Abstract
Background: Since the 1990s, numerous investigations have assessed the diagnostic effectiveness—specifically sensitivity, specificity, and accuracy—of exercise stress testing (EST), stress echocardiography (SE), stress myocardial single-photon emission computed tomography (SPECT), and stress cardiac magnetic resonance imaging (CMR). However, the outcomes of these studies have often been inconsistent and inconclusive. To provide a clearer comparison, we conducted systematic reviews and meta-analyses aimed at quantitatively evaluating and comparing the aggregated diagnostic performance of these four commonly used techniques for detecting coronary artery disease (CAD). Methods: A comprehensive search of PubMed, Scopus, Embase, Cochrane Library, and Web of Science was conducted to identify cohort studies evaluating the diagnostic accuracy of EST, SE, stress myocardial SPECT, and stress CMR in symptomatic patients with suspected or confirmed CAD. The main goal was to compare their diagnostic value by pooling sensitivity and specificity results. Each study’s data were extracted in terms of true positives, false positives, true negatives, and false negatives. Results: A total of 104 studies, comprising 16,824 symptomatic individuals with either suspected or known CAD, met the inclusion criteria. The pooled sensitivities for CAD detection were 0.66 (95% CI: 0.59–0.72, p < 0.001) for EST, 0.81 (95% CI: 0.79–0.83, p < 0.001) for SE, 0.82 (95% CI: 0.78–0.85, p < 0.001) for stress myocardial SPECT, and 0.83 (95% CI: 0.81–0.85, p < 0.001) for stress CMR. Corresponding specificities were 0.61 (95% CI: 0.55–0.67, p < 0.001), 0.85 (95% CI: 0.82–0.87, p < 0.001), 0.74 (95% CI: 0.70–0.78, p < 0.001), and 0.89 (95% CI: 0.86–0.92, p < 0.001), respectively. Considerable heterogeneity was observed across the studies, as reflected by I2 values ranging from 82.5% to 92.5%. Egger’s generalized test revealed statistically significant publication bias (p < 0.05 for all methods), likely due to the influence of smaller studies reporting more favorable results. Despite this, sensitivity analyses supported the overall robustness and reliability of the pooled findings. Conclusions: Among the diagnostic tools assessed, EST demonstrated the lowest accuracy for detecting obstructive CAD, whereas stress CMR exhibited the highest. Although stress myocardial SPECT showed strong sensitivity, its specificity was comparatively limited. SE emerged as the most balanced option, offering good diagnostic accuracy combined with advantages such as broad availability, cost-effectiveness, and the absence of ionizing radiation.
1. Introduction
Coronary artery disease (CAD) remains the leading cause of death globally [1], affecting an estimated 200 million individuals worldwide [2]. Prevalence rates vary by region, with Central and Eastern Europe and Central Asia experiencing the highest burden, while South Asia reports the lowest [3]. The global incidence of CAD is projected to increase through 2025–2034, driven by aging populations and ongoing exposure to risk factors [4]. Early screening plays a vital role in minimizing both morbidity and mortality by enabling timely interventions, such as lifestyle modifications, cardioprotective therapies, and coronary revascularization procedures. Assessing the pre-test probability of CAD—based on variables like age, gender, symptoms, and cardiovascular risk profile—can help guide clinicians toward the most suitable diagnostic approach [5].
According to the 2024 European Guidelines [6], diagnostic testing is advised for symptomatic patients with a moderate (15–50%) or high (50–85%) likelihood of obstructive CAD, while those with a low likelihood (5–15%) may not require immediate testing unless symptoms persist or are unclear. The guidelines particularly endorse coronary computed tomography angiography (CCTA) for symptomatic individuals within the low to moderate (5–50%) pre-test likelihood range. For individuals assessed to have a very low likelihood (≤5%) of obstructive CAD, screening may be postponed unless symptoms continue and non-cardiac origins have been excluded.
Despite these evidence-based recommendations, a substantial number of functional diagnostic tests are still conducted for reasons deemed “rarely appropriate” [7,8,9,10]. This trend may reflect limited adherence to guidelines by healthcare professionals, the comparatively lower availability of CCTA versus conventional functional tests and the influence of defensive medicine, which can lead to unnecessary procedures and inflated healthcare costs [11,12]. Currently, the most widely used noninvasive functional tests for detecting obstructive CAD include exercise stress testing (EST), stress echocardiography (SE), stress myocardial perfusion imaging with single-photon emission computed tomography (SPECT), and stress cardiac magnetic resonance (CMR). These tests employ stress agents—such as physical exercise, dobutamine, dipyridamole, or adenosine—to provoke myocardial ischemia in symptomatic patients.
Since the 1990s, numerous studies have examined the diagnostic performance—sensitivity, specificity, and accuracy—of EST, SE, stress myocardial SPECT, and stress CMR, with results often inconsistent and inconclusive. To address this, we propose conducting systematic reviews and meta-analyses to quantitatively compare the pooled diagnostic metrics of each method used for CAD detection. Furthermore, we will explore the technical and physiological mechanisms underlying the differences in diagnostic accuracy among the four primary testing modalities and their associated ischemic stressors.
2. Materials and Methods
These systematic reviews and meta-analyses adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [13] and were officially registered with the PROSPERO database (registration number: CRD420251118642).
2.1. Search Strategy
Two independent reviewers (A.S. and M.B.) conducted comprehensive literature searches across PubMed, Scopus, Embase, Cochrane, and Web of Science. They reviewed all available studies—without restrictions on publication date—that evaluated the diagnostic sensitivity, specificity, and overall accuracy of four main noninvasive tests: EST, SE, myocardial SPECT, and stress CMR. The analyses also focused on four primary ischemic stress agents: physical exercise, dobutamine, dipyridamole, and adenosine. The search, performed between 3 June and 27 June 2025, incorporated terms such as “sensitivity”, “specificity”, “accuracy”, “coronary artery disease”, or “CAD”, and related keywords for each imaging modality and stress agent. No language filters were applied, and pediatric studies were also considered eligible.
2.2. Inclusion and Exclusion Criteria
Eligible studies were those evaluating the diagnostic accuracy of the four primary tests (EST, SE, stress myocardial SPECT, and stress CMR) in symptomatic individuals with suspected or known CAD. Excluded were studies lacking complete diagnostic data, those without coronary angiography confirmation, animal research, non-original articles, duplicate records, abstracts, case reports, conference materials, reviews, editorials, and letters without primary data.
2.3. Study Selection and Data Extraction
Study selection and data extraction were conducted independently by A.S. and M.B. Extracted data included the following: (1) patient demographics (age and sex); (2) history of CAD (e.g., previous myocardial infarction or revascularization); (3) the threshold used for defining obstructive CAD (≥50% or ≥70% stenosis); (4) sensitivity = true positives (TP)/[TP + false negatives (FN)]; (5) specificity = true negatives (TN)/[TN + false positives (FP)]; (6) diagnostic accuracy = (TP + TN)/total sample; and (7) follow-up details, if available. Any discrepancies were resolved through consultation with a third reviewer (G.L.N.), who also verified all extracted information.
2.4. Assessment of Bias
The risk of bias for included studies was assessed using the National Institutes of Health (NIH) Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies [14]. A.S. and G.L.N. independently rated each study against 14 criteria, classifying overall quality as “good” (11–14 criteria met), “fair” (6–10), or “poor” (0–5). Discrepancies were resolved through discussion. Inter-rater reliability was assessed using Cohen’s Kappa coefficient (k), calculated with the formula k = (po − pe)/(1 − pe), where po is the observed agreement and pe is the expected agreement by chance [15].
2.5. Statistical Methods
The primary objective was to assess the diagnostic performance of the four commonly used tests (EST, SE, stress myocardial SPECT, and stress CMR) by analyzing pooled sensitivity and specificity data [16,17]. TP, TN, FP, and FN were extracted as raw counts for each included study.
A Bayesian bivariate meta-analysis using a binomial–normal framework was applied to jointly model sensitivity and specificity, building on prior research [18,19]. The model used a logit link function with non-informative normal priors (with a mean of zero and large variance) for fixed effects and penalized complexity priors (hyperparameters 3 and 0.05) for random-effect variances. For the Fisher’s z-transformed correlation, a normal prior with a zero mean and large variance was assumed.
Diagnostic performance was visually summarized using forest plots [20], crosshairs plots [21] and summary receiver operating characteristic (SROC) curves [22]. Crosshairs plots were used to visualize study-level sensitivity and specificity estimates, while forest plots presented pooled values graphically. The area under each SROC curve (AUC) was calculated to compare the overall diagnostic effectiveness of different tests and stress agents.
For each meta-analysis of methods, model fitting was refined using 40,000 iterations of the Markov Chain Monte Carlo (MCMC) procedure.
Posterior estimates for sensitivity and specificity were calculated for all four modalities. These estimates were also stratified in subgroup analyses to explore sources of heterogeneity. For each modality, the following subgroups were assessed: (1) EST: studies involving both sexes vs. female-only samples; (2) SE: based on type of stress (exercise, dobutamine, dipyridamole, or dual imaging); (3) SPECT: stratified by stressor (exercise, dobutamine, dipyridamole, or adenosine); and (4) CMR: stratified similarly to SPECT.
The I2 statistic was used to assess the degree of variability among studies in the subgroup analysis, attributing it to heterogeneity rather than random chance, since Bayesian approaches do not offer a sufficient measure of heterogeneity.
Bivariate funnel plots [23] were used to assess the risk of publication bias based on study precision. Egger’s generalized test, suitable for bivariate structures, was employed to statistically evaluate publication bias (https://doi.org/10.48550/arXiv.2209.07270) [24].
To evaluate robustness, sensitivity analyses were performed by iteratively excluding individual studies and re-running the meta-analysis [25]. Posterior credibility intervals and confidence intervals were computed, and a two-sided p value < 0.05 was considered statistically significant where applicable.
All statistical analyses were conducted using Comprehensive Meta-Analysis software version 3.0 (Biostat, Englewood, NJ, USA) and the R programming language (v4.3.2), employing the meta4diag package (v2.1.1) for diagnostic test meta-analysis.
3. Results
3.1. Study Selection
A comprehensive search was conducted across PubMed, Scopus, Embase, Cochrane, and Web of Science databases to identify studies evaluating the sensitivity, specificity, and diagnostic accuracy of EST, SE, stress myocardial SPECT, and stress CMR in detecting obstructive CAD. This initial search yielded 2381 records. After removing 181 duplicate entries (7.6%), an additional 1855 studies (77.9%) were excluded based on title screening, and 145 (6.1%) were removed following abstract review. The remaining 200 studies (8.4%) underwent full-text evaluation for eligibility. Of these, 24 (1%) were excluded due to unavailable full texts and 72 (3%) due to incomplete or missing data. Ultimately, 104 studies (4.4%) [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129] involving a total of 16,824 symptomatic patients with suspected or known CAD were included in the systematic review and meta-analysis. Notably, 42 studies (40.4%) examined more than one diagnostic modality, resulting in 159 total analyses evaluating the performance of EST (n = 25), SE (n = 58), stress myocardial SPECT (n = 42), and stress CMR (n = 34) (Figure 1).
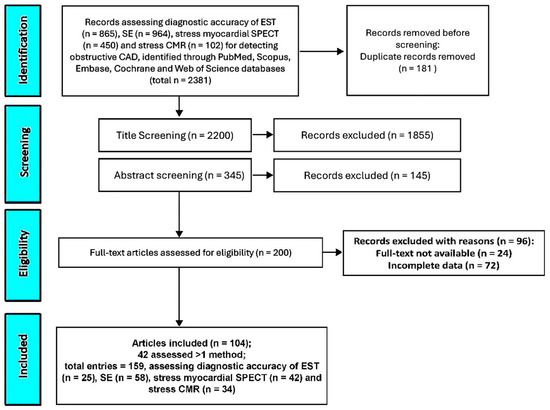
Figure 1.
The PRISMA flow diagram used for identifying the included studies. CAD, coronary artery disease; CMR, cardiac magnetic resonance; EST, exercise stress testing; SE, stress echocardiography; and SPECT, single-photon emission computed tomography.
3.2. Clinical Characteristics, Stress Protocols, and Main Findings of the Included Studies
Table 1 provides an overview of the included studies, outlining key information such as the study name, year of publication, country of origin, study design, sample size, percentage of female participants, average patient age, and whether CAD was suspected or previously diagnosed. It also details the angiographic criteria used to define obstructive CAD, the stress testing protocols applied, and the reported sensitivity, specificity, and accuracy for each myocardial ischemia detection method. For the 42 studies that assessed more than one diagnostic technique, the findings are presented separately for each method examined.

Table 1.
Clinical characteristics, stress protocols, and main findings of the 159 total analyses assessing sensitivity, specificity, and accuracy of EST, SE, stress myocardial SPECT, and stress CMR for detecting obstructive CAD. Acc, accuracy; CAD, coronary artery disease; CFR, coronary flow reserve; CMR, cardiac magnetic resonance; ESE, exercise stress echocardiography; EST, exercise stress testing; IV, intravenous; Monoc., monocentric; NS, not specified; P., prospective; SE, stress echocardiography; Sen, sensitivity; Spe, specificity; SPECT, single-photon emission computed tomography; Tc, Technetium; and Tl, Thallium. For the population study of Cortigiani L. et al. [82], hypertensive * and normotensive ** patients were separately analyzed.
The 104 studies included in the analysis were published between 1990 and 2025. Among these, 27 were conducted in the United States, 16 in Italy, 12 in Germany, 7 each in The Netherlands and the United Kingdom, 4 each in Switzerland and Spain, and 3 in Turkey, Greece, and Japan. Two studies originated from Belgium, Serbia, Poland, China, and Taiwan, while single studies were reported from France, Ireland, Norway, Portugal, Argentina, Chile, Canada, and Iran. The average patient age across all studies was 59.4 years (ranging from 14.1 to 71 years), with women comprising 33.9% of the study population (range: 1.9% to 100%).
Most of the included studies (91.4%) were prospective in design, with only nine (8.6%) being retrospective. Furthermore, 90 studies (86.5%) were single-center investigations, while 14 (13.5%) involved multiple centers. Roughly half of the studies (51.9%) enrolled symptomatic patients without a prior history of CAD, while 6.7% included only individuals with established CAD. The remaining 41.4% evaluated symptomatic patients with either known or suspected CAD. In terms of coronary angiographic criteria, the majority of studies (72.1%) defined significant coronary stenosis as a ≥50% reduction in luminal diameter. Two studies (1.9%) used a ≥60% threshold, 23 studies (22.1%) applied a ≥70% cutoff, and four studies (3.8%) used a ≥75% definition for obstructive CAD.
The stress testing protocols employed varied across modalities. For EST, exercise SPECT, and exercise CMR, the standard Bruce protocol was most frequently used, incorporating 25-Watt incremental workloads every 2 min. Supine bicycle ergometry with the same 25-Watt incremental approach on a tilting table was commonly used in exercise stress echocardiography (ESE). Dobutamine stress testing protocols typically involved an IV infusion starting at 5 mcg/kg/min and increasing to 40 mcg/kg/min in 3 min stages, with the addition of atropine up to 1 mg for dobutamine echo and SPECT studies, and up to 2 mg for CMR protocols. Dipyridamole-based protocols included a two-step IV infusion ranging from 0.56 to 0.84 mg/kg over 10 min plus atropine (0.25–1 mg) for dipyridamole echo and SPECT, while a single-step 0.56 mg/kg over 4 min was applied for dipyridamole CMR. Adenosine was administered at 140 mcg/kg/min over 4–6 min in both SPECT and CMR stress testing protocols.
Among all assessed modalities, EST demonstrated the lowest overall diagnostic performance, with a pooled sensitivity, specificity, and accuracy of 63.6%, 62.3%, and 63.2%, respectively, for detecting obstructive CAD. In contrast, dipyridamole stress CMR showed the highest pooled sensitivity (84.8%) and diagnostic accuracy (87.3%). Notably, exercise CMR yielded the highest pooled specificity (92.2%) across all tests in identifying obstructive CAD.
3.3. NIH Quality Rating
Based on the NIH quality assessment tool, 73 studies were rated as having good methodological quality, while the remaining 31 were classified as fair (see Supplementary Table S1). The level of inter-reviewer agreement for the risk of bias evaluation was substantial, as reflected by a Cohen’s Kappa coefficient of 0.82.
3.4. Diagnostic Accuracy of Exercise Stress Testing for Detecting Obstructive CAD
Figure 2 presents the forest plots depicting the sensitivity and specificity of EST across all included studies, as well as within the two subgroups: studies involving only female participants and those including both sexes.
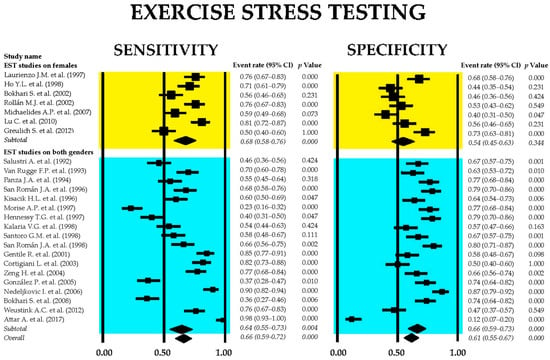
Figure 2.
Forest plots illustrating the sensitivity and specificity of exercise stress testing are provided for the overall set of studies [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50], as well as for the subgroups comprising female-only participants [31,34,39,40,45,47,48] and mixed-gender [26,27,28,29,30,32,33,35,36,37,38,41,42,43,44,45,46,49,50] cohorts.
The combined sensitivity of EST for identifying obstructive CAD was 0.66 (95% CI: 0.59–0.72, p < 0.001), while the pooled specificity was 0.61 (95% CI: 0.55–0.67, p < 0.001). Subgroup analysis indicated that studies focused exclusively on female participants demonstrated low specificity, which did not reach statistical significance. The heterogeneity across studies was substantial, with I2 values of 91.5% for sensitivity and 89.5% for specificity (both p < 0.001).
Figure 3 displays the crosshair plot (A), illustrating the performance of EST in the subgroups of female-only and mixed-gender populations, along with the SROC curve (B), which consolidates the diagnostic performance data from all included EST studies as well as from the respective subgroups.
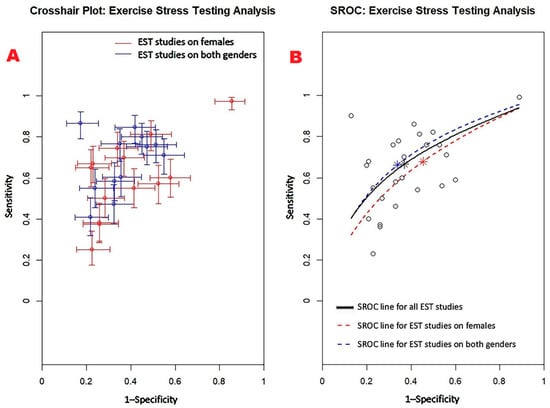
Figure 3.
