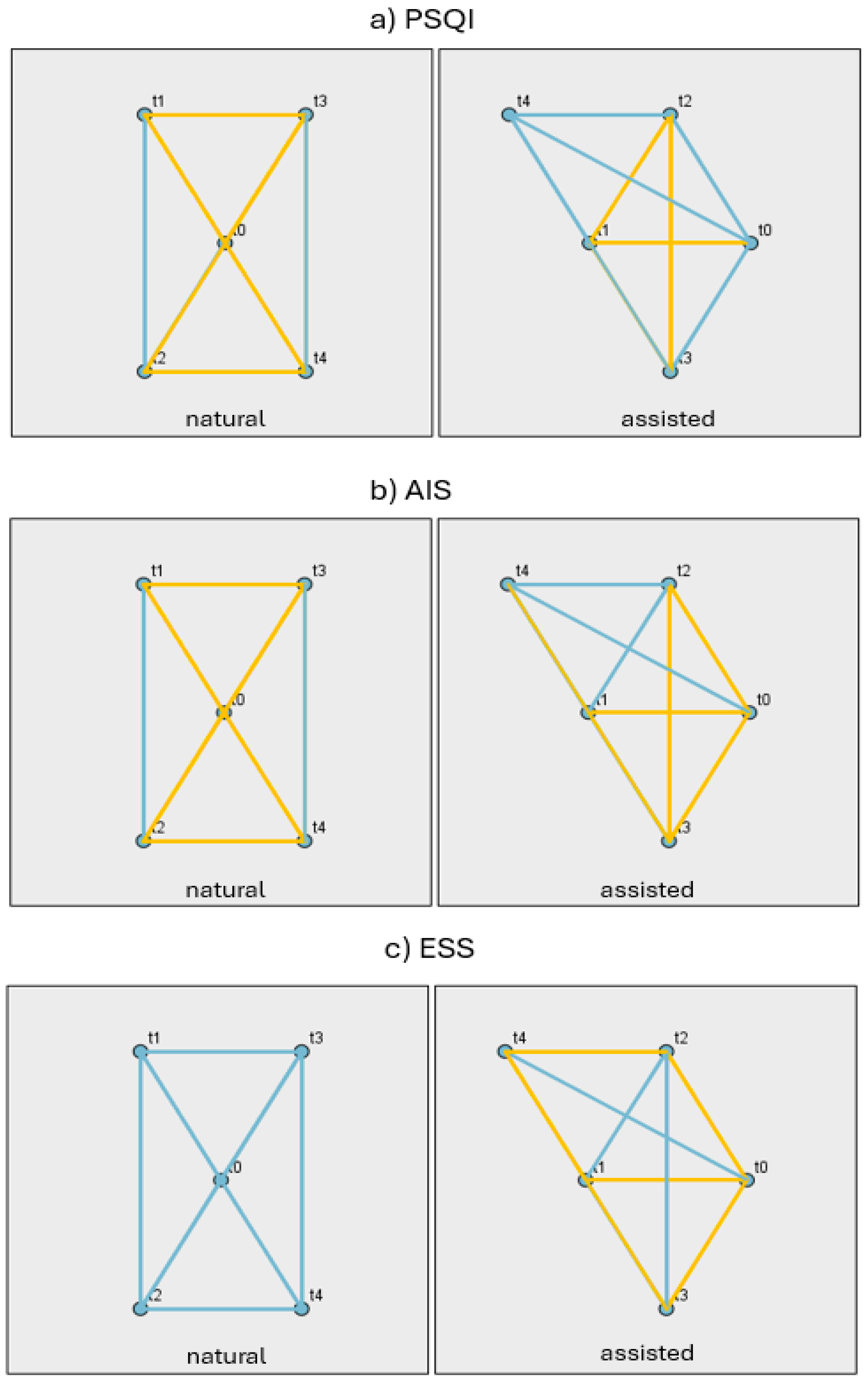
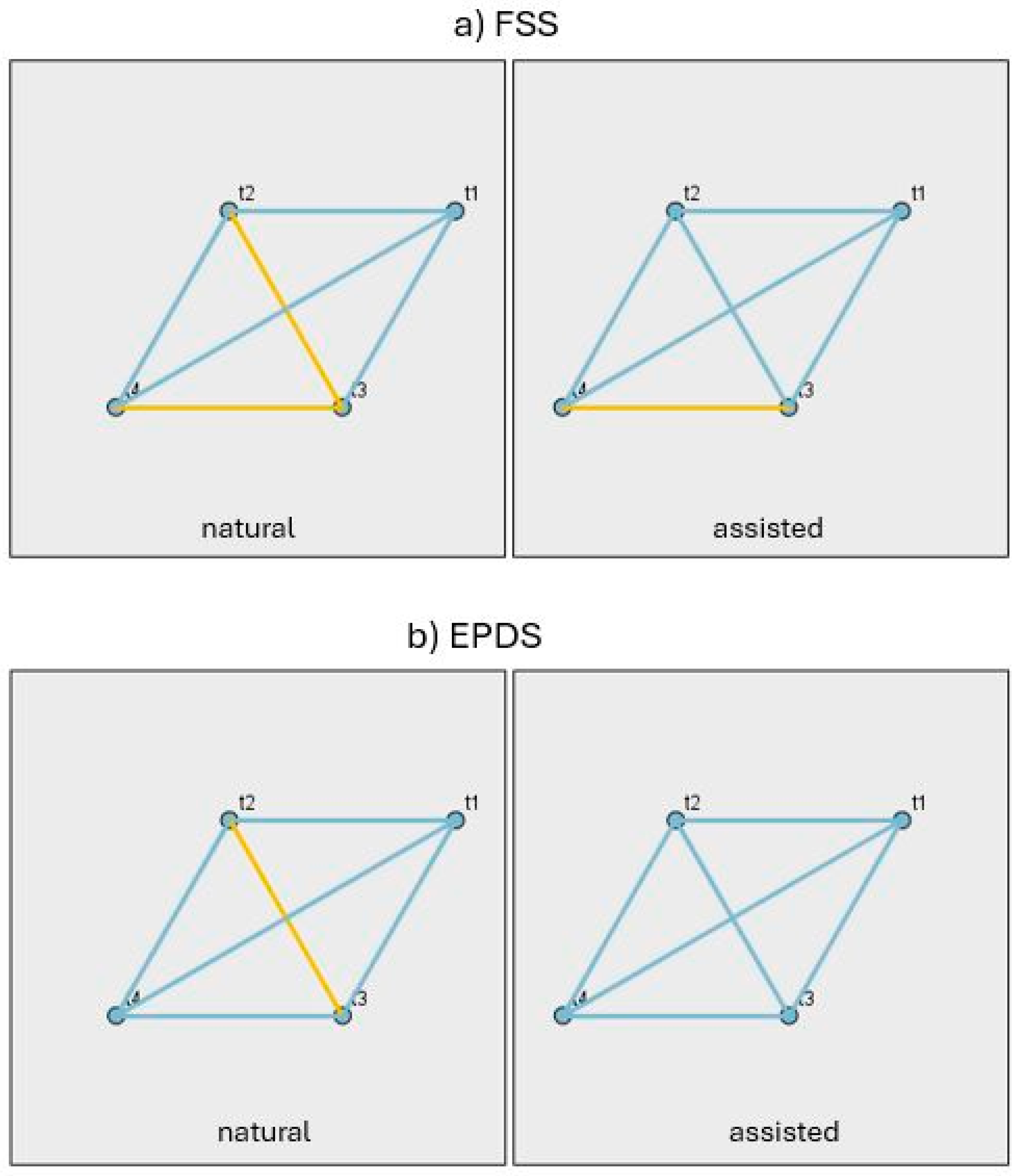
3.2. Changes in Sleep, Emotional Well-Being and Fatigue Indices During Pregnancy and the Postpartum Period
3.3. Differences Between the NC and ART Groups and Changes in Psychological and Physiological Measures at Each Stage (t0–t4)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SDis | Sleep Disturbances |
| NC | Naturally Conceived |
| ART | Assisted Reproductive Treatment |
| UGHA | University General Hospital of Alexandroupolis |
| PSQI | Pittsburgh Sleep Quality Index |
| AIS | Athens Isomnia Scale |
| ESS | Epworth Sleepiness Scale |
| FSS | Fatigue Se`verity Scale |
| EPDS | Edinburgh Postnatal Depression Scale |
References
- Van den Broeck, U.; Emery, M.; Wischmann, T.; Thorn, P. Counselling in Infertility: Individual, Couple and Group Interventions. Patient Educ. Couns. 2010, 81, 422–428. [Google Scholar] [CrossRef]
- Cavanaugh, A. Exploring the Role of Playfulness, Social Support and Self Esteem in Coping with the Transition to Motherhood; Hoffman, M.A., Ed.; ProQuest Dissertations Publishing: Ann Arbor, MI, USA, 2006. [Google Scholar]
- Choi, P.; Henshaw, C.; Baker, S.; Tree, J. Supermum, Superwife, Supereverything: Performing Femininity in the Transition to Motherhood. J. Reprod. Infant Psychol. 2005, 23, 167–180. [Google Scholar] [CrossRef]
- Greil, A.; McQuillan, J.; Slauson-Blevins, K. The Social Construction of Infertility. Sociol. Compass 2011, 5, 736–746. [Google Scholar] [CrossRef]
- Cousineau, T.M.; Domar, A.D. Psychological Impact of Infertility. Best Pract. Res. Clin. Obstet. Gynaecol. 2007, 21, 293–308. [Google Scholar] [CrossRef]
- Kepley, J.M.; Bates, K.; Mohiuddin, S.S. Physiology, Maternal Changes. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK539766/ (accessed on 7 June 2025).
- Chandra, M.; Paray, A.A. Natural Physiological Changes During Pregnancy. Yale J. Biol. Med. 2024, 97, 85–92. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haas, J.S.; Jackson, R.A.; Fuentes-Afflick, E.; Stewart, A.L.; Dean, M.L.; Brawarsky, P.; Escobar, G.J. Changes in the Health Status of Women During and after Pregnancy. J. Gen. Intern. Med. 2005, 20, 45–51. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nissen, M.; Barrios Campo, N.; Flaucher, M.; Jaeger, K.M.; Titzmann, A.; Blunck, D.; Fasching, P.A.; Engelhardt, V.; Eskofier, B.M.; Leutheuser, H. Prevalence and course of pregnancy symptoms using self-reported pregnancy app symptom tracker data. Npj Digit. Med. 2023, 6, 189. [Google Scholar] [CrossRef] [PubMed]
