Is Chronic Whiplash-Associated Disorder Associated with Central Nervous System Impairments? A Controlled Observational Study in a Lithuanian Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Objective Examination
2.3. Questionnaires
- (1)
- Visual Analogue Scale (VAS, 0–100 mm)—to assess pain intensity (no pain–worst pain) and general health perception (best health–worst health). Participants were asked to score their overall pain over the last week as well as pain in different regions of the body.
- (2)
- Quebec Task Force Questionnaire (QTFQ)—to assess symptoms before the accident and acute phase (filled out on the first visit) as well as in the chronic phase [17]. The QTFQ was used to determine the development of a) new symptoms after the collision and b) persistence or development of new symptoms during the follow-up period.
- (3)
- Disability Rating Index (DRI)—to evaluate performance in everyday activities [18]. Evaluation of the DRI represents a sum of scores from 12 scales and ranges from 0 to 1200, where higher scores indicate worse functional status.
- (4)
- Cognitive Failure Questionnaire (CFQ)—to measure a person’s likelihood of committing an error in the completion of an everyday task [19]. The CFQ consists of 25 questions, and the sum of all answers ranges from 0 to 100, with the cut-off score of 43 and above reflecting cognitive impairment.
- (5)
- Hospital Anxiety and Depression (HAD) scale—to determine the levels of anxiety and depression that a person is experiencing [20]. The total sum for both HAD anxiety and HAD depression levels ranges from 0 to 21. A score equal to or more than 10 allows suspicion of clinically significant anxiety or depression symptoms.
2.4. WAD Grade Assessment
2.5. Statistics
3. Results
3.1. Participants and Factors of Collision
3.2. Clinical WAD Grades and Sick Leave in WAD Group
| WAD (n = 34–45) | Control (n = 48–50) | Statistics | |
|---|---|---|---|
| Age, years | 29.5 (19) | 28 (16) | Mann–Whitney U test, p = 0.961 |
| Body mass index, kg/m2 | 23.8 ± 13.3 | 24 ± 4.3 | Student t test, p = 0.841 |
| Sex: | Pearson‘s chi square test, p = 0.858 | ||
| Men | 17 (38%) | 18 (36%) | |
| Women | 28 (62%) | 32 (64%) | |
| Education: | Pearson‘s chi square test, p = 0.428 | ||
| Secondary | 21 (47%) | 20 (40%) | |
| Professional | 11 (24%) | 10 (20%) | |
| University | 12 (27%) | 20 (40%) | |
| Employment status: | Fisher–Freeman–Halton Exact test, p = 0.304 | ||
| Employed | 30 (67%) | 30 (60%) | |
| Unemployed | 3 (7%) | 0 | |
| Not working | 2 (4%) | 2 (4%) | |
| Student | 8 (18%) | 14 (28%) | |
| Employed student | 2 (4%) | 4 (8%) |
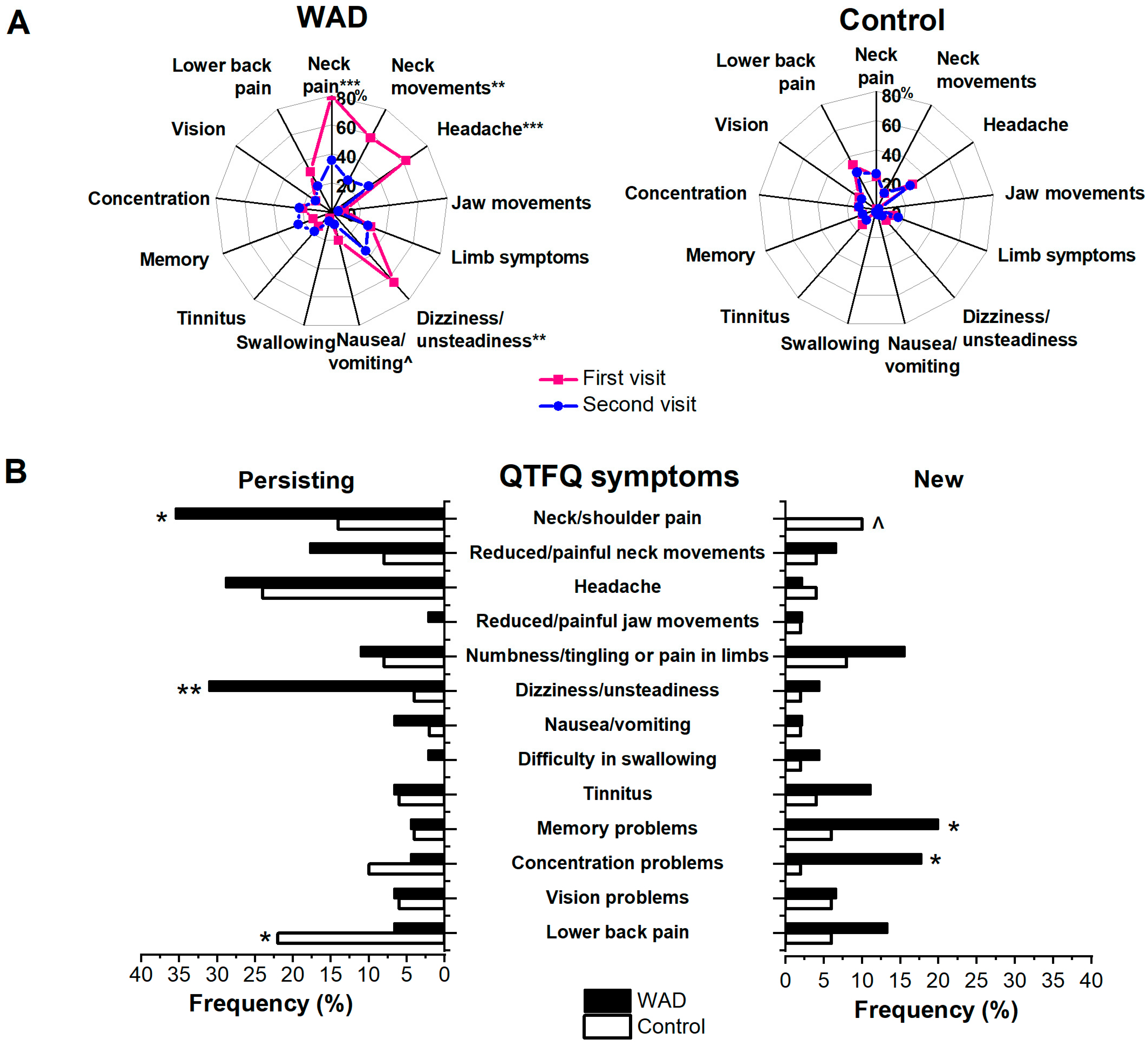
3.3. Specific WAD Symptom Questionnaire (Quebec Task Force Questionnaire, QTFQ)
3.4. Parameters of General Health, Pain, Disability, Cognitive Failure, and Depression/Anxiety
| Parameter | First Visit | Second Visit | Within-Group p Value | Change ([First–Second Visit]) |
|---|---|---|---|---|
| General health perception (VAS, mm) | ||||