(A) Crosshair plot illustrating the results of EST studies conducted in two distinct subgroups—female-only and mixed-gender populations. (B) The SROC curve represents the overall diagnostic performance of EST across all studies (AUC = 0.69; 95% CI: 0.62–0.76), as well as for studies including only women (AUC = 0.65; 95% CI: 0.52–0.80) and those involving both sexes (AUC = 0.71; 95% CI: 0.61–0.78). EST, exercise stress testing; SROC, summary receiver operating characteristic.
Figure 4 shows Begg’s funnel plot for the detection of publication bias in EST studies.
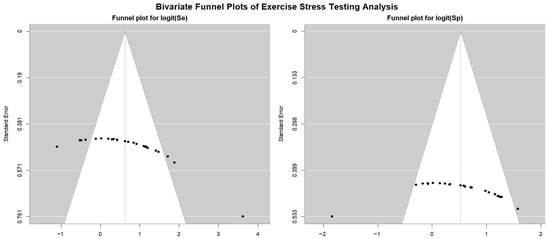
Figure 4.
Begg’s funnel plot for the detection of publication bias in EST studies.
Egger’s generalized test indicated potential publication bias with a p value of 0.02. A sensitivity analysis supported the stability of the study findings. The exclusion of individual studies led to only minor fluctuations in diagnostic estimates. Specifically, in EST studies involving female participants, sensitivity ranged from 0.68 (95% CI: 0.55–0.78, p = 0.007) to 0.73 (95% CI: 0.67–0.79, p < 0.001), while specificity varied from 0.50 (95% CI: 0.41–0.60, p = 0.96) to 0.56 (95% CI: 0.33–0.77, p = 0.61). In studies that included both sexes, sensitivity ranged from 0.52 (95% CI: 0.39–0.64, p = 0.80) to 0.62 (95% CI: 0.52–0.70, p = 0.016), and specificity varied from 0.65 (95% CI: 0.58–0.71, p < 0.001) to 0.72 (95% CI: 0.67–0.77, p < 0.001).
3.5. Diagnostic Accuracy of Stress Echocardiography for Detecting Obstructive CAD
Figure 5 displays the combined sensitivity and specificity estimates for SE and its primary clinical modalities, including ESE, dobutamine stress echocardiography (DSE), dipyridamole stress echocardiography (dipSE), and dual-imaging SE.
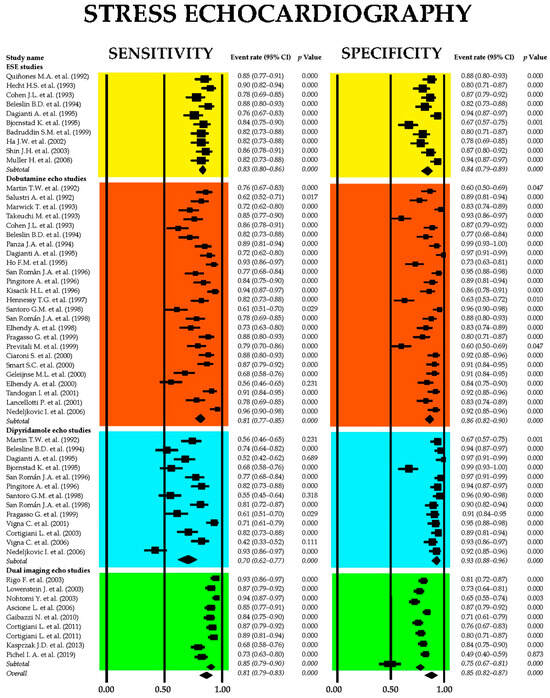
Figure 5.
Forest plots illustrating the sensitivity and specificity of SE and its principal clinical applications, including ESE [51,52,53,54,55,56,57,58,59,60], DSE [26,28,29,30,33,36,37,44,53,54,55,61,62,63,64,65,66,67,68,69,70,71,72,73,74], dipSE [29,36,37,41,44,54,55,56,61,65,67,75,76], and dual-imaging SE [77,78,79,80,81,82,83,84]. DipSE, dipyridamole stress echocardiography; DSE, dobutamine stress echocardiography; ESE, exercise stress echocardiography; and SE, stress echocardiography.
The aggregated sensitivity of SE for identifying obstructive CAD was 0.81 (95% CI: 0.79–0.83, p < 0.001), with a pooled specificity of 0.85 (95% CI: 0.82–0.87, p < 0.001). Among the various SE modalities, dipSE demonstrated the lowest sensitivity at 0.70 (95% CI: 0.62–0.77, p < 0.001), but the highest specificity at 0.93 (95% CI: 0.88–0.96, p < 0.001) for detecting CAD. The I2 statistic indicated significant heterogeneity, with values of 86.3% for sensitivity and 86.6% for specificity (both p < 0.001).
Figure 6 presents (A) the crosshair plot illustrating sensitivity and specificity estimates for each SE modality—ESE, DSE, dipSE, and dual-imaging SE—and (B) the SROC curve, which integrates the diagnostic performance of overall SE and each individual technique.
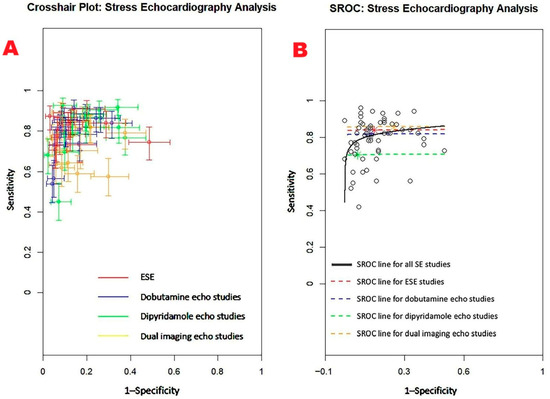
Figure 6.
(A) Crosshair plot displaying the sensitivity and specificity results from studies evaluating various forms of stress echocardiography, including ESE, DSE, dipSE, and dual-imaging SE. (B) SROC curve illustrating the overall diagnostic accuracy of SE (AUC = 0.85; 95% CI: 0.80–0.91) and the individual performance of each technique: ESE (AUC = 0.84; 95% CI: 0.69–0.91), DSE (AUC = 0.82; 95% CI: 0.66–0.89), dipSE (AUC = 0.71; 95% CI: 0.46–0.86), and dual-imaging SE (AUC = 0.86; 95% CI: 0.75–0.91). DipSE, dipyridamole stress echocardiography; DSE, dobutamine stress echocardiography; ESE, exercise stress echocardiography; SE, stress echocardiography; and SROC, summary receiver operating characteristic.
Figure 7 displays the Begg’s funnel plot used to assess potential publication bias among the SE studies.
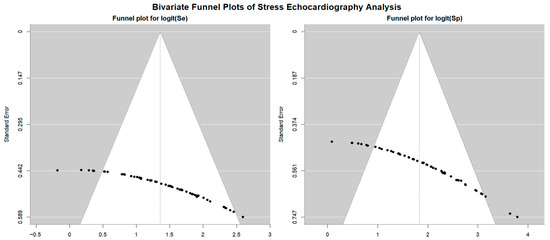
Figure 7.
Begg’s funnel plot for the assessment of publication bias in studies evaluating sensitivity and specificity of SE for detecting obstructive CAD. CAD, coronary artery disease; SE, stress echocardiography.
Egger’s generalized test revealed strong evidence of publication bias, with a p value of less than 0.001.
Sensitivity analyses reinforced the reliability of the overall findings. Stepwise exclusion of individual studies resulted in only minor fluctuations in sensitivity estimates across various stress echocardiography modalities. For ESE, sensitivity ranged from 0.83 (95% CI: 0.80–0.86, p < 0.001) to 0.87 (95% CI: 0.81–0.91, p < 0.001); in DSE studies, sensitivity varied from 0.69 (95% CI: 0.54–0.81, p = 0.01) to 0.82 (95% CI: 0.76–0.86, p < 0.001), while for dipSE studies, it ranged from 0.63 (95% CI: 0.52–0.72, p = 0.02) to 0.70 (95% CI: 0.63–0.76, p < 0.001). Finally, dual-imaging SE showed a sensitivity range between 0.87 (95% CI: 0.80–0.91, p < 0.001) and 0.91 (95% CI: 0.86–0.95, p < 0.001). Specificity estimates showed similar stability upon stepwise exclusion. For ESE, specificity ranged from 0.82 (95% CI: 0.76–0.87, p < 0.001) to 0.86 (95% CI: 0.81–0.90, p < 0.001); DSE studies reported specificity values from 0.88 (95% CI: 0.78–0.93, p < 0.001) down to 0.79 (95% CI: 0.58–0.91, p = 0.01), while for DipSE studies specificity ranged from 0.90 (95% CI: 0.61–0.98, p = 0.01) to 0.95 (95% CI: 0.85–0.98, p < 0.001). Finally, in dual-imaging SE studies specificity varied between 0.73 (95% CI: 0.63–0.81, p < 0.001) and 0.77 (95% CI: 0.66–0.85, p < 0.001).
3.6. Diagnostic Accuracy of Stress Myocardial SPECT for Predicting Obstructive CAD
Figure 8 presents the combined sensitivity and specificity estimates for stress myocardial SPECT, including the main scintigraphic techniques commonly used in clinical settings—exercise, dobutamine, dipyridamole, and adenosine SPECT.
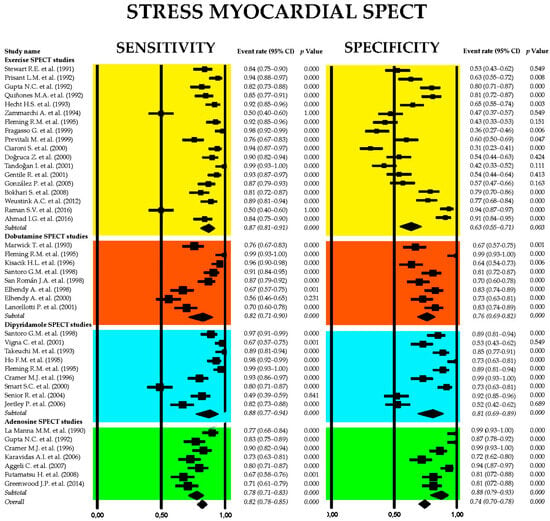
Figure 8.
Forest plots showing the sensitivity and specificity of stress myocardial SPECT and principal stress myocardial scintigraphic techniques adopted in clinical practice: exercise [38,43,46,49,51,52,67,68,69,73,85,86,87,88,89,90,91,92], dobutamine [30,36,37,62,66,72,74,89], dipyridamole [36,63,64,70,76,89,94,95,96], and adenosine [87,94,97,98,99,100,101] myocardial SPECT. SPECT, single-photon emission computed tomography.
The pooled sensitivity of stress myocardial SPECT for detecting obstructive CAD was 0.82 (95% CI: 0.78–0.85, p < 0.001), with a pooled specificity of 0.74 (95% CI: 0.70–0.78, p < 0.001). Among the various stress scintigraphy methods, dipyridamole SPECT demonstrated the highest diagnostic performance, with a sensitivity of 0.88 (95% CI: 0.77–0.94, p < 0.001) and a specificity of 0.81 (95% CI: 0.69–0.89, p < 0.001). In contrast, exercise SPECT exhibited the lowest specificity at 0.63 (95% CI: 0.55–0.71, p = 0.003). Substantial heterogeneity was noted across the studies, with I2 values of 89.3% for sensitivity and 91.5% for specificity (both p < 0.001).
Figure 9 displays the crosshair plot (A), highlighting the sensitivity and specificity results for individual SPECT modalities—including exercise, dobutamine, dipyridamole, and adenosine-based protocols. The SROC curve (B) provides an overview of the diagnostic performance of stress myocardial SPECT and its principal clinical techniques.
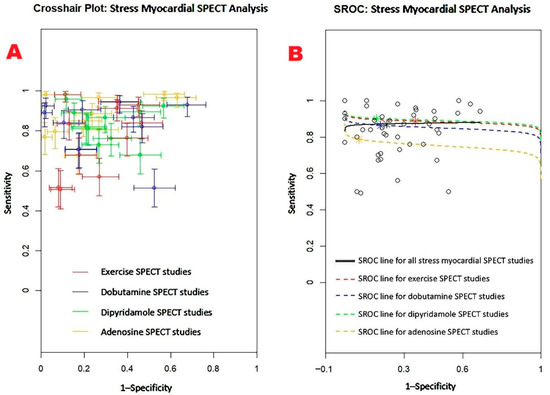
Figure 9.
(A) Crosshair plot presenting the sensitivity and specificity results from studies evaluating stress myocardial SPECT using exercise, dobutamine, dipyridamole, and adenosine protocols. (B) SROC curve illustrating the overall diagnostic accuracy of stress myocardial SPECT (AUC = 0.88; 95% CI: 0.79–0.92), along with the performance of individual techniques: exercise SPECT (AUC = 0.88; 95% CI: 0.79–0.93), dobutamine SPECT (AUC = 0.85; 95% CI: 0.66–0.94), dipyridamole SPECT (AUC = 0.89; 95% CI: 0.73–0.95), and adenosine SPECT (AUC = 0.75; 95% CI: 0.47–0.91). SPECT, single-photon emission computed tomography; SROC, summary receiver operating characteristic.
Begg’s funnel plot for the detection of publication bias in stress myocardial SPECT studies is depicted in Figure 10.
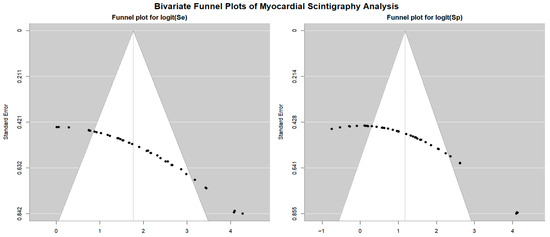
Figure 10.
Begg’s funnel plot for the assessment of publication bias in studies evaluating sensitivity and specificity of stress myocardial SPECT for predicting obstructive CAD. CAD, coronary artery disease; SPECT, single-photon emission computed tomography.
Egger’s generalized test indicated significant publication bias, with a p value of less than 0.001. The sensitivity analyses substantiated the overall stability of the results. The sequential exclusion of individual studies produced only negligible fluctuations in sensitivity across the different myocardial SPECT modalities. For exercise SPECT, sensitivity ranged from 0.84 (95% CI: 0.69–0.92, p < 0.001) to 0.90 (95% CI: 0.74–0.96, p < 0.001); for dobutamine SPECT, it fluctuated between 0.85 (95% CI: 0.72–0.92, p < 0.001) and 0.94 (95% CI: 0.70–0.99, p = 0.005); dipyridamole SPECT sensitivity varied from 0.90 (95% CI: 0.78–0.96, p < 0.001) to 0.97 (95% CI: 0.84–0.99, p < 0.001); and adenosine SPECT showed a range from 0.79 (95% CI: 0.72–0.84, p < 0.001) to 0.84 (95% CI: 0.75–0.90, p < 0.001). Specificity values also showed minimal variation upon sequential study removal. For exercise SPECT, specificity ranged from 0.84 (95% CI: 0.69–0.92, p < 0.001) to 0.90 (95% CI: 0.74–0.96, p < 0.001); dobutamine SPECT ranged from 0.73 (95% CI: 0.62–0.82, p < 0.001) to 0.76 (95% CI: 0.61–0.86, p < 0.001); dipyridamole SPECT showed a range from 0.83 (95% CI: 0.72–0.91, p < 0.001) to 0.87 (95% CI: 0.77–0.93, p < 0.001); and adenosine SPECT specificity varied between 0.90 (95% CI: 0.80–0.95, p < 0.001) and 0.98 (95% CI: 0.76–0.99, p = 0.006).
3.7. Diagnostic Accuracy of Stress CMR for Detecting Obstructive CAD
Figure 11 displays the combined sensitivity and specificity values for stress CMR, including the primary techniques commonly employed in clinical settings—exercise, dobutamine, dipyridamole, and adenosine CMR.
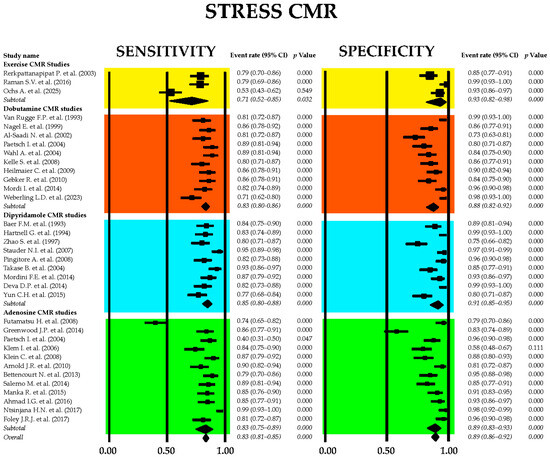
Figure 11.
Forest plots showing the sensitivity and specificity of stress CMR and principal stress CMR methods used in clinical practice: exercise [91,102,103], dobutamine [27,104,105,106,107,108,109,110,111,112], dipyridamole [113,114,115,116,117,118,119,120,121], and adenosine [92,100,101,106,122,123,124,125,126,127,128,129] CMR. CMR, cardiac magnetic resonance.
The combined sensitivity of stress CMR imaging for detecting obstructive CAD was 0.83 (95% CI: 0.81–0.85, p < 0.001), while the overall specificity was 0.89 (95% CI: 0.86–0.92, p < 0.001). Among the different stress CMR modalities, dipyridamole-based CMR exhibited the highest sensitivity at 0.85 (95% CI: 0.80–0.88, p < 0.001), whereas exercise CMR demonstrated the greatest specificity, reaching 0.93 (95% CI: 0.82–0.98, p < 0.001). Significant heterogeneity was observed among the included studies, with I2 values of 83.0% for sensitivity and 82.5% for specificity (both p < 0.001).
Figure 12 displays (A) the crosshair plot summarizing sensitivity and specificity data for each stress CMR method—exercise, dobutamine, dipyridamole, and adenosine—and (B) the SROC curve reflecting the overall diagnostic performance of stress CMR and its primary clinical applications.
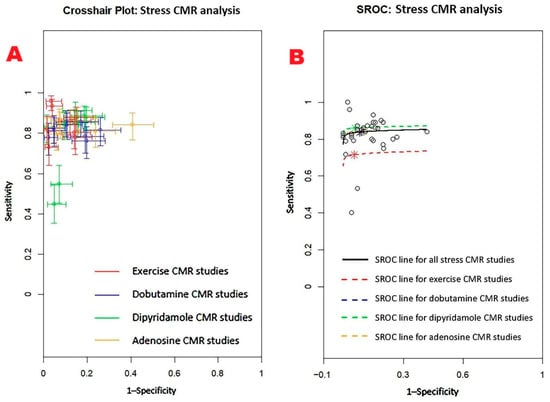
Figure 12.