- Pengo, M.F.; Won, C.H.; Bourjeily, G. Sleep in Women Across the Life Span. Chest 2018, 154, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Badr, M.S. A Role for Sleep Disorders in Pregnancy Complications: Challenges and opportunities. Am. J. Obstet. Gynecol. 2014, 210, 3–11. [Google Scholar] [CrossRef]
- Haney, A.; Buysse, D.J.; Rosario, B.L.; Chen, Y.-F.; Okun, M.L. Sleep Disturbance and Cardiometabolic Risk Factors in Early Pregnancy: A Preliminary Study. Sleep Med. 2014, 15, 444–450. [Google Scholar] [CrossRef]
- Wesström, J.; Skalkidou, A.; Manconi, M.; Fulda, S.; Sundström-Poromaa, I. Pre-Pregnancy Restless Legs Syndrome (Willis-Ekbom Disease) is Associated with Perinatal Depression. J. Clin. Sleep Med. 2014, 10, 527–533. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tomfohr, L.M.; Buliga, E.; Letourneau, N.L.; Campbell, T.S.; Giesbrecht, G.F. Trajectories of Sleep Quality and Associations with Mood During the Perinatal Period. Sleep 2015, 38, 1237–1245. [Google Scholar] [CrossRef]
- Polo-Kantola, P.; Aukia, L.; Karlsson, H.; Karlsson, L.; Paavonen, E.J. Sleep Quality During Pregnancy: Associations with Depressive and Anxiety Symptoms. Acta Obstet. Gynecol. Scand. 2017, 96, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Lateef, O.M.; Akintubosun, M.O. Sleep and Reproductive Health. J. Circadian Rhythm. 2020, 18, 1. [Google Scholar] [CrossRef]
- Haufe, A.; Leeners, B. Sleep Disturbances Across a Woman’s Lifespan: What Is the Role of Reproductive Hormones? J. Endocr. Soc. 2023, 7, bvad036. [Google Scholar] [CrossRef] [PubMed]
- Ramya, S.; Poornima, P.; Jananisri, A.; Geofferina, I.P.; Bavyataa, V.; Divya, M.; Priyanga, P.; Vadivukarasi, J.; Sujitha, S.; Elamathi, S.; et al. Role of Hormones and the Potential Impact of Multiple Stresses on Infertility. Stresses 2023, 3, 454–474. [Google Scholar] [CrossRef]
- Morris, C.J.; Aeschbach, D.; Scheer, F.A. Circadian System, Sleep and Endocrinology. Mol. Cell Endocrinol. 2012, 349, 91–104. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bachelot, A.; Binart, N. Reproductive role of prolactin. Reproduction 2007, 133, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Chueh, K.H.; Lin, J.L. Somatic Symptoms, Sleep Disturbance and Psychological Distress Among Women Undergoing Oocyte Pick-Up and In Vitro Fertilisation-Embryo Transfer. J. Clin. Nurs. 2016, 25, 1748–1756. [Google Scholar] [CrossRef]
- Goldstein, C.A.; Lanham, M.S.; Smith, Y.R.; O’Brien, L.M. Sleep in Women Undergoing In Vitro Fertilization: A Pilot Study. Sleep Med. 2017, 32, 105–113. [Google Scholar] [CrossRef]
