Macrolide Therapy in Patients with Sepsis or Septic Shock: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Inclusion and Exclusion Criteria
2.3. Search Strategy
2.4. Study Selection, Data Extraction
2.5. Quality Assessment and Risk of Bias Evaluation
2.6. Statistical Methods
3. Results
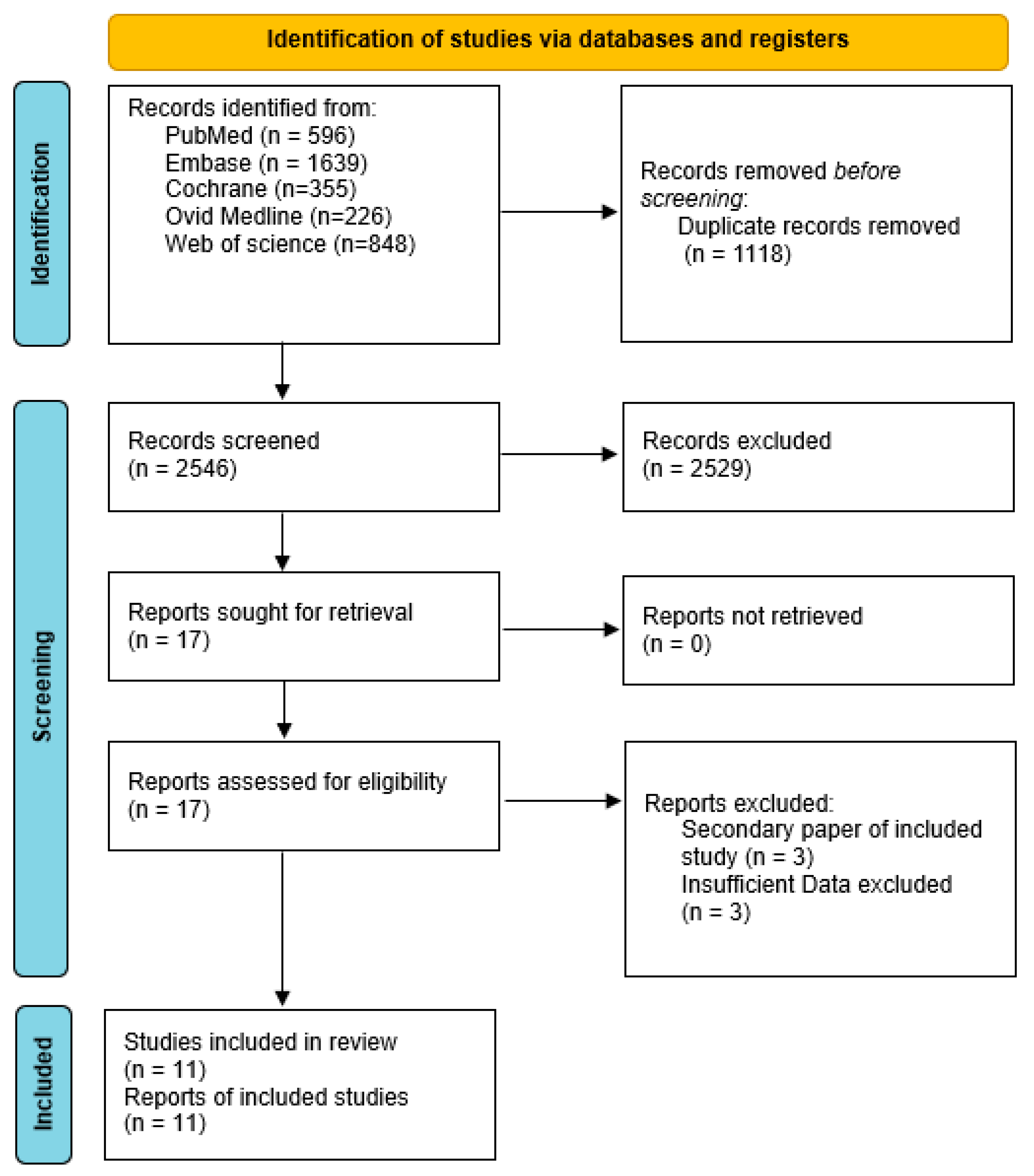
3.1. Study Selection
3.2. Study and Patient’s Characteristics
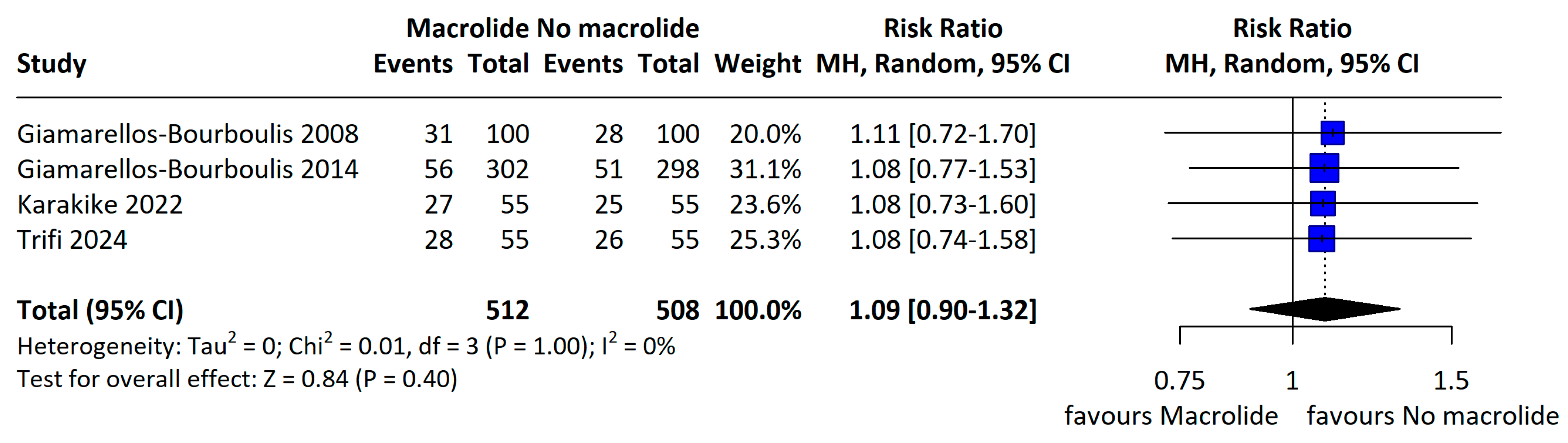
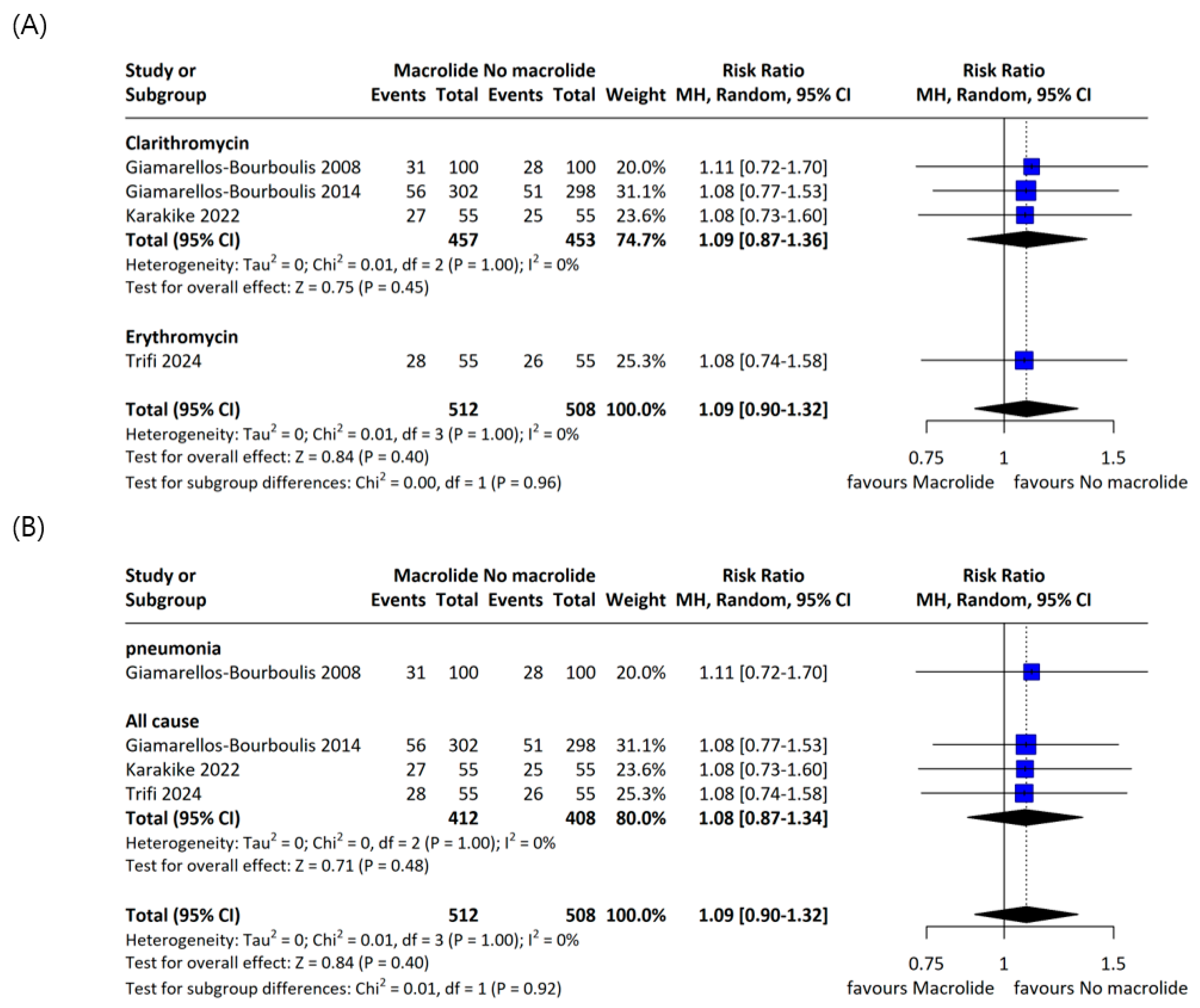
3.3. Primary Outcome
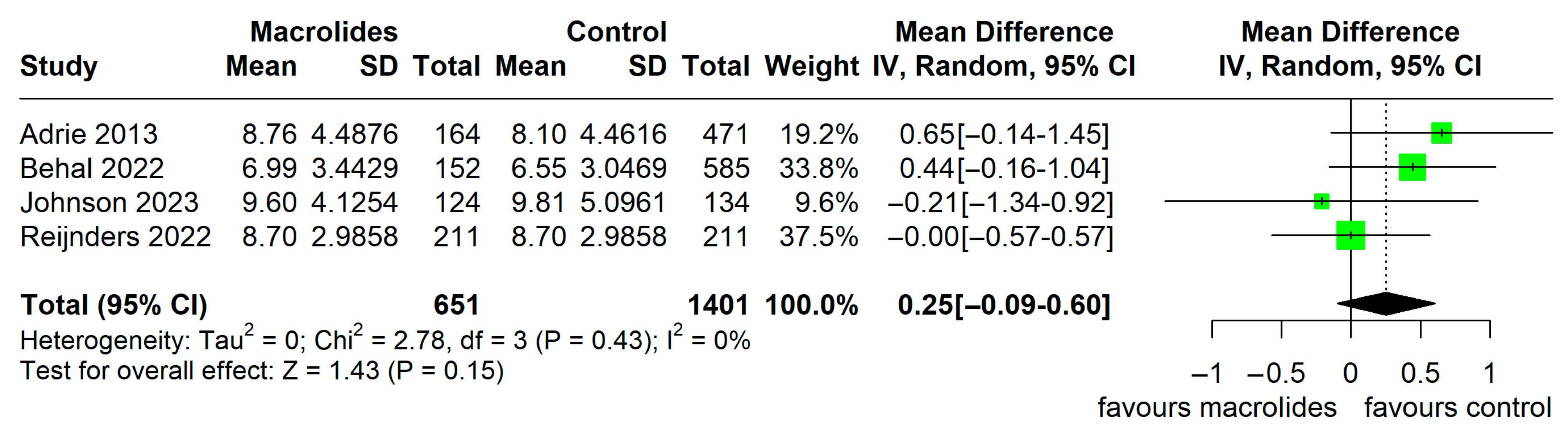
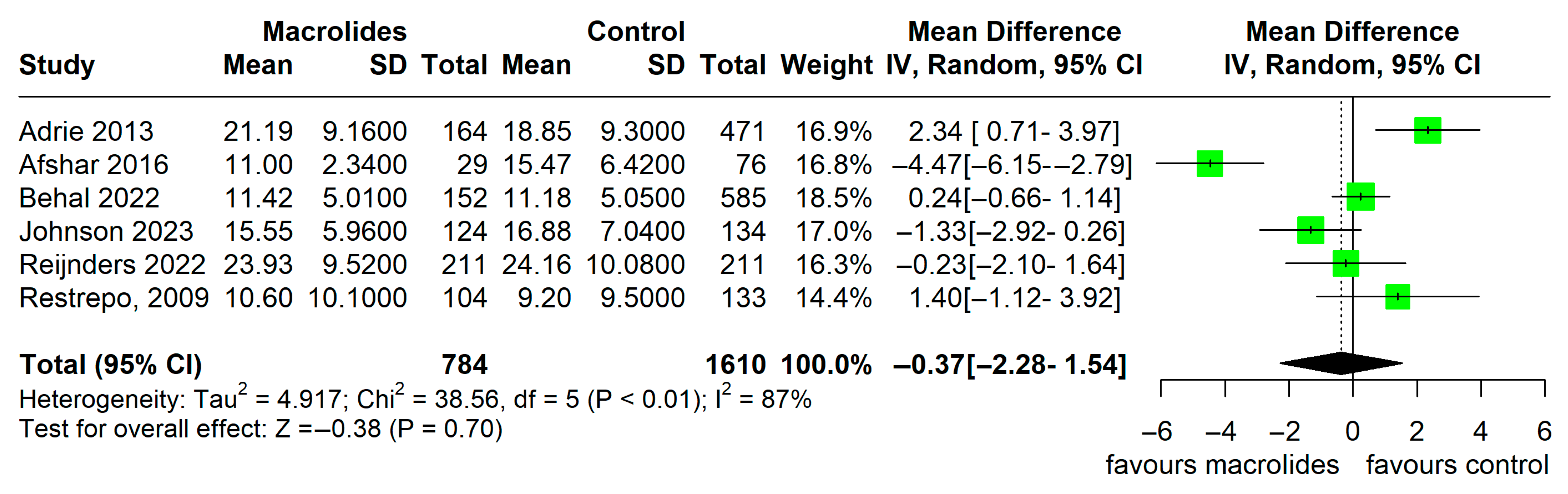
3.4. Secondary Outcome
3.5. Quality of Included Studies and Certainty of Evidence
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bauer, M.; Gerlach, H.; Vogelmann, T.; Preissing, F.; Stiefel, J.; Adam, D. Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019- results from a systematic review and meta-analysis. Crit. Care 2020, 24, 239. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Kamath, S.; Hammad Altaq, H.; Abdo, T. Management of Sepsis and Septic Shock: What Have We Learned in the Last Two Decades? Microorganisms 2023, 11, 2231. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; McIntyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit. Care Med. 2021, 49, e1063–e1143. [Google Scholar] [CrossRef] [PubMed]
- Guarino, M.; Perna, B.; Cesaro, A.E.; Maritati, M.; Spampinato, M.D.; Contini, C.; De Giorgio, R. 2023 Update on Sepsis and Septic Shock in Adult Patients: Management in the Emergency Department. J. Clin. Med. 2023, 12, 3188. [Google Scholar] [CrossRef] [PubMed]