(A) Crosshair plot for stress CMR studies assessing sensitivity and specificity of exercise CMT, dobutamine CMR, dipyridamole CMR, and adenosine CMR. (B) SROC summarizing the overall performance of stress CMR (AUC = 0.85; 95%CI 0.60–0.94) and of each stress CMR technique: exercise (AUC = 0.74; 95%CI 0.30–0.95), dobutamine (AUC = 0.85; 95%CI 0.55–0.95), dipyridamole (AUC = 0.87; 95%CI 0.53–0.97), and adenosine (AUC = 0.85; 95%CI 0.57–0.95) CMR. CMR, cardiac magnetic resonance; SROC, summary receiver operating characteristic.
The Begg’s funnel plot for the detection of publication bias in stress CMR studies is depicted in Figure 13.
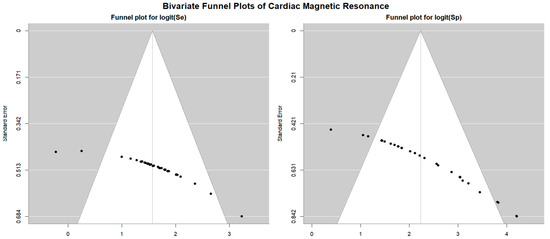
Figure 13.
Begg’s funnel plot for the assessment of publication bias in studies evaluating sensitivity and specificity of stress CMR for identifying obstructive CAD. CAD, coronary artery disease; CMR, cardiac magnetic resonance.
Egger’s generalized test indicated significant publication bias with a p value less than 0.001. The sensitivity analysis affirmed the robustness of the results, with the sequential removal of individual studies yielding only minimal variations in sensitivity among the different CMR stress techniques. For dobutamine CMR, sensitivity ranged from 0.83 (95% CI: 0.78–0.86, p < 0.001) to 0.85 (95% CI: 0.81–0.88, p < 0.001); for dipyridamole CMR, from 0.82 (95% CI: 0.77–0.86, p < 0.001) to 0.86 (95% CI: 0.81–0.89, p < 0.001); and for adenosine CMR, from 0.78 (95% CI: 0.57–0.90, p = 0.01) to 0.83 (95% CI: 0.74–0.90, p < 0.001). Specificity estimates also varied slightly with study exclusion. For dobutamine CMR, specificity ranged from 0.83 (95% CI: 0.71–0.90, p < 0.001) to 0.86 (95% CI: 0.68–0.95, p = 0.001); for dipyridamole CMR, from 0.89 (95% CI: 0.70–0.97, p = 0.001) to 0.97 (95% CI: 0.58–1.00, p = 0.03); and for adenosine CMR, from 0.83 (95% CI: 0.69–0.91, p < 0.001) to 0.88 (95% CI: 0.81–0.93, p < 0.001).
3.8. Posterior Analysis of Sensitivity and Specificity of Methods
Figure 14 and Figure 15 visually present the posterior sensitivity and specificity estimates for the four evaluated techniques—EST, SE, stress SPECT, and stress CMR—in the diagnosis of obstructive CAD.
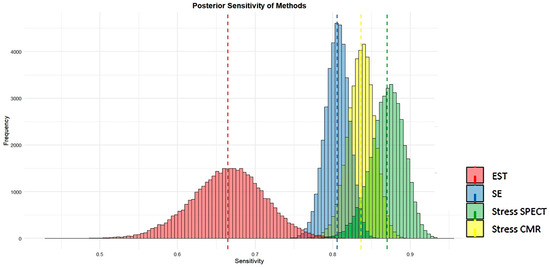
Figure 14.
Posterior estimates of sensitivity of the four methods analyzed (EST, SE, stress SPECT, and stress CMR) for detecting obstructive CAD. CAD, coronary artery disease; CMR, cardiac magnetic resonance; EST, exercise stress testing; SE, stress echocardiography; and SPECT, single-photon emission computed tomography.
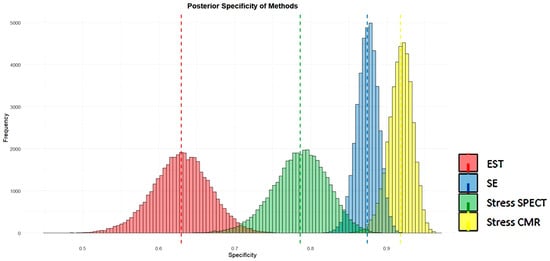
Figure 15.
Posterior estimates of specificity of the four methods analyzed (EST, SE, stress SPECT, and stress CMR) for detecting obstructive CAD. CAD, coronary artery disease; CMR, cardiac magnetic resonance; EST, exercise stress testing; SE, stress echocardiography; and SPECT, single-photon emission computed tomography.
Figure 14 and Figure 15 show that SE and stress CMR exhibit high sensitivity and specificity in identifying CAD, with minimal variability as indicated by their narrow histogram distributions. In contrast, EST showed the lowest sensitivity and specificity accompanied by greater data dispersion, as reflected in its broader histograms. Unlike the other methods, stress myocardial SPECT displayed high sensitivity but comparatively lower specificity for detecting CAD. Overall, both stress CMR and SE outperformed SPECT and EST in terms of diagnostic accuracy.
3.9. Subgroup Analyses by Region, Study Period, and Study Design (By Modality)
The pooled, sample-size-weighted sensitivity and specificity for detecting obstructive CAD were also estimated by geographic region, by study period (1990–2004 vs. 2005–2025), and by study design (prospective vs. retrospective). These results are presented in Supplementary Tables S2–S4. While absolute values varied somewhat across subgroups, the relative ranking of modalities (CMR highest, SE intermediate, SPECT intermediate, and EST lowest) was consistent, supporting the robustness of the overall findings.
4. Discussion
4.1. Principal Findings of These Systematic Reviews and Meta-Analyses
These meta-analyses compared the diagnostic performance of four non-invasive stress testing modalities—EST, SE, stress myocardial SPECT, and stress CMR—for detecting obstructive CAD. EST demonstrated the lowest diagnostic accuracy, with a pooled sensitivity and specificity of 0.66 and 0.61, respectively; subgroup analysis revealed particularly low and statistically non-significant specificity in female-only studies. In contrast, SE achieved higher diagnostic values, showing a pooled sensitivity of 0.81 and specificity of 0.85. Among SE subtypes, dipSE had the lowest sensitivity (0.70) but the highest specificity (0.93), while dual-imaging SE demonstrated both high sensitivity and specificity. Stress myocardial SPECT exhibited a pooled sensitivity of 0.82 and specificity of 0.74; dipyridamole-based SPECT achieved the best performance among scintigraphic techniques, with a sensitivity and specificity of 0.88 and 0.81, respectively, while exercise SPECT had the lowest specificity (0.63). Stress CMR yielded the highest overall diagnostic accuracy, with a pooled sensitivity of 0.83 and specificity of 0.89; dipyridamole CMR provided the highest sensitivity (0.85), and exercise CMR achieved the best specificity (0.93).
Visual assessments supported these numerical findings. SE and CMR demonstrated strong sensitivity and specificity in detecting CAD, with limited variability, as evidenced by the narrow spread of its histogram distributions. Conversely, EST yielded the lowest sensitivity and specificity, along with increased data variability, as indicated by its wider histograms. Myocardial SPECT showed high sensitivity but comparatively lower specificity. Overall, stress SE and CMR were the most accurate techniques for detecting obstructive CAD, clearly outperforming both EST and myocardial SPECT in terms of diagnostic efficacy.
High heterogeneity was observed across the included studies, with I2 values ranging from 82.5% to 92.5%. This substantial between-study variability is likely attributable to differences in study region, time period, and design. Despite this heterogeneity, our additional subgroup analyses confirmed the relative ranking of diagnostic modalities: CMR demonstrated the highest accuracy, SE and SPECT showed intermediate performance, and EST consistently ranked lowest.
Egger’s generalized test indicated a statistically significant presence of publication bias (p < 0.05 across all methods), suggesting that smaller studies tended to report more favorable results, potentially inflating pooled accuracy estimates. Nevertheless, the relative ranking of modalities remained stable, reinforcing the robustness of the comparative findings.
Funnel plot analysis further revealed asymmetry in both sensitivity and specificity across all methods, suggesting that studies with lower accuracy were underrepresented. This underrepresentation may have contributed to overly optimistic pooled estimates, a phenomenon frequently observed in diagnostic accuracy meta-analyses. Despite these concerns, sensitivity analyses confirmed the overall robustness and reliability of the pooled results.
4.2. Factors Influencing the Diagnostic Accuracy of EST for Identifying Obstructive CAD
Evidence from published studies suggests that multiple variables can influence both the sensitivity and specificity of EST in diagnosing obstructive CAD. Sensitivity tends to decrease when myocardial stress is inadequate—often due to insufficient exercise duration or suboptimal exertion levels. This is commonly observed in women, individuals with reduced exercise capacity, patients on medications such as β-blockers, nitrates, or calcium channel blockers that blunt heart rate response and mask ischemic changes, or when ECG monitoring is limited [130,131,132,133]. It is also worth noting that ST-segment depression in inferior limb leads is less predictive of CAD than in lateral precordial leads (V4–V6) [46]. Additionally, caffeine intake before testing may lead to false negatives, as it interferes with adenosine-mediated vasodilation, thus impairing hyperemic response [134]. Left ventricular dysfunction may prevent an observable ischemic response during exercise, also yielding a false-negative result [135]. Other causes of a false-negative EST include male sex, single-vessel disease, involvement of the right coronary or circumflex artery, the presence of serial stenoses, or well-developed collateral circulation, all of which may prevent typical ECG changes despite underlying disease [49,136]. Posterior wall ischemia may be missed if the involved vessel is not adequately represented on standard ECG leads [137]. Similarly, in cases of multivessel disease, widespread but balanced underperfusion may not generate detectable ECG abnormalities [138].
On the other hand, several factors can compromise the specificity of EST. A hypertensive response during exercise is a commonly cited cause of false-positive results [58,139,140,141]. This response may indicate underlying left ventricular hypertrophy (LVH), which is associated with reduced coronary vasodilator reserve and subendocardial ischemia, even in the absence of atherosclerosis [140,141]. Some studies propose that a pronounced increase in systolic blood pressure during exercise may affect atrial repolarization [140,142]. Repeating the test after optimizing blood pressure control has been suggested as a useful strategy in such cases [58]. Female sex is another well-established predictor of false-positive EST results, with women demonstrating higher false-positive rates than men [49,131,132,133,143,144]. Explanations include lower pre-test probability, more significant heart rate increase, and a stronger hypertensive response to exercise [49,131,132,133,144]. Estrogen therapy has also been linked to increased false-positive rates, potentially due to estrogen’s structural similarity to digitalis [145] and its vasoconstrictive effects on coronary arterioles [32,39,133,146]. Other clinical conditions—such as severe valvular disease, mitral valve prolapse, cardiomyopathies, anemia, hypokalemia, arrhythmias, conduction disturbances (e.g., left bundle branch block, pacemaker rhythm, or Wolff–Parkinson–White syndrome), LVH, and digitalis therapy—can cause baseline ECG abnormalities that may be misinterpreted as ischemia during EST [35,47,49,133]. Furthermore, heightened myocardial sensitivity to catecholamines may result in heart rate elevations that mimic ischemic patterns despite normal coronary arteries [147]. A quick return of the ST segment to baseline (within one minute of recovery) often indicates a false-positive result, whereas persistent ST changes are more suggestive of true ischemia [139,148]. Finally, recent studies from our group have shown a link between chest wall deformities—such as a concave chest shape—and false-positive EST findings in symptomatic patients evaluated for suspected CAD [149,150]. This may be due to mechanical effects from the abnormal chest anatomy, potentially causing exercise-induced ECG alterations through cardiac compression, displacement, or electrical dyssynchrony.
Table 2 provides a summary of the various factors that may influence the sensitivity and specificity of EST in the detection of obstructive CAD.

Table 2.
List of the principal factors that may affect sensitivity and specificity of EST in the detection of obstructive CAD. CAD, coronary artery disease; LBBB, left bundle branch block; ECG, electrocardiogram; EST, exercise stress testing; LVH, left ventricular hypertrophy; and WPW, Wolff–Parkinson–White.
4.3. Diagnostic Accuracy of Stress Echocardiography Modalities in Detecting Obstructive CAD
Stress echocardiography has evolved into a cornerstone of non-invasive diagnostic testing for CAD, allowing clinicians to assess inducible ischemia by observing changes in myocardial wall motion under stress. The four principal modalities—ESE, DSE, DipSE, and dual-imaging SE—each have distinct mechanisms, advantages, limitations, and diagnostic profiles.
ESE offers a physiologic and non-invasive method for diagnosing CAD by provoking ischemia through increased myocardial oxygen demand during physical exertion. One of its major advantages lies in its ability to simulate real-life cardiac stress, particularly useful in patients with normal exercise tolerance and interpretable electrocardiograms. ESE has shown a high specificity and a moderate-to-high sensitivity, especially in multivessel and left anterior descending (LAD) territory disease. In comparative studies, ESE and SPECT were concordant in approximately 88% of cases, and when used in combination, sensitivity improved, although at the cost of reduced specificity [51]. While SPECT evaluates perfusion and echocardiography evaluates wall motion, both modalities detect ischemia in overlapping myocardial territories, each capturing abnormalities missed by the other. Notably, ESE demonstrated superior sensitivity for detecting LAD artery lesions and in patients with triple-vessel disease, especially when images were acquired at peak exercise rather than post-exercise [52]. Supine bicycle echocardiography, a variation of ESE, provides increased venous return and oxygen demand, enhancing ischemic burden and improving the visualization of wall motion abnormalities [57]. Despite these strengths, ESE faces several limitations. The accuracy of the test is highly operator-dependent, relying on both the technical skill of the sonographer and the interpretative expertise of the physician. Imaging during exercise can be technically difficult due to hyperventilation and excessive chest wall movement, often necessitating apical-only views and potentially leading to false interpretations [151]. Furthermore, submaximal stress from inadequate exercise capacity, particularly in elderly, hypertensive, or deconditioned patients, may yield non-diagnostic or false-negative results [60]. False-positive results are more frequent in patients with a hypertensive response to exercise, where increased afterload and wall stress may mimic ischemia without significant epicardial stenosis [58]. Women are also more prone to false positives due to smaller heart size, breast attenuation, and apical thinning, although test specificity becomes similar to that of men when verification bias is adjusted [152]. Overall, while ESE is physiologic, widely available, and cost-effective, its limitations in patient subgroups and imaging artifacts must be acknowledged.
DSE serves as a valuable alternative in patients unable to exercise. It increases heart rate, blood pressure, and myocardial contractility, thereby provoking ischemia through elevated oxygen demand [153]. Compared to ESE, DSE has slightly higher sensitivity for detecting CAD, particularly in one-vessel disease and when imaging is technically limited during exercise [53]. It is especially effective in detecting severe and multivessel CAD, with the highest sensitivity reported in patients with stenosis exceeding 70% [154]. Additionally, DSE has the benefit of fewer motion-related artifacts, and ischemia tends to occur at a lower heart rate, enhancing early detection. The diagnostic accuracy of DSE can be further improved by co-administering atropine, particularly in patients on beta-blockers or those with an inadequate chronotropic response [155,156]. Transesophageal DSE can also be used in patients with poor transthoracic acoustic windows, such as the obese, elderly, or post-surgical patients, offering excellent imaging quality and accuracy for LAD and right coronary artery (RCA) territory disease [28]. Nevertheless, DSE has its own limitations. It is less specific than dipyridamole echocardiography and may provoke arrhythmias, including atrial fibrillation and supraventricular tachycardia [65,157]. False positives may occur in non-ischemic cardiomyopathies and patients with resting wall motion abnormalities, particularly in the basal segments [33]. DSE may also underestimate CAD in cases of mild or single-vessel disease and in submaximal tests where side effects limit the full dose [63]. Additionally, DSE has a lower sensitivity in detecting lesions in RCA or left circumflex (LCx) compared to LAD due to the smaller myocardial mass they perfuse [72]. Despite this, DSE remains one of the most widely validated pharmacological stress tests, with a diagnostic accuracy comparable to perfusion scintigraphy, and superior to EST [30].
DipSE, a vasodilator-based modality, is another option for patients unable to exercise. It induces ischemia by increasing coronary flow and creating a subendocardial steal in territories supplied by stenotic arteries. DipSE’s chief advantage is its very high specificity, particularly for multivessel disease, and its safety profile is favorable, with fewer arrhythmic complications than DSE [158]. In hypertensive patients and those with left bundle branch block (LBBB) or pacemakers, DipSE maintains high specificity, outperforming perfusion scintigraphy in these subgroups [69,73]. The test is further enhanced by adding atropine to increase heart rate and sensitivity, especially in patients under beta-blocker therapy [156,159]. Imaging quality is usually superior due to stable hemodynamics and minimal patient motion. However, DipSE is limited by its lower sensitivity, particularly in detecting single-vessel or mild CAD [160,161]. Its effectiveness may also be blunted by concurrent antianginal therapy, which prevents sufficient coronary steal to induce ischemia [54]. Additionally, DipSE primarily affects coronary supply and may not produce the myocardial oxygen mismatch seen with exercise or dobutamine, making wall motion abnormalities less frequent. False negatives are thus more likely when ischemic burden is mild or confined to small myocardial regions [29]. While technically simpler and better tolerated, its diagnostic yield is best in more advanced disease or when combined with other diagnostic approaches.
Dual-imaging SE, typically combining wall motion assessment with coronary flow reserve (CFR) measured via Doppler, represents a novel approach to improving diagnostic sensitivity. The integration of CFR, particularly in the LAD territory, adds significant value in cases where wall motion appears normal but microvascular or early epicardial disease is suspected [162]. The method enhances the detection of mild or single-vessel CAD and improves risk stratification, especially in hypertensive patients with subclinical microvascular dysfunction [82,163]. A normal CFR has high negative predictive value, while an abnormal CFR often precedes detectable wall motion abnormalities, thereby increasing sensitivity [83,164]. The combined analysis of wall motion and CFR outperforms either method alone, and may approach the diagnostic yield of myocardial perfusion imaging, without radiation exposure [81]. Despite its promise, dual-imaging has technical challenges. The acquisition of CFR via transthoracic Doppler requires significant expertise, is time-consuming, and is often limited to the LAD due to anatomical constraints [79]. Furthermore, CFR cannot distinguish between microvascular and macrovascular disease, and its specificity may be reduced in patients with diabetes, hypertension, or hypercholesterolemia, who may have microvascular dysfunction in the absence of epicardial stenosis [165,166,167]. Nevertheless, in selected patients, particularly those with an intermediate likelihood of disease or suboptimal wall motion analysis, the dual-imaging strategy offers a powerful diagnostic and prognostic tool.
Table 3 summarizes the main advantages and technical limitations of ESE, DSE, DipSE, and dual-imaging stress echocardiography for CAD detection in clinical practice.

Table 3.
Strengths and weaknesses of the four stress echocardiographic approaches in clinical practice. CAD, coronary artery disease; CFR, coronary flow reserve; DipSE, dipyridamole stress echocardiography; LAD, left anterior descending artery; and WM, wall motion.