- Li, L.; Ferin, M.; Sauer, M.V.; Lobo, R.A. Serum and Follicular Fluid Ghrelin Levels Negatively Reflect Human Oocyte Quality and In Vitro Embryo Development. Fertil. Steril. 2011, 96, 1116–1120. [Google Scholar] [CrossRef]
- Jana, S.K.; Babu, K.N.; Chattopadhyay, R.; Chakravarty, B.; Chaudhury, K. Upper Control Limit of Reactive Oxygen Species in Follicular Fluid Beyond which Viable Embryo Formation is not Favorable. Reprod. Toxicol. 2010, 29, 447–451. [Google Scholar] [CrossRef]
- Das, S. Reactive Oxygen Species Level in Follicular Fluid—Embryo Quality Marker in IVF? Hum. Reprod. 2006, 21, 2403–24037. [Google Scholar] [CrossRef] [PubMed]
- Sejian, V.; Naqvi, S.M.; Ezeji, T.C.; Lakritz, J.; Lal, R. Environmental Stress and Amelioration in Livestock Production; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Ko, S.H.; Chang, S.C.; Chen, C.H. A Comparative Study of Sleep Quality Between Pregnant and Nonpregnant Taiwanese Women. J. Nurs. Scholarsh. 2010, 42, 23–30. [Google Scholar] [CrossRef]
- Kamysheva, E.; Skouteris, H.; Wertheim, E.H.; Paxton, S.J.; Milgrom, J. A Prospective Investigation of the Relationships Among Sleep Quality, Physical Symptoms, and Depressive Symptoms During Pregnancy. J. Affect. Disord. 2010, 123, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W. Correlation Between Sleep Quality of Third-Trimester Pregnancy and Postpartum Depression. Med. Sci. Monit. 2014, 20, 2740–2745. [Google Scholar] [CrossRef]
- Sharma, V.; Mazmanian, D. Sleep Loss and Postpartum Psychosis. Bipolar Disord. 2003, 5, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Tsuno, N.; Besset, A.; Ritchie, K. Sleep and Depression. J. Clin. Psychiatry 2005, 66, 1254–1269. [Google Scholar] [CrossRef]
- Krystal, A.D. Psychiatric Disorders and Sleep. Neurol. Clin. 2012, 30, 1389–1413. [Google Scholar] [CrossRef]
- Xie, Y.; Peng, R.; Xiao, L.; Li, S.; Li, X. Sleep Quality During Pregnancy Following Assisted Reproductive Technology and Natural Conceiving: A Prospective Birth Cohort Study. Front. Endocrinol. 2025, 15, 1497722. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A New Instrument for Psychiatric Practice and Research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Kotronoulas, G.C.; Papadopoulou, C.N.; Papapetrou, A.; Patiraki, E. Psychometric Evaluation and Feasibility of the Greek Pittsburgh Sleep Quality Index (GR-PSQI) in Patients with Cancer Receiving Chemotherapy. Support. Care Cancer 2011, 19, 1831–1840. [Google Scholar] [CrossRef] [PubMed]
- Perantoni, E.; Steiropoulos, P.; Siopi, D.; Amfilochiou, A.; Michailidis, V.; Christoforatou, K.; Tsara, V. Validation of the Greek Version of Pittsburg Sleep Quality Questionnaire in a Sleep Lab Population. Eur. Respir. J. 2012, 40, 903. [Google Scholar]