| WAD | 32.5 (35) | 18 (31) | 0.041 | −4 (35) |
| Controls | 8 (14) | 8 (17.25) | 0.155 | 1 (10.25) |
| Between-group p value | <0.001 | 0.051 | 0.012 | |
| Pain last week (VAS, mm) | ||||
| WAD | 49 (38) | 8 (33) | <0.001 | −20 (46) |
| Controls | 4 (16) | 5 (19) | 0.489 | 0 (12.75) |
| Between-group p value | <0.001 | 0.456 | <0.001 | |
| Headache (VAS, mm) | ||||
| WAD | 0 (8) | 0 (0) | 0.036 | 0 (0) |
| Controls | 0 (0) | 0 (0) | 0.270 | 0 (0) |
| Between-group p value | 0.018 | 0.569 | 0.074 | |
| Neck pain (VAS, mm) | ||||
| WAD | 23 (61) | 0 (2) | <0.001 | −8 (30.5) |
| Controls | 0 (0) | 0 (0) | 0.050 | 0 (0) |
| Between-group p value | <0.001 | 0.202 | <0.001 | |
| Lower back pain (VAS, mm) | ||||
| WAD | 0 (24) | 0 (0) | 0.076 | 0 (16.75) |
| Controls | 0 (0) | 0 (0.75) | 0.924 | 0 (0) |
| Between-group p value | 0.179 | 0.915 | 0.296 | |
| Disability Rate Index | ||||
| WAD | 315 (461.5) | 26 (102.5) | <0.001 | −231 (448) |
| Controls | 20.5 (60.25) | 9.5 (49) | 0.857 | 0 (24.63) |
| Between-group p value | <0.001 | 0.109 | <0.001 | |
| Cognitive Failure Questionnaire | ||||
| WAD | 30 (13) [n = 8] | 33 (16) [n = 7] | 0.308 | 3 (13) |
| Controls | 27 (13.25) [n = 4] | 25.5 (11) [n = 7] | 0.565 | −1.5 (11.25) |
| Between-group p value | 0.024 | 0.004 | 0.240 | |
| HADS Anxiety | ||||
| WAD | 7 (4) [n = 10] | 5 (4) [n = 6] | 0.002 | −2 (5) |
| Controls | 4.5 (4) [n = 6] | 3 (3) [n = 6] | 0.002 | −1 (2.5) |
| Between-group p value | 0.002 | 0.002 | 0.400 | |
| HADS Depression | ||||
| WAD | 2 (3) [n = 1] | 2 (4) [n = 0] | 0.596 | 0 (2.5) |
| Controls | 1.5 (3) [n = 2] | 1 (2) [n = 2] | 0.044 | 0 (1.5) |
| Between-group p value | 0.088 | 0.010 | 0.351 | |
3.5. Neck Range of Motion (ROM)
3.6. Predicting Chronic WAD
4. Discussion
4.1. Acute WAD
4.2. WAD Symptomatology at Follow-Up
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Versteegen, G.J.; Kingma, J.; Meijler, W.J.; Ten Duis, H.J. Neck sprain in patients injured in car accidents: A retrospective study covering the period 1970–1994. Eur. Spine J. 1998, 7, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Albano, M.; Alpini, D.C.; Carbone, G.V. Whiplash and Sport. In Whiplash Injuries: Diagnosis and Treatment; Alpini, D.C., Brugnoni, G., Cesarani, A., Eds.; Springer: Milan, Italy, 2014; pp. 127–137. [Google Scholar]
- Carroll, L.J.; Holm, L.W.; Hogg-Johnson, S.; Côté, P.; Cassidy, J.D.; Haldeman, S.; Nordin, M.; Hurwitz, E.L.; Carragee, E.J.; van der Velde, G.; et al. Course and prognostic factors for neck pain in whiplash-associated disorders (WAD): Results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. Spine 2008, 33, S83–S92. [Google Scholar] [CrossRef]
- Al-Khazali, H.M.; Ashina, H.; Iljazi, A.; Lipton, R.B.; Ashina, M.; Ashina, S.; Schytz, H.W. Neck pain and headache after whiplash injury: A systematic review and meta-analysis. Pain 2020, 161, 880–888. [Google Scholar] [CrossRef]