4.4. Diagnostic Accuracy of Stress Myocardial SPECT for CAD Detection
Myocardial perfusion imaging with SPECT remains a cornerstone in the non-invasive diagnosis of CAD. The stress modality used—whether physiological (exercise) or pharmacologic (dobutamine, dipyridamole, or adenosine)—significantly influences diagnostic accuracy, tolerance, and image quality. Each modality presents unique advantages and limitations.
Exercise stress SPECT is a physiological method that provides valuable functional information in addition to perfusion assessment. It has demonstrated superior diagnostic performance compared to EST in detecting CAD in both single- and multivessel disease [46]. Its ability to assess physical capacity also adds prognostic value. Moreover, perfusion abnormalities often occur earlier than ischemia detectable by ECG, which enhances sensitivity [168]. However, about 25–30% of patients may not achieve adequate exercise levels due to comorbidities [169]. Attenuation artifacts—particularly diaphragmatic attenuation in men and breast attenuation in women—can result in high false-positive rates, notably affecting the inferior and anterior walls [88,170]. LBBB is another major limitation, as it leads to septal perfusion defects in the absence of CAD [73].
Dobutamine stress SPECT is indicated when exercise is not feasible. It increases myocardial oxygen demand by enhancing heart rate and contractility, mimicking exercise. It has shown very high sensitivity and specificity in limited cohorts [89], particularly for detecting single-vessel CAD and LAD lesions. It is preferred in patients with LBBB or LVH, where perfusion SPECT may yield false positives [62]. Nonetheless, dobutamine may cause pro-arrhythmia, and its uptake may interfere with MIBI imaging in normally perfused areas, potentially leading to an underestimation of flow heterogeneity [171]. The diagnostic performance of dobutamine SPECT is comparable to stress echocardiography, although the latter may have higher specificity in certain subgroups.
Dipyridamole stress SPECT offers a non-exertional alternative by inducing coronary vasodilation. It has shown high sensitivity in CAD detection, particularly in multivessel disease [36]. Its diagnostic efficacy is comparable to that of adenosine, with good agreement in terms of regional tracer uptake and hemodynamic effects [94]. Dipyridamole is especially useful in detecting perfusion abnormalities in territories with mild-to-moderate stenosis, which may not produce echocardiographic changes [172]. However, its specificity is modest due to attenuation artifacts and motion-related false positives [173]. Though side effects like flushing or chest discomfort are common, they are generally reversible with aminophylline.
Adenosine stress SPECT is widely used due to its short half-life, rapid onset of action, and safety profile. Like dipyridamole, it produces vasodilation but with a shorter infusion time and reduced potential for bronchospasm. The diagnostic accuracy of adenosine SPECT is similar to that of exercise SPECT in detecting both fixed and reversible perfusion defects [97]. It shows particular superiority in LBBB patients by minimizing false-positive septal defects [73,174]. Additionally, it has shown higher sensitivity than dobutamine for perfusion imaging, though specificity may be lower in patients with a smaller heart size or single-vessel disease [101]. Continuous ECG and blood pressure monitoring are recommended due to transient side effects such as chest pain, atrioventricular block, or bradycardia [87].
The choice of stress modality in myocardial SPECT should be individualized based on patient characteristics, ability to exercise, comorbidities (e.g., LBBB and LVH), and institutional expertise. Exercise remains the preferred first-line option when feasible, while pharmacologic stressors offer robust alternatives with comparable diagnostic power. Nonetheless, each carries unique artifacts and side effect profiles, influencing diagnostic interpretation and clinical decision-making.
Table 4 summarizes the main advantages and limitations of exercise stress SPECT, dobutamine stress SPECT, dipyridamole stress SPECT, and adenosine stress SPECT for CAD detection.

Table 4.
Comparison of stress modalities in myocardial SPECT imaging. AV, atrioventricular; BP, blood pressure; CAD, coronary artery disease; ECG, electrocardiogram; EST, exercise stress testing; LAD, left anterior descending artery; LBBB, left bundle branch block; LVH, left ventricular hypertrophy; and SPECT, single-photon emission computed tomography.
4.5. Diagnostic Accuracy of Stress CMR for Detecting Obstructive CAD
CMR stress testing has evolved into a powerful non-invasive tool for detecting CAD, utilizing various stressors including exercise, dobutamine, dipyridamole, and adenosine. Exercise stress CMR, though technically demanding, offers physiological relevance by simulating natural exertion. Early studies using treadmill-based protocols demonstrated promising diagnostic accuracy, with sensitivity and specificity reported at 79% and 85%, respectively, for detecting significant coronary stenoses [102]. A larger multicenter study confirmed excellent correlation with angiographic findings and outperformed SPECT in diagnostic performance, with a sensitivity and specificity of 79% and 99%, respectively [91]. Exercise CMR can also identify microvascular dysfunction [175]. However, its clinical adoption has been limited due to logistical hurdles such as delayed imaging after exercise, which may diminish the detection of transient ischemic changes. Supine bicycle ergometry is feasible but often hampered by muscle fatigue in untrained patients. Recently, dynamic handgrip exercise combined with fast strain–encoded imaging (DHE–fSENC) emerged as a viable, shorter, and more accessible stress protocol, showing a sensitivity and specificity of 82% and 89% for obstructive CAD, even in elderly populations [103].
Dobutamine stress cardiac magnetic resonance (DSCMR), a pharmacologic alternative, mimics exercise by increasing heart rate and myocardial oxygen demand. It is well established as a feasible, safe, and highly accurate method for CAD detection, with reported sensitivity and specificity ranging from 81 to 89% and 85 to 100%, respectively [27,109]. Compared to DSE, DSCMR has shown superior diagnostic accuracy due to better spatial resolution, clearer endocardial border delineation, and reduced operator dependency [104]. Technical advances such as steady-state free precession sequences and parallel imaging have further enhanced image quality [176]. DSCMR has proven effective across various subgroups, including patients with multivessel disease, prior stenting, and chronic kidney disease [177]. Its negative predictive value remains high (95%), supporting its utility in ruling out significant CAD [112]. However, limitations include reduced accessibility and limited applicability in patients with claustrophobia or non-MR-compatible devices. Additional concerns involve suboptimal temporal resolution—particularly at high heart rates—and the inability to interpret diagnostic ECGs due to magnetic interference.
Dipyridamole stress CMR primarily assesses perfusion and wall motion abnormalities. Studies have demonstrated comparable diagnostic accuracy to nuclear imaging, with sensitivity and specificity frequently exceeding 85% [113,116]. It is especially sensitive in detecting moderate stenoses via perfusion deficits and more specific for severe disease using wall motion criteria [118]. Dipyridamole-induced perfusion defects correlate strongly with significant CAD and prior myocardial infarction. A fully quantitative perfusion method outperformed both semi-quantitative and qualitative analyses, achieving an AUC of 92%, sensitivity of 87%, and specificity of 93% [119]. This modality is safe and effective even in complex scenarios such as congenital heart disease [120] or post-bypass graft evaluation [117], and offers utility in comprehensive assessments combining cine, perfusion, and LGE imaging.
Adenosine stress CMR is now widely used and regarded for its rapid infusion protocol, high safety profile, and excellent spatial resolution. It offers good sensitivity (84–89%) and specificity (87–98%) for detecting functionally significant CAD, especially when combined with late gadolinium enhancement (LGE) [122]. The multiparametric approach of adenosine perfusion-CMR, incorporating rest perfusion, cine imaging, and LGE, improves specificity and helps differentiate artifacts from true ischemia. While artifacts like dark-rim effects may reduce specificity, innovations such as spiral pulse sequences have minimized their impact [126]. Adenosine stress CMR performs robustly at 3.0T, showing excellent accuracy in both pediatric and adult populations [127,128], and is superior to SPECT, particularly in women and overweight patients, due to higher spatial resolution and reduced attenuation artifacts [101].
Table 5 lists the relevant advantages and limitations of exercise stress CMR, dobutamine stress CMR, dipyridamole, stress CMR, and adenosine stress CMR for predicting obstructive CAD.

Table 5.
Principal advantages and limitations of exercise stress CMR, dobutamine stress CMR, dipyridamole, stress CMR, and adenosine stress CMR in clinical practice. CAD, coronary artery disease; CKD, chronic kidney disease; DSE, dobutamine stress echocardiography; DSCMR, dobutamine stress cardiac magnetic resonance; IV, intravenous; MR, magnetic resonance; and SPECT, single-photon emission computed tomography.
4.6. Clinical Applicability of EST, SE, SPECT, and CMR
In clinical practice, the choice among EST, SE, SPECT, and CMR must balance diagnostic accuracy with availability, patient safety, and resource considerations. EST remains the most widely available and least expensive technique, requiring minimal infrastructure; however, its diagnostic yield is limited, particularly in women, elderly patients, and those unable to achieve adequate exercise capacity. For this reason, its role has shifted toward functional capacity assessment and symptom reproduction rather than the primary diagnosis of obstructive CAD. SE offers an attractive compromise: it is broadly available, relatively inexpensive compared with advanced imaging, and does not involve radiation exposure. Operator expertise is essential, but when performed well, SE achieves robust accuracy and can be integrated into routine workflows. SPECT is still the most common imaging test worldwide, with extensive evidence and standardized protocols. It provides reproducible results and broad accessibility, yet its limitations include radiation exposure, attenuation artifacts, and declining specificity in certain populations. CMR has emerged as the most accurate modality, offering high spatial resolution, no ionizing radiation, and a comprehensive assessment of structure, function, and perfusion. Nonetheless, its uptake is constrained by limited availability, longer examination times, contraindications in patients with devices or claustrophobia, and higher costs. Taken together, SE provides the most pragmatic balance of accuracy, safety, and feasibility, SPECT offers a familiar and standardized tool, CMR represents the accuracy benchmark where available, and EST, although inexpensive, has a marginal role in contemporary diagnostic pathways.
4.7. 2024 ESC Guidelines Recommendations for the Diagnostic Management of Patients with Suspected CAD
The 2024 European guidelines underscore the evolving role of diagnostic testing in patients with suspected chronic coronary syndrome (CCS), emphasizing the stratification of patients based on pre-test probability (PTP) and the appropriate use of non-invasive imaging modalities to diagnose obstructive CAD and estimate the risk of major adverse cardiovascular events (MACE).
EST retains value, particularly for patients with a low clinical likelihood of CAD (>5–15%), where a negative result can reclassify individuals into a very low risk group (≤5%) with a favorable prognosis [178]. It remains clinically relevant for reproducing anginal symptoms, which are themselves prognostic [179,180]. Traditionally, the EST involves graded physical activity until the appearance of limiting symptoms or abnormal ECG findings. While widely used, its diagnostic accuracy is inferior to modern functional imaging techniques and coronary computed tomography angiography (CCTA), with sensitivity and specificity reported at only 58% and 62%, respectively [181]. Data from the SCOT–HEART trial showed that abnormal EST results predict future revascularization and myocardial infarction, yet a large subset of patients with normal or inconclusive tests still had underlying CAD that was better detected with CCTA [182]. Consequently, CCTA is now preferred as a first-line diagnostic strategy, especially in individuals with low or moderate PTP. Nevertheless, EST may remain relevant in regions with limited access to imaging or as a pragmatic option for symptom evaluation and risk stratification. However, the test is not appropriate in patients with baseline ECG abnormalities, such as LBBB, paced rhythms, or digitalis therapy, which interfere with interpretation.
In individuals with moderate or high PTP of obstructive CAD (>15–85%), the guidelines recommend SE, SPECT, or positron emission tomography (PET) myocardial perfusion imaging, or stress CMR, as first-line tests. SE assesses myocardial ischemia by detecting regional wall-thickening abnormalities (RWTA), induced by increased oxygen demand during stress. The technique can utilize exercise, dobutamine, or vasodilators such as adenosine or dipyridamole. Though highly accessible, low-cost, and radiation-free [183,184], its effectiveness depends on operator skill and image quality, which may be compromised in patients with obesity or lung disease. RWTA-based diagnosis may underestimate ischemia in patients with microvascular dysfunction [185], making it less suitable for conditions like angina with non-obstructive coronary arteries (ANOCA) or ischemia with non-obstructive coronary arteries (INOCA). Additional stress echocardiography techniques, such as the measurement of CFR in the LAD artery, offer incremental value in risk stratification [186]. The use of ultrasound contrast agents (microbubbles) is also recommended when two or more myocardial segments are poorly visualized. SE is especially recommended in patients with moderate-to-high PTP to diagnose myocardial ischemia and estimate MACE risk [6].
Myocardial perfusion SPECT imaging evaluates regional myocardial blood flow using radiotracers, with technetium–99m preferred over thallium due to lower radiation exposure. Although traditionally limited by attenuation artifacts and reduced accuracy in multivessel CAD, newer cadmium–zinc telluride (CZT) detectors have improved its spatial resolution, reduced scan time, and enabled myocardial blood flow (MBF) quantification [187]. SPECT remains widely applicable, including for patients unable to exercise. In high-risk populations, it offers good diagnostic and prognostic utility [188,189,190,191]. When SPECT or PET is selected, coronary artery calcium scoring (CACS) from CT attenuation correction imaging is recommended for the enhanced detection of non-obstructive CAD.
Stress CMR, using gadolinium-enhanced perfusion imaging, offers high spatial resolution without radiation and provides a comprehensive evaluation including the assessment of LV function and myocardial scarring via LGE [181,192,193]. It is particularly suited to patients with suspected CAD who require an assessment of both ischemia and myocardial viability. Stress CMR has shown strong prognostic value [194,195,196] and enhances management decisions [191,197]. However, limitations include restricted availability, claustrophobia, longer acquisition times [198], and contraindications in patients with certain implants or advanced renal dysfunction.
Finally, CCTA is recommended as the first-line test in patients with low to moderate PTP (5–50%), based on its high negative predictive value and ability to detect non-obstructive CAD [199,200,201]. It is particularly advantageous in younger patients or those with atypical symptoms. In contrast, functional imaging is preferred when information on ischemia, viability, or microvascular disease is required, and when CCTA’s utility may be limited, such as in cases of severe coronary calcification, arrhythmias, or contrast allergies.
ESC 2024 recommendations regarding the use of EST, SE, stress myocardial SPECT, and stress CMR in clinical settings are outlined in Table 6.

Table 6.
ESC 2024 guideline recommendations for the clinical application of EST, SE, stress myocardial SPECT, and stress CMR. BP, blood pressure; CACS, coronary artery calcium score; CAD, coronary artery disease; CFR, coronary flow reserve; CMR, cardiac magnetic resonance; EST, exercise stress testing; MACE, major adverse cardiovascular events; PET, positron emission tomography; SE, stress echocardiography; and SPECT, single-photon emission computed tomography.
4.8. Innovative Screening Methods for CAD Detection
In recent years, our research group has conducted several studies aimed at examining how chest wall conformation may impact the outcomes of EST and/or ESE. By incorporating a preliminary, noninvasive assessment of chest wall shape using the modified Haller index (MHI)—calculated as the ratio between the transverse external thoracic diameter and the anteroposterior internal thoracic diameter [202]—into PTP evaluation, we improved the identification of patients with a low or extremely low likelihood of CAD and/or an increased chance of a false positive EST or ESE result.
Specifically, a concave chest wall shape—often resulting from varying degrees of sternal depression or pectus excavatum and defined by an MHI greater than 2.5 [203]—was shown to independently predict false positive ESE outcomes [204] and was strongly linked to a favorable cardiovascular prognosis and a low risk of adverse events over mid-to-long-term follow-up [205,206]. Individuals with this chest conformation (MHI > 2.5) are typically women with a smaller body surface area and a lower incidence of traditional cardiovascular risk factors. These patients are frequently referred to echocardiographic labs to rule out CAD due to resting or stress ECG abnormalities.
Common findings in such patients include smaller cardiac chambers, preserved left ventricular geometry, normal left ventricular systolic and diastolic function, and a higher occurrence of mitral valve prolapse. Notably, mitral valve prolapse often coexists with anterior chest wall deformities and thoracic skeletal anomalies, including pectus excavatum, pectus carinatum, scoliosis, straight back syndrome, and Marfan syndrome [207]. Those with an MHI > 2.5 and/or mitral valve prolapse often report atypical chest pain and palpitations and tend to present with resting or exercise-induced premature ventricular contractions and pseudo-ischemic ECG patterns [208].
A reduced anteroposterior chest diameter (typically ≤13.5 cm) [204] may contribute to, or independently cause, dynamic LV dyssynchrony during physical exertion, driven by external compressive forces rather than intrinsic myocardial impairment or actual ischemia. Such dyssynchrony may be misinterpreted as regional wall motion abnormalities suggestive of obstructive CAD, even by experienced echocardiographers. Additionally, atrial and/or ventricular arrhythmias stemming from the sternal compression of cardiac structures are commonly observed in individuals with varying degrees of anterior chest wall deformity.
Clinically, these findings suggest that in symptomatic patients with a low PTP for CAD (<15%), an MHI > 2.5 or an anteroposterior chest diameter ≤ 13.5 cm, and mitral valve prolapse, a positive EST or ESE result is likely to be falsely positive. For such patients, it may be advisable to forego EST, ESE, or further imaging like CCTA or invasive coronary angiography. On the other hand, a circular or convex chest shape (MHI ≤ 2.5 or anteroposterior chest diameter > 13.5 cm) is often associated with enlarged cardiac chambers, atrial fibrillation, and CAD [209]. Therefore, symptomatic individuals with an intermediate-to-high PTP for CAD (15–85%) and MHI ≤ 2.5 warrant further diagnostic evaluation.
According to findings from this meta-analysis, SE (preferably ESE in patients capable of exercise) may serve as the preferred initial test for detecting CAD, given its high diagnostic performance, broad availability, and cost-effectiveness. A clearly negative SE can reliably exclude obstructive CAD. Conversely, a positive or inconclusive SE result may necessitate further imaging like CCTA. This diagnostic pathway may reduce unnecessary exposure to ionizing radiation from myocardial SPECT and may help avoid referrals to stress CMR, which is often less accessible due to its higher cost and time requirements.
A flowchart outlining this proposed screening strategy for CAD in symptomatic patients, stratified by PTP, is shown in Figure 16.
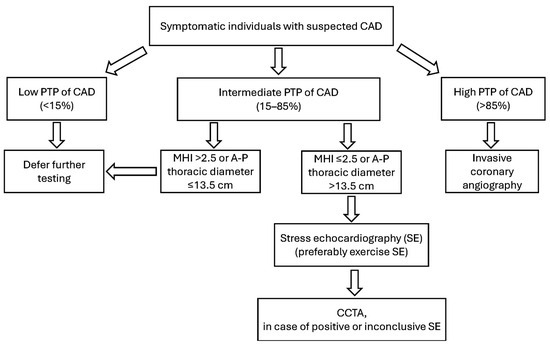
Figure 16.
Innovative screening strategy for symptomatic individuals with suspected CAD. A–P, antero–posterior; CAD, coronary artery disease; CCTA, coronary computed tomography angiography; MHI, modified Haller index; PTP, pre-test probability; and SE, stress echocardiography.