- Soldatos, C.R.; Dikeos, D.G.; Paparrigopoulos, T.J. Athens Insomnia Scale: Validation of an Instrument Based on ICD-10 Criteria. J. Psychosom. Res. 2000, 48, 555–560. [Google Scholar] [CrossRef]
- Tsara, V.; Serasli, E.; Amfilochiou, A.; Constantinidis, T.; Christaki, P. Greek Version of the Epworth Sleepiness Scale. Sleep Breath. 2004, 8, 91–95. [Google Scholar] [CrossRef]
- Bakalidou, D.; Skordilis, E.K.; Giannopoulos, S.; Stamboulis, E.; Voumvourakis, K. Validity and Reliability of the FSS in Greek MS Patients. SpringerPlus 2013, 2, 304. [Google Scholar] [CrossRef]
- Leonardou, A.A.; Zervas, Y.M.; Papageorgiou, C.C.; Marks, M.N.; Tsartsara, E.C.; Antsaklis, A.; Christodoulou, G.N.; Soldatos, C.R. Validation of the Edinburgh Postnatal Depression Scale and Prevalence of Postnatal Depression at Two Months Postpartum in a Sample of Greek Mothers. J. Reprod. Infant Psychol. 2009, 27, 28–39. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: Abingdon, UK, 2013. [Google Scholar] [CrossRef]
- Field, A. Discovering Statistics Using IBM SPSS Statistics; Sage Publications Limited: Thousand Oaks, CA, USA, 2024. [Google Scholar]
- de Melo, M.B.; Daldegan-Bueno, D.; Menezes Oliveira, M.G.; de Souza, A.L. Beyond ANOVA and MANOVA for Repeated Measures: Advantages of Generalized Estimated Equations and Generalized Linear Mixed Models and its use in Neuroscience Research. Eur. J. Neurosci. 2022, 56, 6089–6098. [Google Scholar] [CrossRef] [PubMed]
- Krueger, C.; Tian, L. A Comparison of the General Linear Mixed Model and Repeated Measures ANOVA Using a Dataset with Multiple Missing Data Points. Biol. Res. Nurs. 2004, 6, 151–157. [Google Scholar] [CrossRef]
- Booth, J.G.; Casella, G.; Friedl, H.; Hobert, J.P. Negative Binomial Loglinear Mixed Models. Stat. Model. 2003, 3, 179–191. [Google Scholar] [CrossRef]
- Liu, K.; Case, A. Reproductive Endocrinology and Infertility Committee. Advanced Reproductive Age and Fertility. J. Obstet. Gynaecol. Can. 2011, 33, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Attali, E.; Yogev, Y. The Impact of Advanced Maternal Age on Pregnancy Outcome. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 70, 2–9. [Google Scholar] [CrossRef]
- Wallace, W.H.; Kelsey, T.W. Human Ovarian Reserve from Conception to the Menopause. PLoS ONE 2010, 5, e8772. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tan, T.Y.; Lau, S.K.; Loh, S.F.; Tan, H.H. Female Ageing and Reproductive Outcome in Assisted Reproduction Cycles. Singap. Med. J. 2014, 55, 305–309. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gunby, J.; Daya, S. IVF Directors Group of the Canadian Fertility and Andrology Society. Assisted Reproductive Technologies (ART) in Canada: 2001 results from the Canadian ART register. Fertil. Steril. 2005, 84, 590–599. [Google Scholar] [CrossRef]