- Afshar, M.; Foster, C.L.; Layden, J.E.; Burnham, E.L. Azithromycin use and outcomes in severe sepsis patients with and without pneumonia. J. Crit. Care 2016, 32, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Ichikado, K.; Takaki, M.; Sakata, Y.; Yasuda, Y.; Shingu, N.; Tanaka, A.; Hisanaga, J.; Eguchi, Y.; Anan, K.; et al. Efficacy of azithromycin in sepsis-associated acute respiratory distress syndrome: A retrospective study and propensity score analysis. Springerplus 2016, 5, 1193. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J. Immunomodulatory therapies for sepsis: Unexpected effects with macrolides. Int. J. Antimicrob. Agents 2008, 32, S39–S43. [Google Scholar] [CrossRef]
- Carlyn, C.J.; Andersen, N.J.; Baltch, A.L.; Smith, R.; Reilly, A.A.; Lawrence, D.A. Analysis of septic biomarker patterns: Prognostic value in predicting septic state. Diagn. Microbiol. Infect. Dis. 2015, 83, 312–318. [Google Scholar] [CrossRef]
- Parnham, M.J. Immunomodulatory effects of antimicrobials in the therapy of respiratory tract infections. Curr. Opin. Infect. Dis. 2005, 18, 125–131. [Google Scholar] [CrossRef]
- Zimmermann, P.; Ziesenitz, V.C.; Curtis, N.; Ritz, N. The Immunomodulatory Effects of Macrolides-A Systematic Review of the Underlying Mechanisms. Front. Immunol. 2018, 9, 302. [Google Scholar] [CrossRef]
- Sligl, W.I.; Asadi, L.; Eurich, D.T.; Tjosvold, L.; Marrie, T.J.; Majumdar, S.R. Macrolides and Mortality in Critically Ill Patients With Community-Acquired Pneumonia. Crit. Care Med. 2014, 42, 420–432. [Google Scholar] [CrossRef]
- Albert, R.K.; Schuller, J.L. Macrolide Antibiotics and the Risk of Cardiac Arrhythmias. Am. J. Respir. Crit. Care Med. 2014, 189, 1173–1180. [Google Scholar] [CrossRef]
- Chi, K.Y.; Li, M.Y.; Chen, C.; Kang, E.; Cochrane, T. Ten circumstances and solutions for finding the sample mean and standard deviation for meta-analysis. Syst. Rev. 2023, 12, 62. [Google Scholar] [CrossRef]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef]