4.9. Limitations of the Studies Included in the Present Meta-Analysis
A key limitation of the studies included in this analysis was their generally small sample sizes and the fact that most were conducted at single centers. Additionally, 8.6% of the studies were retrospective in design. The I2 values for EST, SE, stress myocardial SPECT, and stress CMR studies ranged from 82.5% to 92.5%, reflecting substantial heterogeneity among the studies. This variability is partly attributable to differences in the populations studied—symptomatic patients from different countries, age groups, and sexes, with varying degrees of CAD severity. Approximately half of the studies enrolled patients with suspected CAD only, whereas the remainder included those with known CAD or mixed populations. Since diagnostic accuracy often appears higher in known CAD, this mixture may have biased the pooled estimates upward. In addition, the angiographic thresholds used to define obstructive CAD varied (≥50% vs. ≥70% stenosis), further limiting the direct comparability of results across studies. Furthermore, the studies used differing stress protocols, varying ischemic stressor doses, and distinct angiographic criteria for defining obstructive CAD. The presence of “small study effects”—where smaller trials (which made up a large proportion of this meta-analysis) tend to show more favorable or exaggerated outcomes compared to larger studies [210]—was evident; consequently, Egger’s generalized test indicated significant publication bias in the data sets for EST, SE, stress myocardial SPECT, and stress CMR. Nevertheless, sensitivity analyses affirmed the stability and reliability of the findings across all diagnostic meta-analyses. Finally, the present meta-analysis did not include studies assessing coronary microvascular dysfunction in patients with suspected CAD. In this regard, recent findings suggest that exercise-induced ischemic ECG alterations are highly specific for detecting coronary microvascular dysfunction in patients with ANOCA [211].
5. Conclusions
Among the diagnostic tools assessed, EST demonstrated the least effective diagnostic performance for identifying obstructive CAD, whereas stress CMR exhibited the highest overall accuracy. Although stress myocardial SPECT was characterized by strong sensitivity, its specificity was relatively limited. SE emerged as a favorable middle ground, offering a reliable balance of diagnostic accuracy and practical advantages, including broad accessibility, cost-efficiency, and the absence of radiation exposure.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/jcm14176238/s1: Table S1: RoB assessment of the included studies. Tables S2–S4: subgroup analyses by region, period, and design, stratified by modality.
Author Contributions
Conceptualization, A.S. and G.L.N.; methodology, A.S., A.P. and G.B.-Z.; software, A.S.; validation, G.B.-Z.; formal analysis, A.S.; investigation, A.S.; resources, A.S.; data curation, A.S. and A.P.; writing—original draft preparation, A.S.; writing—review and editing, A.P., G.L.N. and G.B.-Z.; visualization, M.B. and G.B.-Z.; supervision, M.L. and G.B.-Z.; project administration, A.S.; funding acquisition, A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Italian Ministry of Health, Ricerca Corrente IRCCS MultiMedica.
Institutional Review Board Statement
This study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines and was registered in the PROSPERO database (registration number: CRD420251118642), date of approval 3 August 2025.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data extracted from included studies will be publicly available on Zenodo (https://zenodo.org, accessed on 1 August 2025).
Acknowledgments
The authors wish to thank Monica Fumagalli and Lorenzo Bonarini for their graphical support.
Conflicts of Interest
Andrea Sonaglioni, Alessio Polymeropoulos, Massimo Baravelli, Gian Luigi Nicolosi, and Michele Lombardo declare no conflicts of interest. Giuseppe Biondi-Zoccai has consulted, lectured, and/or served as an advisory board member for Abiomed, Advanced Nanotherapies, Aleph, Amarin, AstraZeneca, Balmed, Cardionovum, Cepton, Crannmedical, Endocore Lab, Eukon, Guidotti, Innovheart, Meditrial, Menarini, Microport, Opsens Medical, Synthesa, Terumo, and Translumina, outside of the present work.
References
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Safiri, S.; Karamzad, N.; Singh, K.; Carson-Chahhoud, K.; Adams, C.; Nejadghaderi, S.A.; Almasi-Hashiani, A.; Sullman, M.J.M.; Mansournia, M.A.; Bragazzi, N.L.; et al. Burden of ischemic heart disease and its attributable risk factors in 204 countries and territories, 1990–2019. Eur. J. Prev. Cardiol. 2022, 29, 420–431. [Google Scholar] [CrossRef]
- Guan, C.; Wu, S.; Xu, W.; Zhang, J. Global, regional, and national burden of ischaemic heart disease and its trends, 1990–2019. Public Health 2023, 223, 57–66. [Google Scholar] [CrossRef]
- Khan, M.A.; Hashim, M.J.; Mustafa, H.; Baniyas, M.Y.; Al Suwaidi, S.K.B.M.; AlKatheeri, R.; Alblooshi, F.M.K.; Almatrooshi, M.E.A.H.; Alzaabi, M.E.H.; Al Darmaki, R.S.; et al. Global epidemiology of ischemic heart disease: Results from the Global Burden of Disease Study. Cureus 2020, 12, e9349. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar] [CrossRef] [PubMed]
- McCully, R.B.; Pellikka, P.A.; Hodge, D.O.; Araoz, P.A.; Miller, T.D.; Gibbons, R.J. Applicability of appropriateness criteria for stress imaging: Similarities and differences between stress echocardiography and single-photon emission computed tomography myocardial perfusion imaging criteria. Circ. Cardiovasc. Imaging 2009, 2, 213–218. [Google Scholar] [CrossRef]
- Mansour, I.N.; Lang, R.M.; Aburuwaida, W.M.; Bhave, N.M.; Ward, R.P. Evaluation of the clinical application of the ACCF/ASE appropriateness criteria for stress echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, R.J.; Askew, J.W.; Hodge, D.; Kaping, B.; Carryer, D.J.; Miller, T. Appropriate use criteria for stress single-photon emission computed tomography sestamibi studies: A quality improvement project. Circulation 2011, 123, 499–503. [Google Scholar] [CrossRef]
- Willens, H.J.; Nelson, K.; Hendel, R.C. Appropriate use criteria for stress echocardiography: Impact of updated criteria on appropriateness ratings, correlation with pre-authorization guidelines, and effect of temporal trends and an educational initiative on utilization. JACC Cardiovasc. Imaging 2013, 6, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Karbach, U.; Schubert, I.; Hagemeister, J.; Ernstmann, N.; Pfaff, H.; Höpp, H.W. Physicians’ knowledge of and compliance with guidelines: An exploratory study in cardiovascular diseases. Dtsch. Arztebl. Int. 2011, 108, 61–69. [Google Scholar]
- Eftekhari, M.H.; Parsapoor, A.; Ahmadi, A.; Yavari, N.; Larijani, B.; Gooshki, E.S. Exploring defensive medicine: Examples, underlying and contextual factors, and potential strategies—A qualitative study. BMC Med. Ethics 2023, 24, 82. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Ma, L.L.; Wang, Y.Y.; Yang, Z.H.; Huang, D.; Weng, H.; Zeng, X.T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: What are they and which is better? Mil. Med. Res. 2020, 7, 7. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Reitsma, J.B.; Glas, A.S.; Rutjes, A.W.; Scholten, R.J.; Bossuyt, P.M.; Zwinderman, A.H. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J. Clin. Epidemiol. 2005, 58, 982–990. [Google Scholar] [CrossRef]
- Chu, H.; Cole, S.R. Bivariate meta-analysis of sensitivity and specificity with sparse data: A generalized linear mixed model approach. J. Clin. Epidemiol. 2006, 59, 1331–1332. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Riebler, A.; Rue, H. Bayesian bivariate meta-analysis of diagnostic test studies with interpretable priors. Stat. Med. 2017, 36, 3039–3058. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Riebler, A.; Bachmann, L.M.; Rue, H.; Held, L. Bayesian bivariate meta-analysis of diagnostic test studies using integrated nested Laplace approximations. Stat. Med. 2010, 29, 1325–1339. [Google Scholar] [CrossRef] [PubMed]
- Cuzick, J. Forest plots and the interpretation of subgroups. Lancet 2005, 365, 1308. [Google Scholar] [CrossRef]
- Phillips, B.; Stewart, L.A.; Sutton, A.J. ‘Cross hairs’ plots for diagnostic meta-analysis. Res. Synth. Methods 2010, 1, 308–315. [Google Scholar] [CrossRef]
- Rosman, A.S.; Korsten, M.A. Application of summary receiver operating characteristics (sROC) analysis to diagnostic clinical testing. Adv. Med. Sci. 2007, 52, 76–82. [Google Scholar]
- Hunter, J.P.; Saratzis, A.; Sutton, A.J.; Boucher, R.H.; Sayers, R.D.; Bown, M.J. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J. Clin. Epidemiol. 2014, 67, 897–903. [Google Scholar] [CrossRef]
- Harbord, R.M.; Deeks, J.J.; Egger, M.; Whiting, P.; Sterne, J.A. A unification of models for meta-analysis of diagnostic accuracy studies. Biostatistics 2007, 8, 239–251. [Google Scholar] [CrossRef]
- Thabane, L.; Mbuagbaw, L.; Zhang, S.; Samaan, Z.; Marcucci, M.; Ye, C.; Thabane, M.; Giangregorio, L.; Dennis, B.; Kosa, D.; et al. A tutorial on sensitivity analyses in clinical trials: The what, why, when and how. BMC Med. Res. Methodol. 2013, 13, 92. [Google Scholar] [CrossRef] [PubMed]
- Salustri, A.; Fioretti, P.M.; Pozzoli, M.M.; McNeill, A.J.; Roelandt, J.R. Dobutamine stress echocardiography: Its role in the diagnosis of coronary artery disease. Eur. Heart J. 1992, 13, 70–77. [Google Scholar] [CrossRef] [PubMed]
- van Rugge, F.P.; van der Wall, E.E.; de Roos, A.; Bruschke, A.V. Dobutamine stress magnetic resonance imaging for detection of coronary artery disease. J. Am. Coll. Cardiol. 1993, 22, 431–439. [Google Scholar] [CrossRef]
- Panza, J.A.; Laurienzo, J.M.; Curiel, R.V.; Quyyumi, A.A.; Cannon, R.O., III. Transesophageal dobutamine stress echocardiography for evaluation of patients with coronary artery disease. J. Am. Coll. Cardiol. 1994, 24, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- San Román, J.A.; Vilacosta, I.; Castillo, J.A.; Rollán, M.J.; Peral, V.; Sánchez-Harguindey, L.; Fernández-Avilés, F. Dipyridamole and dobutamine-atropine stress echocardiography in the diagnosis of coronary artery disease. Chest 1996, 110, 1248–1254. [Google Scholar] [CrossRef]
- Kisacik, H.L.; Ozdemir, K.; Altinyay, E.; Oğuzhan, A.; Kural, T.; Kir, M.; Kütük, E.; Göksel, S. Comparison of exercise stress testing with simultaneous dobutamine stress echocardiography and technetium-99m isonitrile single-photon emission computerized tomography for diagnosis of coronary artery disease. Eur. Heart J. 1996, 17, 113–119. [Google Scholar] [CrossRef][Green Version]
- Laurienzo, J.M.; Cannon, R.O., III; Quyyumi, A.A.; Dilsizian, V.; Panza, J.A. Improved specificity of transesophageal dobutamine stress echocardiography compared to standard tests for evaluation of coronary artery disease in women presenting with chest pain. Am. J. Cardiol. 1997, 80, 1402–1407. [Google Scholar] [CrossRef]
- Morise, A.P.; Beto, R. The specificity of exercise electrocardiography in women grouped by estrogen status. Int. J. Cardiol. 1997, 60, 55–65. [Google Scholar] [CrossRef]
- Hennessy, T.G.; Codd, M.B.; Hennessy, M.S.; Kane, G.; McCarthy, C.; McCann, H.A.; Sugrue, D.D. Comparison of dobutamine stress echocardiography and treadmill exercise electrocardiography for detection of coronary artery disease. Coron. Artery Dis. 1997, 8, 689–695. [Google Scholar] [CrossRef]
- Ho, Y.L.; Wu, C.C.; Huang, P.J.; Lin, L.C.; Chieng, P.U.; Chen, W.J.; Chen, M.F.; Lee, Y.T. Assessment of coronary artery disease in women by dobutamine stress echocardiography: Comparison with stress thallium-201 single-photon emission computed tomography and exercise electrocardiography. Am. Heart J. 1998, 135, 655–662. [Google Scholar] [CrossRef]
- Kalaria, V.G.; Dwyer, E.M. Ability of the exercise electrocardiogram test to detect ischemia in stable coronary artery disease patients with ST-segment depression on the resting electrocardiogram. Am. Heart J. 1998, 135, 901–906. [Google Scholar] [CrossRef]
- Santoro, G.M.; Sciagrà, R.; Buonamici, P.; Consoli, N.; Mazzoni, V.; Zerauschek, F.; Bisi, G.; Fazzini, P.F. Head-to-head comparison of exercise stress testing, pharmacologic stress echocardiography, and perfusion tomography as first-line examination for chest pain in patients without history of coronary artery disease. J. Nucl. Cardiol. 1998, 5, 19–27. [Google Scholar] [CrossRef]
- San Román, J.A.; Vilacosta, I.; Castillo, J.A.; Rollán, M.J.; Hernández, M.; Peral, V.; Garcimartín, I.; de la Torre, M.M.; Fernández-Avilés, F. Selection of the optimal stress test for the diagnosis of coronary artery disease. Heart 1998, 80, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Gentile, R.; Vitarelli, A.; Schillaci, O.; Laganà, B.; Gianni, C.; Rossi-Fanelli, F.; Fedele, F. Diagnostic accuracy and prognostic implications of stress testing for coronary artery disease in the elderly. Ital. Heart J. 2001, 2, 539–545. [Google Scholar]
- Bokhari, S.; Bergmann, S.R. The effect of estrogen compared to estrogen plus progesterone on the exercise electrocardiogram. J. Am. Coll. Cardiol. 2002, 40, 1092–1096. [Google Scholar] [CrossRef] [PubMed]
- Rollán, M.J.; San Román, J.A.; Vilacosta, I.; Ortega, J.R.; Bratos, J.L. Dobutamine stress echocardiography in the diagnosis of coronary artery disease in women with chest pain: Comparison with different noninvasive tests. Clin. Cardiol. 2002, 25, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Cortigiani, L.; Bigi, R.; Rigo, F.; Landi, P.; Baldini, U.; Mariani, P.R.; Picano, E.; Echo Persantine International Cooperative Study Group. Diagnostic value of exercise electrocardiography and dipyridamole stress echocardiography in hypertensive and normotensive chest pain patients with right bundle branch block. J. Hypertens. 2003, 21, 2189–2194. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Ge, K.Y.; Nie, Y.; Yan, W.; Yang, X.K. [Factors related to false positive results of treadmill electrocardiogram test for the detection of coronary heart disease]. Zhonghua Nei Ke Za Zhi 2004, 43, 669–671. (In Chinese) [Google Scholar]
- González, P.; Massardo, T.; Jofré, M.J.; Yovanovich, J.; Prat, H.; Muñoz, A.; Arriagada, M.; Anzoátegui, W.; Carmona, A.R. 201Tl myocardial SPECT detects significant coronary artery disease between 50% and 75% angiogram stenosis. Rev. Esp. Med. Nucl. 2005, 24, 305–311. [Google Scholar] [CrossRef]
- Nedeljkovic, I.; Ostojic, M.; Beleslin, B.; Djordjevic-Dikic, A.; Stepanovic, J.; Nedeljkovic, M.; Stojkovic, S.; Stankovic, G.; Saponjski, J.; Petrasinovic, Z.; et al. Comparison of exercise, dobutamine-atropine and dipyridamole-atropine stress echocardiography in detecting coronary artery disease. Cardiovasc. Ultrasound 2006, 4, 22. [Google Scholar] [CrossRef][Green Version]
- Michaelides, A.P.; Fourlas, C.A.; Chatzistamatiou, E.I.; Andrikopoulos, G.K.; Soulis, D.; Psomadaki, Z.D.; Stefanadis, C.I. QRS score improves diagnostic ability of treadmill exercise testing in women. Coron. Artery Dis. 2007, 18, 313–318. [Google Scholar] [CrossRef]
- Bokhari, S.; Shahzad, A.; Bergmann, S.R. Superiority of exercise myocardial perfusion imaging compared with the exercise ECG in the diagnosis of coronary artery disease. Coron. Artery Dis. 2008, 19, 399–404. [Google Scholar] [CrossRef]
- Lu, C.; Lu, F.; Fragasso, G.; Dabrowski, P.; Di Bello, V.; Chierchia, S.L.; Gianolli, L.; Marzilli, M.; Balbarini, A. Comparison of exercise electrocardiography, technetium-99m sestamibi single photon emission computed tomography, and dobutamine and dipyridamole echocardiography for detection of coronary artery disease in hypertensive women. Am. J. Cardiol. 2010, 105, 1254–1260. [Google Scholar] [CrossRef]
- Greulich, S.; Bruder, O.; Parker, M.; Schumm, J.; Grün, S.; Schneider, S.; Klem, I.; Sechtem, U.; Mahrholdt, H. Comparison of exercise electrocardiography and stress perfusion CMR for the detection of coronary artery disease in women. J. Cardiovasc. Magn. Reson. 2012, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Weustink, A.C.; Neefjes, L.A.; Rossi, A.; Meijboom, W.B.; Nieman, K.; Capuano, E.; Boersma, E.; Mollet, N.R.; Krestin, G.P.; de Feyter, P.J. Diagnostic performance of exercise bicycle testing and single-photon emission computed tomography: Comparison with 64-slice computed tomography coronary angiography. Int. J. Cardiovasc. Imaging 2012, 28, 675–684. [Google Scholar] [CrossRef][Green Version]