- Shreffler, K.M.; Johnson, D.R. Fertility Intentions, Career Considerations and Subsequent Births: The Moderating Effects of Women’s Work Hours. J. Fam. Econ. Issues 2013, 34, 285–295. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hewlett, S.A. Creating a Life: Professional Women and the Quest for Children; Miramax: New York, NY, USA, 2002. [Google Scholar]
- Simoni, M.K.; Mu, L.; Collins, S.C. Women’s Career Priority is Associated with Attitudes Towards Family Planning and Ethical Acceptance of Reproductive Technologies. Hum. Reprod. 2017, 32, 2069–2075. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, L.A.; Karp, C.; Thiongo, M.; Gichangi, P.; Guiella, G.; Gemmill, A.; Moreau, C.; Bell, S.O. Stability and Change in Fertility Intentions in Response to the COVID-19 Pandemic in Kenya. PLoS Glob. Public Health 2022, 2, e0000147. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cooke, A.; Mills, T.A.; Lavender, T. Advanced Maternal Age: Delayed Childbearing is Rarely a Conscious Choice a Qualitative Study of Women’s Views and Experiences. Int. J. Nurs. Stud. 2012, 49, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Glavin, P.; Young, M.; Schieman, S. Labor Market Influences on Women’s Fertility Decisions: Longitudinal Evidence from Canada. Soc. Sci. Res. 2020, 88, 102417. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.M.; Décieux, F.; Zartler, U.; Schnor, C. What makes a good mother? Two Decades of Research Reflecting Social Norms of Motherhood. J. Fam. Theory Rev. 2023, 15, 57–77. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Williamson, T.; Wagstaff, D.L.; Goodwin, J.; Smith, N. Mothering Ideology: A Qualitative Exploration of Mothers’ Perceptions of Navigating Motherhood Pressures and Partner Relationships. Sex Roles 2023, 88, 101–117. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Institute of Medicine (US) Committee on Unintended Pregnancy; Brown, S.S.; Eisenberg, L. (Eds.) The Best Intentions: Unintended Pregnancy and the Well-Being of Children and Families; Consequences of Unintended Pregnancy; National Academies Press: Washington, DC, USA, 1995; p. 3. Available online: https://www.ncbi.nlm.nih.gov/books/NBK232137/ (accessed on 12 June 2025).