- Adrie, C.; Schwebel, C.; Garrouste-Orgeas, M.; Vignoud, L.; Planquette, B.; Azoulay, E.; Kallel, H.; Darmon, M.; Souweine, B.; Dinh-Xuan, A.T.; et al. Initial use of one or two antibiotics for critically ill patients with community-acquired pneumonia: Impact on survival and bacterial resistance. Crit. Care 2013, 17, R265. [Google Scholar] [CrossRef] [PubMed]
- Behal, M.L.; Nguyen, J.L.; Li, X.; Feola, D.J.; Neyra, J.A.; Flannery, A.H. Azithromycin and Major Adverse Kidney Events in Critically Ill Patients with Sepsis-Associated Acute Kidney Injury. Shock 2022, 57, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Giamarellos-Bourboulis, E.J.; Mylona, V.; Antonopoulou, A.; Tsangaris, I.; Koutelidakis, I.; Marioli, A.; Raftogiannis, M.; Kopterides, P.; Lymberopoulou, K.; Mouktaroudi, M.; et al. Effect of clarithromycin in patients with suspected Gram-negative sepsis: Results of a randomized controlled trial. J. Antimicrob. Chemother. 2014, 69, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Giamarellos-Bourboulis, E.J.; Pechère, J.C.; Routsi, C.; Plachouras, D.; Kollias, S.; Raftogiannis, M.; Zervakis, D.; Baziaka, F.; Koronaios, A.; Antonopoulou, A.; et al. Effect of clarithromycin in patients with sepsis and ventilator-associated pneumonia. Clin. Infect. Dis. 2008, 46, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.M.; Yost, R.J.; Pangrazzi, M.H.; Golden, K.A.; Soubani, A.O.; Wahby, K.A. Azithromycin and Septic Shock Outcomes. J. Pharm. Pract. 2023, 36, 559–565. [Google Scholar] [CrossRef]
- Karakike, E.; Scicluna, B.P.; Roumpoutsou, M.; Mitrou, I.; Karampela, N.; Karageorgos, A.; Psaroulis, K.; Massa, E.; Pitsoulis, A.; Chaloulis, P.; et al. Effect of intravenous clarithromycin in patients with sepsis, respiratory and multiple organ dysfunction syndrome: A randomized clinical trial. Crit. Care 2022, 26, 183. [Google Scholar] [CrossRef] [PubMed]