- Attar, A.; Mehrzadeh, A.; Foulad, M.; Aldavood, D.; Fallahzadeh, M.A.; Assadian Rad, M.; Khosropanah, S. Accuracy of exercise tolerance test in the diagnosis of coronary artery disease in patients with left dominant coronary circulation. Indian Heart J. 2017, 69, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, M.A.; Verani, M.S.; Haichin, R.M.; Mahmarian, J.J.; Suarez, J.; Zoghbi, W.A. Exercise echocardiography versus 201Tl single-photon emission computed tomography in evaluation of coronary artery disease. Analysis of 292 patients. Circulation 1992, 85, 1026–1031. [Google Scholar] [CrossRef]
- Hecht, H.S.; DeBord, L.; Shaw, R.; Chin, H.; Dunlap, R.; Ryan, C.; Myler, R.K. Supine bicycle stress echocardiography versus tomographic thallium-201 exercise imaging for the detection of coronary artery disease. J. Am. Soc. Echocardiogr. 1993, 6, 177–185. [Google Scholar] [CrossRef]
- Cohen, J.L.; Ottenweller, J.E.; George, A.K.; Duvvuri, S. Comparison of dobutamine and exercise echocardiography for detecting coronary artery disease. Am. J. Cardiol. 1993, 72, 1226–1231. [Google Scholar] [CrossRef]
- Beleslin, B.D.; Ostojic, M.; Stepanovic, J.; Djordjevic-Dikic, A.; Stojkovic, S.; Nedeljkovic, M.; Stankovic, G.; Petrasinovic, Z.; Gojkovic, L.; Vasiljevic-Pokrajcic, Z.; et al. Stress echocardiography in the detection of myocardial ischemia. Head-to-head comparison of exercise, dobutamine, and dipyridamole tests. Circulation 1994, 90, 1168–1176. [Google Scholar] [CrossRef]
- Dagianti, A.; Penco, M.; Agati, L.; Sciomer, S.; Dagianti, A.; Rosanio, S.; Fedele, F. Stress echocardiography: Comparison of exercise, dipyridamole and dobutamine in detecting and predicting the extent of coronary artery disease. J. Am. Coll. Cardiol. 1995, 26, 18–25. [Google Scholar] [CrossRef]
- Bjørnstad, K.; Aakhus, S.; Hatle, L. Comparison of digital dipyridamole stress echocardiography and upright bicycle stress echocardiography for identification of coronary artery stenosis. Cardiology 1995, 86, 514–520. [Google Scholar] [CrossRef]
- Badruddin, S.M.; Ahmad, A.; Mickelson, J.; Abukhalil, J.; Winters, W.L.; Nagueh, S.F.; Zoghbi, W.A. Supine bicycle versus post-treadmill exercise echocardiography in the detection of myocardial ischemia: A randomized single-blind crossover trial. J. Am. Coll. Cardiol. 1999, 33, 1485–1490. [Google Scholar] [CrossRef]
- Ha, J.W.; Juracan, E.M.; Mahoney, D.W.; Oh, J.K.; Shub, C.; Seward, J.B.; Pellikka, P.A. Hypertensive response to exercise: A potential cause for new wall motion abnormality in the absence of coronary artery disease. J. Am. Coll. Cardiol. 2002, 39, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Shiota, T.; Kim, Y.J.; Kwan, J.; Qin, J.X.; Eto, Y.; Rodriguez, L.L.; Thomas, J.D. False-positive exercise echocardiograms: Impact of sex and blood pressure response. Am. Heart J. 2003, 146, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Müller, H.; Lerch, R. Tilt table exercise echocardiography assessment in the diagnosis of coronary artery disease. Arch. Cardiovasc. Dis. 2008, 101, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.W.; Seaworth, J.F.; Johns, J.P.; Pupa, L.E.; Condos, W.R. Comparison of adenosine, dipyridamole, and dobutamine in stress echocardiography. Ann. Intern. Med. 1992, 116, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Marwick, T.; D’Hondt, A.M.; Baudhuin, T.; Willemart, B.; Wijns, W.; Detry, J.M.; Melin, J. Optimal use of dobutamine stress for the detection and evaluation of coronary artery disease: Combination with echocardiography or scintigraphy, or both? J. Am. Coll. Cardiol. 1993, 22, 159–167. [Google Scholar] [CrossRef]
- Takeuchi, M.; Araki, M.; Nakashima, Y.; Kuroiwa, A. Comparison of dobutamine stress echocardiography and stress thallium-201 single-photon emission computed tomography for detecting coronary artery disease. J. Am. Soc. Echocardiogr. 1993, 6, 593–602. [Google Scholar] [CrossRef]
- Ho, F.M.; Huang, P.J.; Liau, C.S.; Lee, F.K.; Chieng, P.U.; Su, C.T.; Lee, Y.T. Dobutamine stress echocardiography compared with dipyridamole thallium-201 single-photon emission computed tomography in detecting coronary artery disease. Eur. Heart J. 1995, 16, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Pingitore, A.; Picano, E.; Colosso, M.Q.; Reisenhofer, B.; Gigli, G.; Lucarini, A.R.; Petix, N.; Previtali, M.; Bigi, R.; Chiarandà, G.; et al. The atropine factor in pharmacologic stress echocardiography. Echo Persantine (EPIC) and Echo Dobutamine International Cooperative (EDIC) Study Groups. J. Am. Coll. Cardiol. 1996, 27, 1164–1170. [Google Scholar] [CrossRef]
- Elhendy, A.; van Domburg, R.T.; Bax, J.J.; Nierop, P.R.; Geleijnse, M.L.; Ibrahim, M.M.; Roelandt, J.R. Noninvasive diagnosis of coronary artery stenosis in women with limited exercise capacity: Comparison of dobutamine stress echocardiography and 99mTc sestamibi single-photon emission CT. Chest 1998, 114, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Fragasso, G.; Lu, C.; Dabrowski, P.; Pagnotta, P.; Sheiban, I.; Chierchia, S.L. Comparison of stress/rest myocardial perfusion tomography, dipyridamole and dobutamine stress echocardiography for the detection of coronary disease in hypertensive patients with chest pain and positive exercise test. J. Am. Coll. Cardiol. 1999, 34, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Previtali, M.; Cannizzaro, G.; Lanzarini, L.; Calsamiglia, G.; Poli, A.; Fetiveau, R. Comparison of dobutamine stress echocardiography and exercise stress Thallium-201 SPECT for detection of myocardial ischemia after acute myocardial infarction treated with thrombolysis. Int. J. Card. Imaging 1999, 15, 195–204. [Google Scholar] [CrossRef]
- Ciaroni, S.; Bloch, A.; Albrecht, L.; Vanautryve, B. Diagnosis of coronary artery disease in patients with permanent cardiac pacemaker by dobutamine stress echocardiography or exercise thallium-201 myocardial tomography. Echocardiography 2000, 17, 675–679. [Google Scholar] [CrossRef]
- Smart, S.C.; Bhatia, A.; Hellman, R.; Stoiber, T.; Krasnow, A.; Collier, B.D.; Sagar, K.B. Dobutamine-atropine stress echocardiography and dipyridamole sestamibi scintigraphy for the detection of coronary artery disease: Limitations and concordance. J. Am. Coll. Cardiol. 2000, 36, 1265–1273. [Google Scholar] [CrossRef]
- Geleijnse, M.L.; Vigna, C.; Kasprzak, J.D.; Rambaldi, R.; Salvatori, M.P.; Elhendy, A.; Cornel, J.H.; Fioretti, P.M.; Roelandt, J.R. Usefulness and limitations of dobutamine-atropine stress echocardiography for the diagnosis of coronary artery disease in patients with left bundle branch block. A multicentre study. Eur. Heart J. 2000, 21, 1666–1673. [Google Scholar] [CrossRef]
- Elhendy, A.; van Domburg, R.T.; Bax, J.J.; Poldermans, D.; Sozzi, F.B.; Roelandt, J.R. Accuracy of dobutamine technetium 99m sestamibi SPECT imaging for the diagnosis of single-vessel coronary artery disease: Comparison with echocardiography. Am. Heart J. 2000, 139, 224–230. [Google Scholar] [CrossRef]
- Tandoğan, I.; Yetkin, E.; Yanik, A.; Ulusoy, F.V.; Temizhan, A.; Cehreli, S.; Sasmaz, A. Comparison of thallium-201 exercise SPECT and dobutamine stress echocardiography for diagnosis of coronary artery disease in patients with left bundle branch block. Int. J. Cardiovasc. Imaging 2001, 17, 339–345. [Google Scholar] [CrossRef]
- Lancellotti, P.; Benoit, T.; Rigo, P.; Pierard, L.A. Dobutamine stress echocardiography versus quantitative technetium-99m sestamibi SPECT for detecting residual stenosis and multivessel disease after myocardial infarction. Heart 2001, 86, 510–515. [Google Scholar] [CrossRef][Green Version]
- Vigna, C.; Stanislao, M.; De Rito, V.; Russo, A.; Natali, R.; Santoro, T.; Loperfido, F. Dipyridamole stress echocardiography vs dipyridamole sestamibi scintigraphy for diagnosing coronary artery disease in left bundle-branch block. Chest 2001, 120, 1534–1539. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vigna, C.; Stanislao, M.; De Rito, V.; Russo, A.; Santoro, T.; Fusilli, S.; Valle, G.; Natali, R.; Fanelli, R.; Lotrionte, M.; et al. Inaccuracy of dipyridamole echocardiography or scintigraphy for the diagnosis of coronary artery disease in patients with both left bundle branch block and left ventricular dysfunction. Int. J. Cardiol. 2006, 110, 116–118. [Google Scholar] [CrossRef] [PubMed]
- Rigo, F.; Richieri, M.; Pasanisi, E.; Cutaia, V.; Zanella, C.; Della Valentina, P.; Di Pede, F.; Raviele, A.; Picano, E. Usefulness of coronary flow reserve over regional wall motion when added to dual-imaging dipyridamole echocardiography. Am. J. Cardiol. 2003, 91, 269–273. [Google Scholar] [CrossRef]
- Lowenstein, J.; Tiano, C.; Marquez, G.; Presti, C.; Quiroz, C. Simultaneous analysis of wall motion and coronary flow reserve of the left anterior descending coronary artery by transthoracic Doppler echocardiography during dipyridamole stress echocardiography. J. Am. Soc. Echocardiogr. 2003, 16, 607–613. [Google Scholar] [CrossRef]
- Nohtomi, Y.; Takeuchi, M.; Nagasawa, K.; Arimura, K.; Miyata, K.; Kuwata, K.; Yamawaki, T.; Kondo, S.; Yamada, A.; Okamatsu, S. Simultaneous assessment of wall motion and coronary flow velocity in the left anterior descending coronary artery during dipyridamole stress echocardiography. J. Am. Soc. Echocardiogr. 2003, 16, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Ascione, L.; De Michele, M.; Accadia, M.; Granata, G.; Sacra, C.; D’Andrea, A.; Guarini, P.; Tuccillo, B. Incremental diagnostic value of ultrasonographic assessment of coronary flow reserve with high-dose dipyridamole in patients with acute coronary syndrome. Int. J. Cardiol. 2006, 106, 313–318. [Google Scholar] [CrossRef]
- Gaibazzi, N.; Rigo, F.; Reverberi, C. Detection of coronary artery disease by combined assessment of wall motion, myocardial perfusion and coronary flow reserve: A multiparametric contrast stress-echocardiography study. J. Am. Soc. Echocardiogr. 2010, 23, 1242–1250. [Google Scholar] [CrossRef]
- Cortigiani, L.; Rigo, F.; Galderisi, M.; Gherardi, S.; Bovenzi, F.; Picano, E.; Sicari, R. Diagnostic and prognostic value of Doppler echocardiographic coronary flow reserve in the left anterior descending artery in hypertensive and normotensive patients. Heart 2011, 97, 1758–1765. [Google Scholar] [CrossRef]
- Kasprzak, J.D.; Wejner-Mik, P.; Nouri, A.; Szymczyk, E.; Krzemińska-Pakuła, M.; Lipiec, P. Transthoracic measurement of left coronary artery flow reserve improves the diagnostic value of routine dipyridamole-atropine stress echocardiogram. Arch. Med. Sci. 2013, 9, 802–807. [Google Scholar] [CrossRef]
- Pichel, I.Á.; Fernández Cimadevilla, O.C.; de la Hera Galarza, J.M.; Pasanisi, E.; Ruiz, J.M.G.; Molina, B.D.; Rodriguez, J.L.L.; Sicari, R.; Fernández, M.M. Usefulness of dual imaging stress echocardiography for the diagnosis of coronary allograft vasculopathy in heart transplant recipients. Int. J. Cardiol. 2019, 296, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.E.; Schwaiger, M.; Molina, E.; Popma, J.; Gacioch, G.M.; Kalus, M.; Squicciarini, S.; al-Aouar, Z.R.; Schork, A.; Kuhl, D.E. Comparison of rubidium-82 positron emission tomography and thallium-201 SPECT imaging for detection of coronary artery disease. Am. J. Cardiol. 1991, 67, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Prisant, L.M.; von Dohlen, T.W.; Houghton, J.L.; Carr, A.A.; Frank, M.J. A negative thallium (+/− dipyridamole) stress test excludes significant obstructive epicardial coronary artery disease in hypertensive patients. Am. J. Hypertens. 1992, 5, 71–75. [Google Scholar] [CrossRef]
- Gupta, N.C.; Esterbrooks, D.J.; Hilleman, D.E.; Mohiuddin, S.M. Comparison of adenosine and exercise thallium-201 single-photon emission computed tomography (SPECT) myocardial perfusion imaging. The GE SPECT Multicenter Adenosine Study Group. J. Am. Coll. Cardiol. 1992, 19, 248–257. [Google Scholar] [CrossRef]
- Zammarchi, A.; Pitscheider, W.; Crepaz, R.; Oberhollenzer, R.; Erlicher, A.; Unterhuber, E.; Osele, L.; Braito, E. La scintigrafia miocardica da sforzo con 201-tallio in presenza di blocco di branca sinistra [Exercise 201-thallium myocardial scintigraphy in left bundle branch block]. G. Ital. Cardiol. 1994, 24, 1103–1113. [Google Scholar]
- Fleming, R.M.; Rose, C.H.; Feldmann, K.M. Comparing a high-dose dipyridamole SPECT imaging protocol with dobutamine and exercise stress testing protocols. Angiology 1995, 46, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Doğruca, Z.; Kabasakal, L.; Yapar, F.; Nisil, C.; Vural, V.A.; Onsel, Q. A comparison of Tl-201 stress-reinjection-prone SPECT and Tc-99m-sestamibi gated SPECT in the differentiation of inferior wall defects from artifacts. Nucl. Med. Commun. 2000, 21, 719–727. [Google Scholar] [CrossRef]
- Raman, S.V.; Dickerson, J.A.; Mazur, W.; Wong, T.C.; Schelbert, E.B.; Min, J.K.; Scandling, D.; Bartone, C.; Craft, J.T.; Thavendiranathan, P.; et al. Diagnostic performance of treadmill exercise cardiac magnetic resonance: The prospective, multicenter Exercise CMR’s Accuracy for Cardiovascular Stress Testing (EXACT) Trial. J. Am. Heart Assoc. 2016, 5, e003811. [Google Scholar] [CrossRef]
- Ahmad, I.G.; Abdulla, R.K.; Klem, I.; Margulis, R.; Ivanov, A.; Mohamed, A.; Judd, R.M.; Borges-Neto, S.; Kim, R.J.; Heitner, J.F. Comparison of stress cardiovascular magnetic resonance imaging (CMR) with stress nuclear perfusion for the diagnosis of coronary artery disease. J. Nucl. Cardiol. 2016, 23, 287–297. [Google Scholar] [CrossRef]
- Olszowska, M.; Kostkiewicz, M.; Tracz, W.; Przewlocki, T. Assessment of myocardial perfusion in patients with coronary artery disease. Comparison of myocardial contrast echocardiography and 99mTc MIBI single photon emission computed tomography. Int. J. Cardiol. 2003, 90, 49–55. [Google Scholar] [CrossRef]
- Cramer, M.J.; Verzijlbergen, J.F.; van der Wall, E.E.; Vermeersch, P.H.; Niemeyer, M.G.; Zwinderman, A.H.; Ascoop, C.A.; Pauwels, E.K. Comparison of adenosine and high-dose dipyridamole both combined with low-level exercise stress for 99Tcm-MIBI SPET myocardial perfusion imaging. Nucl. Med. Commun. 1996, 17, 97–104. [Google Scholar] [CrossRef]
- Senior, R.; Lepper, W.; Pasquet, A.; Chung, G.; Hoffman, R.; Vanoverschelde, J.L.; Cerqueira, M.; Kaul, S. Myocardial perfusion assessment in patients with medium probability of coronary artery disease and no prior myocardial infarction: Comparison of myocardial contrast echocardiography with 99mTc single-photon emission computed tomography. Am. Heart J. 2004, 147, 1100–1105. [Google Scholar] [CrossRef] [PubMed]
- Jeetley, P.; Hickman, M.; Kamp, O.; Lang, R.M.; Thomas, J.D.; Vannan, M.A.; Vanoverschelde, J.L.; van der Wouw, P.A.; Senior, R. Myocardial contrast echocardiography for the detection of coronary artery stenosis: A prospective multicenter study in comparison with single-photon emission computed tomography. J. Am. Coll. Cardiol. 2006, 47, 141–145. [Google Scholar] [CrossRef] [PubMed]
- LaManna, M.M.; Mohama, R.; Slavich, I.L., III; Lumia, F.J.; Cha, S.D.; Rambaran, N.; Maranhao, V. Intravenous adenosine (adenoscan) versus exercise in the noninvasive assessment of coronary artery disease by SPECT. Clin. Nucl. Med. 1990, 15, 804–805. [Google Scholar] [CrossRef] [PubMed]
- Karavidas, A.I.; Matsakas, E.P.; Lazaros, G.A.; Brestas, P.S.; Avramidis, D.A.; Zacharoulis, A.A.; Fotiadis, I.N.; Korres, D.A.; Zacharoulis, A.A. Comparison of myocardial contrast echocardiography with SPECT in the evaluation of coronary artery disease in asymptomatic patients with LBBB. Int. J. Cardiol. 2006, 112, 334–340. [Google Scholar] [CrossRef]
- Aggeli, C.; Christoforatou, E.; Giannopoulos, G.; Roussakis, G.; Kokkinakis, C.; Barbetseas, J.; Vlachopoulos, C.; Stefanadis, C. The diagnostic value of adenosine stress-contrast echocardiography for diagnosis of coronary artery disease in hypertensive patients: Comparison to Tl-201 single-photon emission computed tomography. Am. J. Hypertens. 2007, 20, 533–538. [Google Scholar] [CrossRef][Green Version]