- Ranjbar, M.; Rahimi, M.K.; Heidari, E.; Bahariniya, S.; Alimondegari, M.; Lotfi, M.H.; Shafaghat, T. What Factors Influence Couples’ Decisions to have Children? Evidence from a systematic scoping review. BMC Pregnancy Childbirth 2024, 24, 223. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Silva, J.F.; Ocarino, N.M.; Serakides, R. Thyroid Hormones and Female Reproduction. Biol. Reprod. 2018, 99, 907–921. [Google Scholar] [CrossRef]
- Dosiou, C. Thyroid and Fertility: Recent Advances. Thyroid 2020, 30, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Concepción-Zavaleta, M.J.; Coronado-Arroyo, J.C.; Quiroz-Aldave, J.E.; Concepción-Urteaga, L.A.; Paz-Ibarra, J. Thyroid Dysfunction and Female Infertility. A Compr. Rev. Diabetes Metab. Syndr. 2023, 17, 102876. [Google Scholar] [CrossRef] [PubMed]
- Al-Musharaf, S. Changes in Sleep Patterns during Pregnancy and Predictive Factors: A Longitudinal Study in Saudi Women. Nutrients 2022, 14, 2633. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dugas, C.; Slane, V.H. Miscarriage (Archived) 27 June 2022. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Cohain, J.S.; Buxbaum, R.E.; Mankuta, D. Spontaneous First Trimester Miscarriage Rates Per Woman Among Parous Women with 1 or More Pregnancies of 24 Weeks or More. BMC Pregnancy Childbirth 2017, 17, 437. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Naud, K.; Ouellet, A.; Brown, C.; Pasquier, J.C.; Moutquin, J.M. Is Sleep Disturbed in Pregnancy? J. Obstet. Gynaecol. Can. 2010, 32, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Nyer, M.; Farabaugh, A.; Fehling, K.; Soskin, D.; Holt, D.; Papakostas, G.I.; Pedrelli, P.; Fava, M.; Pisoni, A.; Vitolo, O.; et al. Relationship between Sleep Disturbance and Depression, Anxiety, and Functioning in College Students. Depress. Anxiety 2013, 30, 873–880. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chellappa, S.L.; Aeschbach, D. Sleep and Anxiety: From Mechanisms to Interventions. Sleep Med. Rev. 2022, 61, 101583. [Google Scholar] [CrossRef] [PubMed]
- Ben Simon, E.; Rossi, A.; Harvey, A.G.; Walker, M.P. Overanxious and Underslept. Nat. Hum. Behav. 2020, 4, 100–110, Erratum in Nat. Hum. Behav. 2020, 4, 1321. [Google Scholar] [CrossRef] [PubMed]
- Rumble, M.E.; White, K.H.; Benca, R.M. Sleep Disturbances in Mood Disorders. Psychiatr. Clin. N. Am. 2015, 38, 743–759. [Google Scholar] [CrossRef] [PubMed]
- Sameer, H.M.; Imran, N.; Tarar, T.N.; Khawaja, I.S. Association of Excessive Daytime Sleepiness with Psychological Distress in Medical Students. Prim. Care Companion CNS Disord. 2020, 22, 19m02531. [Google Scholar] [CrossRef] [PubMed]