- Reijnders, T.D.Y.; Peters-Sengers, H.; van Vught, L.A.; Uhel, F.; Bonten, M.J.M.; Cremer, O.L.; Schultz, M.J.; Stuiver, M.M.; van der Poll, T.; MARS Consortium. Effect of erythromycin on mortality and the host response in critically ill patients with sepsis: A target trial emulation. Crit. Care 2022, 26, 151. [Google Scholar] [CrossRef]
- Restrepo, M.I.; Mortensen, E.M.; Waterer, G.W.; Wunderink, R.G.; Coalson, J.J.; Anzueto, A. Impact of macrolide therapy on mortality for patients with severe sepsis due to pneumonia. Eur. Respir. J. 2009, 33, 153–159. [Google Scholar] [CrossRef]
- Trifi, A.; Tlili, B.; Kallel Sellami, M.; Feki, M.; Mehdi, A.; Seghir, E.; Messaoud, L.; Abdellatif, S.; Ben Lakhal, S. Immunologic effect and clinical impact of erythromycin in septic patients: A randomized clinical trial. J. Crit. Care 2024, 81, 154533. [Google Scholar] [CrossRef]
- Spyridaki, A.; Raftogiannis, M.; Antonopoulou, A.; Tsaganos, T.; Routsi, C.; Baziaka, F.; Karagianni, V.; Mouktaroudi, M.; Koutoukas, P.; Pelekanou, A.; et al. Effect of clarithromycin in inflammatory markers of patients with ventilator-associated pneumonia and sepsis caused by Gram-negative bacteria: Results from a randomized clinical study. Antimicrob. Agents Chemother. 2012, 56, 3819–3825. [Google Scholar] [CrossRef]
- Simonis, F.D.; de Iudicibus, G.; Cremer, O.L.; Ong, D.S.Y.; van der Poll, T.; Bos, L.D.; Schultz, M.J.; MARS Consortium. Macrolide therapy is associated with reduced mortality in acute respiratory distress syndrome (ARDS) patients. Ann. Transl. Med. 2018, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.H. Macrolides; Treasure Island: London, UK, 2023. [Google Scholar]