- Futamatsu, H.; Klassen, C.; Pilla, M.; Wilke, N.; Angiolillo, D.J.; Smalheiser, S.; Siuciak, A.; Suzuki, N.; Bass, T.A.; Costa, M.A. Diagnostic accuracy of quantitative cardiac MRI evaluation compared to stress single-photon-emission computed tomography. Int. J. Cardiovasc. Imaging 2008, 24, 293–299. [Google Scholar] [CrossRef]
- Greenwood, J.P.; Motwani, M.; Maredia, N.; Brown, J.M.; Everett, C.C.; Nixon, J.; Bijsterveld, P.; Dickinson, C.J.; Ball, S.G.; Plein, S. Comparison of cardiovascular magnetic resonance and single-photon emission computed tomography in women with suspected coronary artery disease from the Clinical Evaluation of Magnetic Resonance Imaging in Coronary Heart Disease (CE-MARC) Trial. Circulation 2014, 129, 1129–1138. [Google Scholar] [CrossRef]
- Rerkpattanapipat, P.; Gandhi, S.K.; Darty, S.N.; Williams, R.T.; Davis, A.D.; Mazur, W.; Clark, H.P.; Little, W.C.; Link, K.M.; Hamilton, C.A.; et al. Feasibility to detect severe coronary artery stenoses with upright treadmill exercise magnetic resonance imaging. Am. J. Cardiol. 2003, 92, 603–606. [Google Scholar] [CrossRef]
- Ochs, A.; Nippes, M.; Salatzki, J.; Weberling, L.D.; Osman, N.; Riffel, J.; Katus, H.A.; Friedrich, M.G.; Frey, N.; Ochs, M.M.; et al. Dynamic handgrip exercise for the detection of myocardial ischemia using fast Strain-ENCoded cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2025, 27, 101879. [Google Scholar] [CrossRef]
- Nagel, E.; Lehmkuhl, H.B.; Bocksch, W.; Klein, C.; Vogel, U.; Frantz, E.; Ellmer, A.; Dreysse, S.; Fleck, E. Noninvasive diagnosis of ischemia-induced wall motion abnormalities with the use of high-dose dobutamine stress MRI: Comparison with dobutamine stress echocardiography. Circulation 1999, 99, 763–770. [Google Scholar] [CrossRef]
- al-Saadi, N.; Gross, M.; Paetsch, I.; Schnackenburg, B.; Bornstedt, A.; Fleck, E.; Nagel, E. Dobutamine induced myocardial perfusion reserve index with cardiovascular MR in patients with coronary artery disease. J. Cardiovasc. Magn. Reson. 2002, 4, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Paetsch, I.; Jahnke, C.; Wahl, A.; Gebker, R.; Neuss, M.; Fleck, E.; Nagel, E. Comparison of dobutamine stress magnetic resonance, adenosine stress magnetic resonance, and adenosine stress magnetic resonance perfusion. Circulation 2004, 110, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Wahl, A.; Paetsch, I.; Roethemeyer, S.; Klein, C.; Fleck, E.; Nagel, E. High-dose dobutamine-atropine stress cardiovascular MR imaging after coronary revascularization in patients with wall motion abnormalities at rest. Radiology 2004, 233, 210–216. [Google Scholar] [CrossRef]
- Kelle, S.; Hamdan, A.; Schnackenburg, B.; Köhler, U.; Klein, C.; Nagel, E.; Fleck, E. Dobutamine stress cardiovascular magnetic resonance at 3 Tesla. J. Cardiovasc. Magn. Reson. 2008, 10, 44. [Google Scholar] [CrossRef]
- Heilmaier, C.; Bruder, O.; Meier, F.; Jochims, M.; Forsting, M.; Sabin, G.V.; Barkhausen, J.; Schlosser, T.W. Dobutamine stress cardiovascular magnetic resonance imaging in patients after invasive coronary revascularization with stent placement. Acta Radiol. 2009, 50, 1134–1141. [Google Scholar] [CrossRef]
- Gebker, R.; Jahnke, C.; Hucko, T.; Manka, R.; Mirelis, J.G.; Hamdan, A.; Schnackenburg, B.; Fleck, E.; Paetsch, I. Dobutamine stress magnetic resonance imaging for the detection of coronary artery disease in women. Heart 2010, 96, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Mordi, I.; Stanton, T.; Carrick, D.; McClure, J.; Oldroyd, K.; Berry, C.; Tzemos, N. Comprehensive dobutamine stress CMR versus echocardiography in LBBB and suspected coronary artery disease. JACC Cardiovasc. Imaging 2014, 7, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Weberling, L.D.; Seitz, S.; Salatzki, J.; Ochs, A.; Haney, A.C.; Siry, D.; Heins, J.; Steen, H.; Frey, N.; André, F. Safety, accuracy, and prediction of prognosis in patients with end-stage chronic kidney disease undergoing dobutamine stress cardiac magnetic resonance imaging. Front. Cardiovasc. Med. 2023, 10, 1228691. [Google Scholar] [CrossRef]
- Baer, F.M.; Smolarz, K.; Theissen, P.; Voth, E.; Schicha, H.; Sechtem, U. Identification of hemodynamically significant coronary artery stenoses by dipyridamole-magnetic resonance imaging and 99mTc-methoxyisobutyl-isonitrile-SPECT. Int. J. Card. Imaging 1993, 9, 133–145. [Google Scholar] [CrossRef]
- Hartnell, G.; Cerel, A.; Kamalesh, M.; Finn, J.P.; Hill, T.; Cohen, M.; Tello, R.; Lewis, S. Detection of myocardial ischemia: Value of combined myocardial perfusion and cineangiographic MR imaging. AJR Am. J. Roentgenol. 1994, 163, 1061–1067. [Google Scholar] [CrossRef]
- Zhao, S.; Croisille, P.; Janier, M.; Roux, J.P.; Plana, A.; Magnin, I.; Revel, D. Comparison between qualitative and quantitative wall motion analyses using dipyridamole stress breath-hold cine magnetic resonance imaging in patients with severe coronary artery stenosis. Magn. Reson. Imaging 1997, 15, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Takase, B.; Nagata, M.; Kihara, T.; Kameyawa, A.; Noya, K.; Matsui, T.; Ohsuzu, F.; Ishihara, M.; Kurita, A. Whole-heart dipyridamole stress first-pass myocardial perfusion MRI for the detection of coronary artery disease. Jpn. Heart J. 2004, 45, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Stauder, N.I.; Klumpp, B.; Stauder, H.; Blumenstock, G.; Fenchel, M.; Küttner, A.; Claussen, C.D.; Miller, S. Assessment of coronary artery bypass grafts by magnetic resonance imaging. Br. J. Radiol. 2007, 80, 975–983. [Google Scholar] [CrossRef]
- Pingitore, A.; Lombardi, M.; Scattini, B.; De Marchi, D.; Aquaro, G.D.; Positano, V.; Picano, E. Head to head comparison between perfusion and function during accelerated high-dose dipyridamole magnetic resonance stress for the detection of coronary artery disease. Am. J. Cardiol. 2008, 101, 8–14. [Google Scholar] [CrossRef]
- Mordini, F.E.; Haddad, T.; Hsu, L.Y.; Kellman, P.; Lowrey, T.B.; Aletras, A.H.; Bandettini, W.P.; Arai, A.E. Diagnostic accuracy of stress perfusion CMR in comparison with quantitative coronary angiography: Fully quantitative, semiquantitative, and qualitative assessment. JACC Cardiovasc. Imaging 2014, 7, 14–22. [Google Scholar] [CrossRef]
- Deva, D.P.; Torres, F.S.; Wald, R.M.; Roche, S.L.; Jimenez-Juan, L.; Oechslin, E.N.; Crean, A.M. The value of stress perfusion cardiovascular magnetic resonance imaging for patients referred from the adult congenital heart disease clinic: 5-year experience at the Toronto General Hospital. Cardiol. Young 2014, 24, 822–830. [Google Scholar] [CrossRef]
- Yun, C.H.; Tsai, J.P.; Tsai, C.T.; Mok, G.S.; Sun, J.Y.; Hung, C.L.; Wu, T.H.; Huang, W.T.; Yang, F.S.; Lee, J.J.; et al. Qualitative and semi-quantitative evaluation of myocardium perfusion with 3 T stress cardiac MRI. BMC Cardiovasc. Disord. 2015, 15, 164. [Google Scholar] [CrossRef]
- Klem, I.; Heitner, J.F.; Shah, D.J.; Sketch, M.H., Jr.; Behar, V.; Weinsaft, J.; Cawley, P.; Parker, M.; Elliott, M.; Judd, R.M.; et al. Improved detection of coronary artery disease by stress perfusion cardiovascular magnetic resonance with the use of delayed enhancement infarction imaging. J. Am. Coll. Cardiol. 2006, 47, 1630–1638. [Google Scholar] [CrossRef]
- Klein, C.; Gebker, R.; Kokocinski, T.; Dreysse, S.; Schnackenburg, B.; Fleck, E.; Nagel, E. Combined magnetic resonance coronary artery imaging, myocardial perfusion and late gadolinium enhancement in patients with suspected coronary artery disease. J. Cardiovasc. Magn. Reson. 2008, 10, 45. [Google Scholar] [CrossRef]
- Arnold, J.R.; Karamitsos, T.D.; Pegg, T.J.; Francis, J.M.; Olszewski, R.; Searle, N.; Senior, R.; Neubauer, S.; Becher, H.; Selvanayagam, J.B. Adenosine stress myocardial contrast echocardiography for the detection of coronary artery disease: A comparison with coronary angiography and cardiac magnetic resonance. JACC Cardiovasc. Imaging 2010, 3, 934–943. [Google Scholar] [CrossRef]
- Bettencourt, N.; Ferreira, N.; Chiribiri, A.; Schuster, A.; Sampaio, F.; Santos, L.; Melica, B.; Rodrigues, A.; Braga, P.; Teixeira, M.; et al. Additive value of magnetic resonance coronary angiography in a comprehensive cardiac magnetic resonance stress-rest protocol for detection of functionally significant coronary artery disease: A pilot study. Circ. Cardiovasc. Imaging 2013, 6, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Salerno, M.; Taylor, A.; Yang, Y.; Kuruvilla, S.; Ragosta, M.; Meyer, C.H.; Kramer, C.M. Adenosine stress cardiovascular magnetic resonance with variable-density spiral pulse sequences accurately detects coronary artery disease: Initial clinical evaluation. Circ. Cardiovasc. Imaging 2014, 7, 639–646. [Google Scholar] [CrossRef]
- Manka, R.; Wissmann, L.; Gebker, R.; Jogiya, R.; Motwani, M.; Frick, M.; Reinartz, S.; Schnackenburg, B.; Niemann, M.; Gotschy, A.; et al. Multicenter evaluation of dynamic three-dimensional magnetic resonance myocardial perfusion imaging for the detection of coronary artery disease defined by fractional flow reserve. Circ. Cardiovasc. Imaging 2015, 8, e003061. [Google Scholar] [CrossRef] [PubMed]
- Ntsinjana, H.N.; Tann, O.; Hughes, M.; Derrick, G.; Secinaro, A.; Schievano, S.; Muthurangu, V.; Taylor, A.M. Utility of adenosine stress perfusion CMR to assess paediatric coronary artery disease. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.R.J.; Kidambi, A.; Biglands, J.D.; Maredia, N.; Dickinson, C.J.; Plein, S.; Greenwood, J.P. A comparison of cardiovascular magnetic resonance and single photon emission computed tomography (SPECT) perfusion imaging in left main stem or equivalent coronary artery disease: A CE-MARC substudy. J. Cardiovasc. Magn. Reson. 2017, 19, 84. [Google Scholar] [CrossRef]
- Southard, J.; Baker, L.; Schaefer, S. In Search of the False-Negative Exercise Treadmill Testing: Evidence-Based Use of Exercise Echocardiography. Clin. Cardiol. 2008, 31, 35–40. [Google Scholar] [CrossRef]
- Sketch, M.H.; Mohiuddin, S.M.; Lynch, J.D.; Zencka, A.E.; Runco, V. Significant Sex Differences in the Correlation of Electrocardiographic Exercise Testing and Coronary Arteriograms. Am. J. Cardiol. 1975, 36, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Ellestad, M.H.; Savitz, S.; Bergdall, D.; Teske, J. The False Positive Stress Test: Multivariate Analysis of 215 Subjects with Hemodynamic, Angiographic and Clinical Data. Am. J. Cardiol. 1977, 40, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, B.T.; Scalia, W.M.; Scalia, G.M. Female False Positive Exercise Stress ECG Testing—Fact Versus Fiction. Heart Lung Circ. 2019, 28, 735–741. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, R.; Ties, D.; Kuijpers, D.; van der Harst, P.; Oudkerk, M. Effects of Caffeine on Myocardial Blood Flow: A Systematic Review. Nutrients 2018, 10, 1083. [Google Scholar] [CrossRef]
- Kramer, N.; Susmano, A.; Shekelle, R.B. The “False Negative” Treadmill Exercise Test and Left Ventricular Dysfunction. Circulation 1978, 57, 763–768. [Google Scholar] [CrossRef]
- Hlatky, M.A.; Pryor, D.B.; Harrell, F.E., Jr.; Califf, R.M.; Mark, D.B.; Rosati, R.A. Factors Affecting Sensitivity and Specificity of Exercise Electrocardiography: Multivariable Analysis. Am. J. Med. 1984, 77, 64–71. [Google Scholar] [CrossRef]
- Gibbons, R.J.; Balady, G.J.; Beasley, J.W.; Bricker, J.T.; Duvernoy, W.F.; Froelicher, V.F.; Mark, D.B.; Marwick, T.H.; McCallister, B.D.; Thompson, P.D.; et al. ACC/AHA Guidelines for Exercise Testing: Executive Summary. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing). Circulation 1997, 96, 345–354. [Google Scholar] [CrossRef]
- Raposeiras-Roubin, S.; Garrido-Pumar, M.; Pubul-Nuñez, V.; Peña-Gil, C.; Argibay-Vázquez, S.; Agra-Bermejo, R.M.; Abu-Assi, E.; Martínez-Monzonís, A.; Vega, M.; Ruibal-Morell, A.; et al. Discrepancy between Stress Electrocardiographic Changes and Nuclear Myocardial Perfusion Defects in the Prognostic Assessment of Patients with Chest Pain. Rev. Port. Cardiol. 2013, 32, 761–768. [Google Scholar] [CrossRef]
- Towashiraporn, K.; Krittayaphong, R.; Yindeengam, A. Predicting Factors for a False Positive Treadmill Exercise Stress Test. J. Med. Assoc. Thai 2013, 96 (Suppl. S2), S133–S138. [Google Scholar]
- Miller, T.D.; Christian, T.F.; Allison, T.G.; Squires, R.W.; Hodge, D.O.; Gibbons, R.J. Is Rest or Exercise Hypertension a Cause of a False-Positive Exercise Test? Chest 2000, 117, 226–232. [Google Scholar] [CrossRef]
- Faisal, A.W.; Abid, A.R.; Azhar, M. Exercise Tolerance Test: A Comparison between True Positive and False Positive Test Results. J. Ayub Med. Coll. Abbottabad 2007, 19, 71–74. [Google Scholar] [PubMed]
- Naimark, B.J.; Tate, R.B.; Manfreda, J.; Stephens, N.L.; Mymin, D. The Association between Exercise Blood Pressure and the Prevalence of ECG Abnormalities. Can. J. Cardiol. 1990, 6, 267–273. [Google Scholar]
- Gettes, L.S.; Sapin, P. Concerning Falsely Negative and Falsely Positive Electrocardiographic Responses to Exercise. Br. Heart J. 1993, 70, 205–207. [Google Scholar] [CrossRef]
- Özkaramanlı Gür, D.; Akyüz, A.; Alpsoy, Ş.; Güler, N. Tools to Improve the Diagnostic Accuracy of Exercise Electrocardiograms in Patients with Atypical Angina Pectoris. Turk. Kardiyol. Dern. Ars. 2018, 46, 375–384. [Google Scholar] [CrossRef]
- Colucci, W.S.; Gimbrone, M.A., Jr.; McLaughlin, M.K.; Halpern, W.; Alexander, R.W. Increased Vascular Catecholamine Sensitivity and Alpha-Adrenergic Receptor Affinity in Female and Estrogen-Treated Male Rats. Circ. Res. 1982, 50, 805–811. [Google Scholar] [CrossRef]
- Mashchak, C.A.; Lobo, R.A.; Dozono-Takano, R.; Eggena, P.; Nakamura, R.M.; Brenner, P.F.; Mishell, D.R., Jr. Comparison of Pharmacodynamic Properties of Various Estrogen Formulations. Am. J. Obstet. Gynecol. 1982, 144, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Taggart, P.; Carruthers, M.; Joseph, S.; Kelly, H.B.; Marcomichelakis, J.; Noble, D.; O’Neill, G.; Somerville, W. Electrocardiographic Changes Resembling Myocardial Ischaemia in Asymptomatic Men with Normal Coronary Arteriograms. Br. Heart J. 1979, 41, 214–225. [Google Scholar] [CrossRef]
- Chow, R.; Fordyce, C.B.; Gao, M.; Chan, S.; Gin, K.; Bennett, M. The Significance of Early Post-Exercise ST Segment Normalization. J. Electrocardiol. 2015, 48, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Nicolosi, G.L.; Lombardo, M.; Anzà, C.; Ambrosio, G. False-Positive Electrocardiographic Changes during Exercise Test in a Patient with Pectus Excavatum. J. Clin. Ultrasound 2020, 48, 579–584. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Nicolosi, G.L.; Lombardo, M. Exercise-Induced Pseudo-Ischaemic Electrocardiographic Changes in a Female with Concave-Shaped Chest Wall. Eur. Heart J. Case Rep. 2024, 8, ytae123. [Google Scholar] [CrossRef]
- Feigenbaum, H. Exercise Echocardiography. J. Am. Soc. Echocardiogr. 1988, 1, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Roger, V.L.; Pellikka, P.A.; Bell, M.R.; Chow, C.W.; Bailey, K.R.; Seward, J.B. Sex and Test Verification Bias: Impact on the Diagnostic Value of Exercise Echocardiography. Circulation 1997, 95, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Piérard, L.A.; Berthe, C.; Albert, A.; Carlier, J.; Kulbertus, H.E. Haemodynamic Alterations during Ischaemia Induced by Dobutamine Stress Testing. Eur. Heart J. 1989, 10, 783–790. [Google Scholar] [CrossRef]
- Fung, A.Y.; Gallagher, K.P.; Buda, A.J. The Physiologic Basis of Dobutamine as Compared with Dipyridamole Stress Interventions in the Assessment of Critical Coronary Stenosis. Circulation 1987, 76, 943–951. [Google Scholar] [CrossRef]
- McNeill, A.J.; Fioretti, P.M.; el-Said, S.M.; Salustri, A.; Forster, T.; Roelandt, J.R. Enhanced Sensitivity for Detection of Coronary Artery Disease by Addition of Atropine to Dobutamine Stress Echocardiography. Am. J. Cardiol. 1992, 70, 41–46. [Google Scholar] [CrossRef]