- Manning, K.; Kauffman, B.Y.; Rogers, A.H.; Garey, L.; Zvolensky, M.J. Fatigue Severity and Fatigue Sensitivity: Relations to Anxiety, Depression, Pain Catastrophizing, and Pain Severity Among Adults with Severe Fatigue and Chronic Low Back Pain. Behav. Med. 2022, 48, 181–189. [Google Scholar] [CrossRef]
- Baattaiah, B.A.; Alharbi, M.D.; Babteen, N.M.; Al-Maqbool, H.M.; Babgi, F.A.; Albatati, A.A. The Relationship Between Fatigue, Sleep Quality, Resilience, and the Risk of Postpartum Depression: An emphasis on maternal mental health. BMC Psychol. 2023, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, G.; Tadi, P. Physiology, Postpartum Changes. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK555904/?utm_source=chatgpt.com (accessed on 15 June 2025).

| Variable | Type of Conception | |||||
|---|---|---|---|---|---|---|
| NC | ART | Total | ||||
| Continuous | ||||||
| Mean | SD | Mean | SD | Mean | SD | |
| Age | 32.444 | 4.116 | 35.964 | 4.643 | 34.571 | 4.745 |
| Categorical | ||||||
| Yes | No | Yes | No | Yes | No | |
| Employment | 35 | 2 | 33 | 24 | 68 | 26 |
| Married | 24 | 13 | 55 | 2 | 79 | 15 |
| History of depression | 6 | 31 | 3 | 54 | 9 | 85 |
| Family history of mood disorders | 7 | 30 | 9 | 48 | 16 | 78 |
| Medical conditions | 5 | 32 | 28 | 29 | 33 | 61 |
| Psychotropic medication | 5 | 32 | 0 | 57 | 5 | 89 |
| Thyroid medication | 4 | 33 | 20 | 37 | 24 | 70 |
| Other medication (prescribed for medical conditions other than psychiatric or thyroid disorders) | 0 | 36 | 5 | 50 | 5 | 86 |
| Variable | Test | df | p | Effect Size |
|---|---|---|---|---|
| Continuous | ||||
| t | df | p | Cohen’s d | |
| Age | −3.789 | 81.111 | <0.001 | −0.792 |
| Categorical | ||||
| x2 | df | p | Cramer’s V | |
| Employment | 15.103 | 1 | <0.001 | 0.401 |
| Married | 16.733 | 1 | <0.001 | 0.422 |
| History of depression | 3.109 | 1 | 0.078 | 0.182 |
| Family history of mood disorders | 0.156 | 1 | 0.693 | 0.041 |
| Medical conditions | 12.488 | 1 | <0.001 | 0.364 |
| Psychotropic medication | 0.008 | 0.294 | ||
| Thyroid medication | 6.955 | 1 | 0.008 | 0.272 |
| Other medication (prescribed for medical conditions other than psychiatric or thyroid disorders) | 0.153 | 0.195 | ||
| Conception Type | Variable | Time | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| t0 | t1 | t2 | t3 | t4 | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| NC | PSQI | 4.89 | 2.90 | 5.92 | 3.14 | 6.59 | 3.81 | 8.23 | 4.53 | 8.51 | 3.78 |
| AIS | 4.53 | 4.09 | 6.25 | 3.81 | 6.89 | 4.68 | 10.17 | 6.30 | 10.03 | 4.88 | |
| ESS | 4.97 | 3.92 | 5.91 | 4.47 | 5.11 | 3.84 | 5.29 | 4.45 | 5.54 | 4.08 | |