- Patel, A.; Joseph, J.; Periasamy, H.; Mokale, S. Azithromycin in Combination with Ceftriaxone Reduces Systemic Inflammation and Provides Survival Benefit in a Murine Model of Polymicrobial Sepsis. Antimicrob. Agents Chemother. 2018, 62, e00752-18. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-T.; Upadhyay, K.; Hiregoudar, B.; Meals, E.; English, B.K.; Talati, A.J. Combination therapy with ampicillin and azithromycin improved outcomes in a mouse model of group B streptococcal sepsis. PLoS ONE 2017, 12, e0182023. [Google Scholar] [CrossRef]
- Yoshioka, D.; Kajiwara, C.; Ishii, Y.; Umeki, K.; Hiramatsu, K.; Kadota, J.-I.; Tateda, K. Efficacy of β-Lactam-plus-Macrolide Combination Therapy in a Mouse Model of Lethal Pneumococcal Pneumonia. Antimicrob. Agents Chemother. 2016, 60, 6146–6154. [Google Scholar] [CrossRef] [PubMed]
- Pollock, J.; Chalmers, J.D. The immunomodulatory effects of macrolide antibiotics in respiratory disease. Pulm. Pharmacol. Ther. 2021, 71, 102095. [Google Scholar] [CrossRef]
- Kanoh, S.; Rubin, B.K. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin. Microbiol. Rev. 2010, 23, 590–615. [Google Scholar] [CrossRef] [PubMed]
- Postma, D.F.; van Werkhoven, C.H.; van Elden, L.J.; Thijsen, S.F.; Hoepelman, A.I.; Kluytmans, J.A.; Boersma, W.G.; Compaijen, C.J.; van der Wall, E.; Prins, J.M.; et al. Antibiotic treatment strategies for community-acquired pneumonia in adults. N. Engl. J. Med. 2015, 372, 1312–1323. [Google Scholar] [CrossRef] [PubMed]
- Niedrig, D.; Maechler, S.; Hoppe, L.; Corti, N.; Kovari, H.; Russmann, S. Drug safety of macrolide and quinolone antibiotics in a tertiary care hospital: Administration of interacting co-medication and QT prolongation. Eur. J. Clin. Pharmacol. 2016, 72, 859–867. [Google Scholar] [CrossRef] [PubMed]


| Study, Year | Study Design | Site | Total n (Macrolide/Control) | Male, n | Age, Mean ± SD or Median (IQR), Years | Type of Drug | Dose of Macrolide (mg) | Cause of Sepsis | Follow-Up Duration |