- Picano, E.; Pingitore, A.; Conti, U.; Kozàkovà, M.; Boem, A.; Cabani, E.; Ciuti, M.; Distante, A.; L’Abbate, A. Enhanced Sensitivity for Detection of Coronary Artery Disease by Addition of Atropine to Dipyridamole Echocardiography. Eur. Heart J. 1993, 14, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Poldermans, D.; Fioretti, P.M.; Boersma, E.; Forster, T.; van Urk, H.; Cornel, J.H.; Arnese, M.; Roelandt, R.T. Safety of Dobutamine-Atropine Stress Echocardiography in Patients with Suspected or Proven Coronary Artery Disease. Am. J. Cardiol. 1994, 73, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Picano, E.; Marini, C.; Pirelli, S.; Maffei, S.; Bolognese, L.; Chiriatti, G.; Chiarella, F.; Orlandini, A.; Seveso, G.; Colosso, M.Q.; et al. Safety of Intravenous High-Dose Dipyridamole Echocardiography. The Echo-Persantine International Cooperative Study Group. Am. J. Cardiol. 1992, 70, 252–258. [Google Scholar] [CrossRef]
- Lim, H.E.; Shim, W.J.; Rhee, H.; Kim, S.M.; Hwang, G.S.; Kim, Y.H.; Seo, H.S.; Oh, D.J.; Ro, Y.M. Assessment of Coronary Flow Reserve with Transthoracic Doppler Echocardiography: Comparison among Adenosine, Standard-Dose Dipyridamole, and High-Dose Dipyridamole. J. Am. Soc. Echocardiogr. 2000, 13, 264–270. [Google Scholar] [CrossRef]
- Previtali, M.; Lanzarini, L.; Ferrario, M.; Tortorici, M.; Mussini, A.; Montemartini, C. Dobutamine versus Dipyridamole Echocardiography in Coronary Artery Disease. Circulation 1991, 83 (Suppl. S5), III27–III31. [Google Scholar]
- Lattanzi, F.; Picano, E.; Bolognese, L.; Piccinino, C.; Sarasso, G.; Orlandini, A.; L’Abbate, A. Inhibition of Dipyridamole-Induced Ischemia by Antianginal Therapy in Humans: Correlation with Exercise Electrocardiography. Circulation 1991, 83, 1256–1262. [Google Scholar] [CrossRef]
- Rigo, F.; Pratali, L.; Pálinkás, A.; Picano, E.; Cutaia, V.; Venneri, L.; Raviele, A. Coronary Flow Reserve and Brachial Artery Reactivity in Patients with Chest Pain and “False Positive” Exercise-Induced ST-Segment Depression. Am. J. Cardiol. 2002, 89, 1141–1144. [Google Scholar] [CrossRef] [PubMed]
- Galderisi, M.; Capaldo, B.; Sidiropulos, M.; D’Errico, A.; Ferrara, L.; Turco, A.; Guarini, P.; Riccardi, G.; de Divitiis, O. Determinants of Reduction of Coronary Flow Reserve in Patients with Type 2 Diabetes Mellitus or Arterial Hypertension without Angiographically Determined Epicardial Coronary Stenosis. Am. J. Hypertens. 2007, 20, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Rigo, F. Coronary Flow Reserve in Stress-Echo Lab: From Pathophysiologic Toy to Diagnostic Tool. Cardiovasc. Ultrasound 2005, 3, 8. [Google Scholar] [CrossRef]
- Nahser, P.J., Jr.; Brown, R.E.; Oskarsson, H.; Winniford, M.D.; Rossen, J.D. Maximal Coronary Flow Reserve and Metabolic Coronary Vasodilation in Patients with Diabetes Mellitus. Circulation 1995, 91, 635–640. [Google Scholar] [CrossRef]
- Schwartzkopff, B.; Motz, W.; Frenzel, H.; Vogt, M.; Knauer, S.; Strauer, B.E. Structural and Functional Alterations of the Intramyocardial Coronary Arterioles in Patients with Arterial Hypertension. Circulation 1993, 88, 993–1003. [Google Scholar] [CrossRef]
- Yokoyama, I.; Ohtake, T.; Momomura, S.; Nishikawa, J.; Sasaki, Y.; Omata, M. Reduced Coronary Flow Reserve in Hypercholesterolemic Patients without Overt Coronary Stenosis. Circulation 1996, 94, 3232–3238. [Google Scholar] [CrossRef] [PubMed]
- Fox, K.; Garcia, M.A.; Ardissino, D.; Buszman, P.; Camici, P.G.; Crea, F.; Daly, C.; De Backer, G.; Hjemdahl, P.; Lopez-Sendon, J.; et al. Guidelines on the Management of Stable Angina Pectoris: Executive Summary. Eur. Heart J. 2006, 27, 1341–1381. [Google Scholar] [CrossRef]
- Gould, K.L.; Westcott, R.J.; Albro, P.C.; Hamilton, G.W. Noninvasive Assessment of Coronary Stenoses by Myocardial Imaging during Pharmacologic Coronary Vasodilatation. II. Clinical Methodology and Feasibility. Am. J. Cardiol. 1978, 41, 279–287. [Google Scholar] [CrossRef]
- Goodgold, H.M.; Rehder, J.G.; Samuels, L.D.; Chaitman, B.R. Improved Interpretation of Exercise Tl-201 Myocardial Perfusion Scintigraphy in Women: Characterization of Breast Attenuation Artifacts. Radiology 1987, 165, 361–366. [Google Scholar] [CrossRef]
- Calnon, D.A.; Glover, D.K.; Beller, G.A.; Vanzetto, G.; Smith, W.H.; Watson, D.D.; Ruiz, M. Effects of Dobutamine Stress on Myocardial Blood Flow, 99mTc Sestamibi Uptake, and Systolic Wall Thickening in the Presence of Coronary Artery Stenoses: Implications for Dobutamine Stress Testing. Circulation 1997, 96, 2353–2360. [Google Scholar] [CrossRef] [PubMed]
- Forster, T.; McNeill, A.J.; Salustri, A.; Reijs, A.E.; el-Said, E.S.; Roelandt, J.R.; Fioretti, P.M. Simultaneous Dobutamine Stress Echocardiography and Technetium-99m Isonitrile Single-Photon Emission Computed Tomography in Patients with Suspected Coronary Artery Disease. J. Am. Coll. Cardiol. 1993, 21, 1591–1596. [Google Scholar] [CrossRef]
- Friedman, J.; Berman, D.S.; Van Train, K.; Garcia, E.V.; Bietendorf, J.; Prigent, F.; Rozanski, A.; Waxman, A.; Maddahi, J. Patient Motion in Thallium-201 Myocardial SPECT Imaging: An Easily Identified Frequent Source of Artifactual Defect. Clin. Nucl. Med. 1988, 13, 321–324. [Google Scholar] [CrossRef]
- O’Keefe, J.H., Jr.; Bateman, T.M.; Barnhart, C.S. Adenosine Thallium-201 Is Superior to Exercise Thallium-201 for Detecting Coronary Artery Disease in Patients with Left Bundle Branch Block. J. Am. Coll. Cardiol. 1993, 21, 1332–1338. [Google Scholar] [CrossRef]
- Raman, S.V.; Richards, D.R.; Jekic, M.; Dickerson, J.A.; Kander, N.H.; Foster, E.L.; Simonetti, O.P. Treadmill Stress Cardiac Magnetic Resonance Imaging: First In Vivo Demonstration of Exercise-Induced Apical Ballooning. J. Am. Coll. Cardiol. 2008, 52, 1884. [Google Scholar] [CrossRef]
- Thiele, H.; Nagel, E.; Paetsch, I.; Schnackenburg, B.; Bornstedt, A.; Kouwenhoven, M.; Wahl, A.; Schuler, G.; Fleck, E. Functional Cardiac MR Imaging with Steady-State Free Precession (SSFP) Significantly Improves Endocardial Border Delineation without Contrast Agents. J. Magn. Reson. Imaging 2001, 14, 362–367. [Google Scholar] [CrossRef]
- Charoenpanichkit, C.; Hundley, W.G. The 20 Year Evolution of Dobutamine Stress Cardiovascular Magnetic Resonance. J. Cardiovasc. Magn. Reson. 2010, 12, 59. [Google Scholar] [CrossRef]
- Rasmussen, L.D.; Schmidt, S.E.; Knuuti, J.; Newby, D.E.; Singh, T.; Nieman, K.; Galema, T.W.; Vrints, C.; Bøttcher, M.; Winther, S. Exercise Electrocardiography for Pre-Test Assessment of the Likelihood of Coronary Artery Disease. Heart 2024, 110, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.P.; Ellestad, M.H. Significance of Chest Pain during Treadmill Exercise: Correlation with Coronary Events. Am. J. Cardiol. 1978, 41, 227–232. [Google Scholar] [CrossRef]
- Lindow, T.; Ekström, M.; Brudin, L.; Carlén, A.; Elmberg, V.; Hedman, K. Typical Angina during Exercise Stress Testing Improves the Prediction of Future Acute Coronary Syndrome. Clin. Physiol. Funct. Imaging 2021, 41, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Ballo, H.; Juarez-Orozco, L.E.; Saraste, A.; Kolh, P.; Rutjes, A.W.S.; Jüni, P.; Windecker, S.; Bax, J.J.; Wijns, W. The Performance of Non-Invasive Tests to Rule-In and Rule-Out Significant Coronary Artery Stenosis in Patients with Stable Angina: A Meta-Analysis Focused on Post-Test Disease Probability. Eur. Heart J. 2018, 39, 3322–3330. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Bing, R.; Dweck, M.R.; van Beek, E.J.R.; Mills, N.L.; Williams, M.C.; Villines, T.C.; Newby, D.E.; Adamson, P.D. Exercise Electrocardiography and Computed Tomography Coronary Angiography for Patients with Suspected Stable Angina Pectoris: A Post Hoc Analysis of the Randomized SCOT-HEART Trial. JAMA Cardiol. 2020, 5, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Picano, E.; Mathias, W., Jr.; Pingitore, A.; Bigi, R.; Previtali, M. Safety and Tolerability of Dobutamine-Atropine Stress Echocardiography: A Prospective, Multicentre Study. Lancet 1994, 344, 1190–1192. [Google Scholar] [CrossRef]
- Pellikka, P.A.; Arruda-Olson, A.; Chaudhry, F.A.; Chen, M.H.; Marshall, J.E.; Porter, T.R.; Sawada, S.G. Guidelines for Performance, Interpretation, and Application of Stress Echocardiography in Ischemic Heart Disease: From the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2020, 33, 1–41.e8. [Google Scholar] [CrossRef]
- Kunadian, V.; Chieffo, A.; Camici, P.G.; Berry, C.; Escaned, J.; Maas, A.H.E.M.; Prescott, E.; Karam, N.; Appelman, Y.; Fraccaro, C.; et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur. Heart J. 2020, 41, 3504–3520. [Google Scholar] [CrossRef]
- Ciampi, Q.; Zagatina, A.; Cortigiani, L.; Gaibazzi, N.; Borguezan Daros, C.; Zhuravskaya, N.; Wierzbowska-Drabik, K.; Kasprzak, J.D.; de Castro E Silva Pretto, J.L.; D’Andrea, A.; et al. Functional, Anatomical, and Prognostic Correlates of Coronary Flow Velocity Reserve During Stress Echocardiography. J. Am. Coll. Cardiol. 2019, 74, 2278–2291. [Google Scholar] [CrossRef]
- Cantoni, V.; Green, R.; Acampa, W.; Zampella, E.; Assante, R.; Nappi, C.; Gaudieri, V.; Mannarino, T.; Cuocolo, R.; Di Vaia, E.; et al. Diagnostic Performance of Myocardial Perfusion Imaging with Conventional and CZT SPECT in Detecting Coronary Artery Disease: A Meta-Analysis. J. Nucl. Cardiol. 2021, 28, 698–715. [Google Scholar] [CrossRef]
- Yang, Z.; Zheng, H.; Zhou, T.; Yang, L.F.; Hu, X.F.; Peng, Z.H.; Jiang, Y.Z.; Li, M.; Sun, G. Diagnostic Performance of Myocardial Perfusion Imaging with SPECT, CT and MR Compared to Fractional Flow Reserve as Reference Standard. Int. J. Cardiol. 2015, 190, 103–105. [Google Scholar] [CrossRef]
- Takx, R.A.; Blomberg, B.A.; El Aidi, H.; Habets, J.; de Jong, P.A.; Nagel, E.; Hoffmann, U.; Leiner, T. Diagnostic Accuracy of Stress Myocardial Perfusion Imaging Compared to Invasive Coronary Angiography with Fractional Flow Reserve: Meta-Analysis. Circ. Cardiovasc. Imaging 2015, 8, e002666. [Google Scholar] [CrossRef]
- Mowatt, G.; Brazzelli, M.; Gemmell, H.; Hillis, G.S.; Metcalfe, M.; Vale, L.; Aberdeen Technology Assessment Review Group. Systematic Review of the Prognostic Effectiveness of SPECT Myocardial Perfusion Scintigraphy in Patients with Suspected or Known Coronary Artery Disease and Following Myocardial Infarction. Nucl. Med. Commun. 2005, 26, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, J.P.; Ripley, D.P.; Berry, C.; McCann, G.P.; Plein, S.; Bucciarelli-Ducci, C.; Dall’Armellina, E.; Prasad, A.; Bijsterveld, P.; Foley, J.R.; et al. Effect of Care Guided by Cardiovascular Magnetic Resonance, Myocardial Perfusion Scintigraphy, or NICE Guidelines on Subsequent Unnecessary Angiography Rates: The CE-MARC 2 Randomized Clinical Trial. JAMA 2016, 316, 1051–1060. [Google Scholar] [CrossRef]
- Dai, N.; Zhang, X.; Zhang, Y.; Hou, L.; Li, W.; Fan, B.; Zhang, T.; Xu, Y. Enhanced Diagnostic Utility Achieved by Myocardial Blood Analysis: A Meta-Analysis of Noninvasive Cardiac Imaging in the Detection of Functional Coronary Artery Disease. Int. J. Cardiol. 2016, 221, 665–673. [Google Scholar] [CrossRef]
- Ripley, D.P.; Motwani, M.; Brown, J.M.; Nixon, J.; Everett, C.C.; Bijsterveld, P.; Maredia, N.; Plein, S.; Greenwood, J.P. Individual Component Analysis of the Multi-Parametric Cardiovascular Magnetic Resonance Protocol in the CE-MARC Trial. J. Cardiovasc. Magn. Reson. 2015, 17, 59. [Google Scholar] [CrossRef]
- Gargiulo, P.; Dellegrottaglie, S.; Bruzzese, D.; Savarese, G.; Scala, O.; Ruggiero, D.; D’Amore, C.; Paolillo, S.; Agostoni, P.; Bossone, E.; et al. The Prognostic Value of Normal Stress Cardiac Magnetic Resonance in Patients with Known or Suspected Coronary Artery Disease: A Meta-Analysis. Circ. Cardiovasc. Imaging 2013, 6, 574–582. [Google Scholar] [CrossRef]
- Iwata, K.; Nakagawa, S.; Ogasawara, K. The Prognostic Value of Normal Stress Cardiovascular Magnetic Resonance Imaging. J. Comput. Assist. Tomogr. 2014, 38, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Ricci, F.; Khanji, M.Y.; Bisaccia, G.; Cipriani, A.; Di Cesare, A.; Ceriello, L.; Mantini, C.; Zimarino, M.; Fedorowski, A.; Gallina, S.; et al. Diagnostic and Prognostic Value of Stress Cardiovascular Magnetic Resonance Imaging in Patients with Known or Suspected Coronary Artery Disease: A Systematic Review and Meta-Analysis. JAMA Cardiol. 2023, 8, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Nagel, E.; Greenwood, J.P.; McCann, G.P.; Bettencourt, N.; Shah, A.M.; Hussain, S.T.; Perera, D.; Plein, S.; Bucciarelli-Ducci, C.; Paul, M.; et al. Magnetic Resonance Perfusion or Fractional Flow Reserve in Coronary Disease. N. Engl. J. Med. 2019, 380, 2418–2428. [Google Scholar] [CrossRef]
- Feger, S.; Rief, M.; Zimmermann, E.; Richter, F.; Roehle, R.; Dewey, M.; Schönenberger, E. Patient Satisfaction with Coronary CT Angiography, Myocardial CT Perfusion, Myocardial Perfusion MRI, SPECT Myocardial Perfusion Imaging and Conventional Coronary Angiography. Eur. Radiol. 2015, 25, 2115–2124. [Google Scholar] [CrossRef] [PubMed]
- Reeh, J.; Therming, C.B.; Heitmann, M.; Højberg, S.; Sørum, C.; Bech, J.; Husum, D.; Dominguez, H.; Sehestedt, T.; Hermann, T.; et al. Prediction of Obstructive Coronary Artery Disease and Prognosis in Patients with Suspected Stable Angina. Eur. Heart J. 2019, 40, 1426–1435. [Google Scholar] [CrossRef]
- Winther, S.; Schmidt, S.E.; Mayrhofer, T.; Bøtker, H.E.; Hoffmann, U.; Douglas, P.S.; Wijns, W.; Bax, J.; Nissen, L.; Lynggaard, V.; et al. Incorporating Coronary Calcification into Pre-Test Assessment of the Likelihood of Coronary Artery Disease. J. Am. Coll. Cardiol. 2020, 76, 2421–2432. [Google Scholar] [CrossRef]
- Boerhout, C.K.M.; Feenstra, R.G.T.; Somsen, G.A.; Appelman, Y.; Ong, P.; Beijk, M.A.M.; Hofstra, L.; van de Hoef, T.P.; Piek, J.J. Coronary Computed Tomographic Angiography as Gatekeeper for New-Onset Stable Angina. Neth. Heart J. 2021, 29, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Baravelli, M.; Vincenti, A.; Trevisan, R.; Zompatori, M.; Nicolosi, G.L.; Lombardo, M.; Anzà, C. A new modified anthropometric Haller index obtained without radiological exposure. Int. J. Cardiovasc. Imaging 2018, 34, 1505–1509. [Google Scholar] [CrossRef] [PubMed]
- Archer, J.E.; Gardner, A.; Berryman, F.; Pynsent, P. The measurement of the normal thorax using the Haller index methodology at multiple vertebral levels. J. Anat. 2016, 229, 577–581. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Nicolosi, G.L.; Rigamonti, E.; Lombardo, M.; Gensini, G.F.; Ambrosio, G. Does chest shape influence exercise stress echocardiographic results in patients with suspected coronary artery disease? Intern. Emerg. Med. 2022, 17, 101–112. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Rigamonti, E.; Nicolosi, G.L.; Lombardo, M. Prognostic value of modified Haller index in patients with suspected coronary artery disease referred for exercise stress echocardiography. J. Cardiovasc. Echogr. 2021, 31, 85–95. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Rigamonti, E.; Nicolosi, G.L.; Lombardo, M. Appropriate use criteria implementation with modified Haller index for predicting stress echocardiographic results and outcome in a population of patients with suspected coronary artery disease. Int. J. Cardiovasc. Imaging 2021, 37, 2917–2930. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Nicolosi, G.L.; Lombardo, M. The relationship between mitral valve prolapse and thoracic skeletal abnormalities in clinical practice: A systematic review. J. Cardiovasc. Med. 2024, 25, 353–363. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Nicolosi, G.L.; Bruno, A.; Lombardo, M.; Muti, P. Echocardiographic assessment of mitral valve prolapse prevalence before and after the year 1999: A systematic review. J. Clin. Med. 2024, 13, 6160. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Grasso, E.; Nicolosi, G.L.; Lombardo, M. Modified Haller index is inversely associated with asymptomatic status in atrial fibrillation patients undergoing electrical cardioversion: A preliminary observation. Minerva Cardiol. Angiol. 2024, 72, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Phua, Q.S.; Bacchi, S.; Goh, R.; Gupta, A.K.; Kovoor, J.G.; Ovenden, C.D.; To, M.S. Small study effects in diagnostic imaging accuracy: A meta-analysis. JAMA Netw. Open 2022, 5, e2228776. [Google Scholar] [CrossRef]
- Sinha, A.; Dutta, U.; Demir, O.M.; De Silva, K.; Ellis, H.; Belford, S.; Ogden, M.; Li Kam Wa, M.; Morgan, H.P.; Shah, A.M.; et al. Rethinking False Positive Exercise Electrocardiographic Stress Tests by Assessing Coronary Microvascular Function. J. Am. Coll. Cardiol. 2024, 83, 291–299. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).