| FSS | 36.97 | 12.74 | 36.67 | 14.31 | 42.09 | 15.71 | 34.29 | 13.29 | |||
| EPDS | 6.32 | 5.99 | 5.53 | 5.60 | 6.66 | 6.21 | 6.09 | 5.20 | |||
| ART | PSQI | 5.00 | 4.00 | 7.38 | 3.25 | 8.75 | 2.71 | 11.57 | 3.41 | 7.29 | 4.50 |
| AIS | 3.56 | 4.61 | 10.50 | 4.54 | 8.00 | 4.38 | 13.43 | 7.48 | 7.00 | 7.01 | |
| ESS | 2.78 | 2.39 | 6.75 | 4.13 | 5.00 | 2.51 | 7.29 | 4.54 | 3.00 | 1.67 | |
| FSS | 38.13 | 17.72 | 33.75 | 16.12 | 43.29 | 18.02 | 32.17 | 18.67 | |||
| EPDS | 5.00 | 4.28 | 6.25 | 6.90 | 8.43 | 9.64 | 7.00 | 5.18 | |||
| Total | PSQI | 4.91 | 3.10 | 6.18 | 3.17 | 7.00 | 3.70 | 8.79 | 4.51 | 8.31 | 3.88 |
| AIS | 4.33 | 4.16 | 7.02 | 4.23 | 7.09 | 4.59 | 10.71 | 6.53 | 9.59 | 5.25 | |
| ESS | 4.53 | 3.75 | 6.07 | 4.37 | 5.09 | 3.61 | 5.62 | 4.47 | 5.17 | 3.92 | |
| FSS | 37.18 | 13.52 | 36.14 | 14.51 | 42.29 | 15.89 | 33.98 | 13.93 | |||
| EPDS | 6.09 | 5.70 | 5.66 | 5.78 | 6.95 | 6.78 | 6.22 | 5.15 | |||
| Contrast | TIME | PSQI | AIS | ESS | FSS | EPDS |
|---|---|---|---|---|---|---|
| NC-ART | t0 | 0.959 | 0.402 | 0.079 | ||
| t1 | 0.15 | 0.02 | 0.437 | 0.908 | 0.383 | |
| t2 | 0.131 | 0.419 | 0.608 | 0.542 | 0.807 | |
| t3 | 0.095 | 0.35 | 0.19 | 0.825 | 0.659 | |
| t4 | 0.541 | 0.104 | 0.056 | 0.592 | 0.896 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evagorou, O.; Arvaniti, A.; Plakias, S.; Koutlaki, N.; Katsikidou, M.; Sfelinioti, S.; Steiropoulos, P.; Samakouri, M. Natural vs. Assisted Conception: Sleep and Emotional Health from Pregnancy to Postpartum—An Exploratory Study. J. Clin. Med. 2025, 14, 6310. https://doi.org/10.3390/jcm14176310
Evagorou O, Arvaniti A, Plakias S, Koutlaki N, Katsikidou M, Sfelinioti S, Steiropoulos P, Samakouri M. Natural vs. Assisted Conception: Sleep and Emotional Health from Pregnancy to Postpartum—An Exploratory Study. Journal of Clinical Medicine. 2025; 14(17):6310. https://doi.org/10.3390/jcm14176310
Chicago/Turabian StyleEvagorou, Olympia, Aikaterini Arvaniti, Spyridon Plakias, Nikoleta Koutlaki, Magdalini Katsikidou, Sofia Sfelinioti, Paschalis Steiropoulos, and Maria Samakouri. 2025. "Natural vs. Assisted Conception: Sleep and Emotional Health from Pregnancy to Postpartum—An Exploratory Study" Journal of Clinical Medicine 14, no. 17: 6310. https://doi.org/10.3390/jcm14176310
APA StyleEvagorou, O., Arvaniti, A., Plakias, S., Koutlaki, N., Katsikidou, M., Sfelinioti, S., Steiropoulos, P., & Samakouri, M. (2025). Natural vs. Assisted Conception: Sleep and Emotional Health from Pregnancy to Postpartum—An Exploratory Study. Journal of Clinical Medicine, 14(17), 6310. https://doi.org/10.3390/jcm14176310