|---|---|---|---|---|---|---|---|---|---|
| Adrie, 2013 [16] | PC | 12 ICUs, France | 635 (164/471) | 411 | 62 (46–76) | NR | NR | Pneumonia | 60 days |
| Afshar, 2016 [6] | RC | Single center, USA | 105 (29/76) | 63 | 55.2 ± 16.4 | Azithromycin | NR | All cause | NR |
| Behal, 2022 [17] | RC | Single center, USA | 737 (152/585) | 411 | 61 (50–70)/ 56 (45–66) | Azithromycin | NR | All cause | NR |
| Giamarellos-Bourboulis, 2008 [8] | RCT | Multicenter, Greece | 200 (100/100) | 147 | 58.4 ± 19.1 | Clarithromycin | 1000 | Pneumonia | 28 days |
| Giamarellos-Bourboulis, 2014 [18] | RCT | Multicenter, Greece | 600 (302/298) | 270 | 66.9 ± 19.6 | Clarithromycin | 1000 | All cause | 28 days |
| Johnson, 2023 [20] | RC | 3 ICUs, USA | 258 (124/134) | 147 | 62.2 ± 12.8 | Azithromycin | NR | All cause | NR |
| Karakike, 2022 [21] | RCT | Multicenter, Greece and Belgium | 110 (55/55) | 72 | 74 (62–80) | Clarithromycin | 1000 | All cause | 90 days |
| Kawamura, 2016 [7] | RC | Single center, Japan | 125 (29/96) | 85 | 75 (66–82) | Azithromycin | NR | All cause | 60 days |
| Reijnders, 2022 [22] | PC | 2 ICUs, The Netherlands | 705 (235/470) | 447 | 60.9 ± 14.8 | Erythromycin | 120–250 mg (up to 600 mg/d) | All cause | 90 days |
| Restrepo, 2009 [23] | RC | 2 ICUs, USA | 237 (104/133) | 189 | 61.3 ± 16.7 | NR | NR | Pneumonia | 90 days |
| Trifi, 2024 [24] | RCT | Single center, Tunisia | 110 (55/55) | 70 | 63 (51–78)/64 (54–77) | Erythromycin | 3000 | All cause | 28 days |
| Study, Year | Overall Mortality, n (Macrolide/Control) | 28/30-Day Mortality, n (Macrolide/ Control) | 60-Day Mortality, n (Macrolide/ Control) | 90-Day Mortality, n (Macrolide/ Control) | ICU LOS, Mean ± SD or Median (IQR), Days (Macrolide/ Control) | Hospital LOS, Mean ± SD or Median (IQR), Days (Macrolide/ Control) |
|---|---|---|---|---|---|---|
| Adrie, 2013 [16] | 35/123 | NR | 35/123 | NR | 7 (3.5–15.5)/6 (3–15) | 17.5 (10.5–35)/15 (8–33) |
| Afshar, 2016 [6] | 1/13 | 1/13 | NR | NR | NR | 11 (8–14)/13 (8–25) |
| Behal, 2022 [17] | 41/189 | NR | NR | NR | 5.8 (2.9–12.1)/5.5 (2.9–11.1) | 10.3 (5.2–18.6)/9.5 (5.1–18.7) |
| Giamarellos-Bourboulis, 2008 [8] | 21/24 | 21/24 | NR | NR | 23.4 (±7.1)/21.6 (±8.22) | NR |
| Giamarellos-Bourboulis, 2014 [18] | 56/51 | 56/51 | NR | NR | NR | NR |
| Johnson, 2023 [20] | 46/51 | NR | NR | NR | 8.4 (4.6–15.6)/8.4 (3.6–17.2) | 13.9 (8.3–24.2)/15.4 (8.1–26.9) |
| Karakike, 2022 [21] | 41/38 | 27/25 | NR | 41/38 | NR | NR |
| Kawamura, 2016 [7] | 10/48 | NR | 10/48 | NR | NR | NR |
| Reijnders, 2022 [22] | 67/74 | 54/56 | NR | 67/74 | 8 (5–13)/8 (5–13) | 22 (12–37.5)/21 (12–39) |
| Restrepo, 2009 [23] | 13/45 | 11/37 | NR | 13/45 | NR | 10.6 (±10.1)/9.2 (±9.5) |
| Trifi, 2024 [24] | 28/26 | 28/26 | NR | NR | 15 (12–18)/18 (11–28) | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.; Lee, J. Macrolide Therapy in Patients with Sepsis or Septic Shock: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 6254. https://doi.org/10.3390/jcm14176254
Kim K, Lee J. Macrolide Therapy in Patients with Sepsis or Septic Shock: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(17):6254. https://doi.org/10.3390/jcm14176254
Chicago/Turabian StyleKim, Kyeongdeok, and Jihye Lee. 2025. "Macrolide Therapy in Patients with Sepsis or Septic Shock: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 17: 6254. https://doi.org/10.3390/jcm14176254
APA StyleKim, K., & Lee, J. (2025). Macrolide Therapy in Patients with Sepsis or Septic Shock: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(17), 6254. https://doi.org/10.3390/jcm14176254