- Côté, P.; Cassidy, J.D.; Carroll, L.; Frank, J.W.; Bombardier, C. A systematic review of the prognosis of acute whiplash and a new conceptual framework to synthesize the literature. Spine 2001, 26, E445–E458. [Google Scholar] [CrossRef] [PubMed]
- Sarrami, P.; Armstrong, E.; Naylor, J.M.; Harris, I.A. Factors predicting outcome in whiplash injury: A systematic meta-review of prognostic factors. J. Orthop. Traumatol. 2017, 18, 9–16. [Google Scholar] [CrossRef]
- Walton, D.M.; Macdermid, J.C.; Giorgianni, A.A.; Mascarenhas, J.C.; West, S.C.; Zammit, C.A. Risk factors for persistent problems following acute whiplash injury: Update of a systematic review and meta-analysis. J. Orthop. Sports Phys. Ther. 2013, 43, 31–43. [Google Scholar] [CrossRef]
- Aarnio, M.; Fredrikson, M.; Lampa, E.; Sorensen, J.; Gordh, T.; Linnman, C. Whiplash injuries associated with experienced pain and disability can be visualized with [11C]-D-deprenyl positron emission tomography and computed tomography. Pain 2022, 163, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Van Oosterwijck, J.; Nijs, J.; Meeus, M.; Paul, L. Evidence for central sensitization in chronic whiplash: A systematic literature review. Eur. J. Pain 2013, 17, 299–312. [Google Scholar] [CrossRef]
- Coppieters, I.; Ickmans, K.; Cagnie, B.; Nijs, J.; De Pauw, R.; Noten, S.; Meeus, M. Cognitive Performance Is Related to Central Sensitization and Health-related Quality of Life in Patients with Chronic Whiplash-Associated Disorders and Fibromyalgia. Pain Physician 2015, 18, E389–E401. [Google Scholar]
- Favaretto, N.; Lionello, M.; Boscolo-Berto, R.; Giacomelli, L.; Rondinelli, R.; Marioni, G. Video-nystagmographic evidence in more than 700 consecutive cases of road traffic whiplash injury. Am. J. Otolaryngol. 2021, 42, 102909. [Google Scholar] [CrossRef]
- Cygaite, L.; Lingyte, I.; Ratkeviciute, K. The analysis of implementation automation traffic speed control. In Proceedings of the Environmental Engineering, Vilnius, Lithuania, 19–20 May 2011; pp. 1057–1063. [Google Scholar]
- Lietuvos Keliu Policijos Tarnyba. Eismo Įvykių, Kuriuose Nukentėjo Žmonės, Lietuvoje Suvestinė. 2020. Available online: https://policija.lrv.lt/uploads/lkpt.policija/documents/files/statistika/2019/2019.pdf (accessed on 1 March 2025).
- Pajediene, E.; Janusauskaite, J.; Samusyte, G.; Stasaitis, K.; Petrikonis, K.; Bileviciute-Ljungar, I. Patterns of acute whiplash-associated disorder in the Lithuanian population after road traffic accidents. J. Rehabil. Med. 2015, 47, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Noll-Hussong, M. Whiplash Syndrome Reloaded: Digital Echoes of Whiplash Syndrome in the European Internet Search Engine Context. JMIR Public Health Surveill. 2017, 3, e15. [Google Scholar] [CrossRef]
- Reese, N.; Bandy, W. Joint Range of Motion and Muscle Length Testing; Saunders: Philadelphia, PA, USA, 2002. [Google Scholar]
- Spitzer, W.O.; Skovron, M.L.; Salmi, L.R.; Cassidy, J.D.; Duranceau, J.; Suissa, S.; Zeiss, E. Scientific monograph of the Quebec Task Force on Whiplash-Associated Disorders: Redefining “whiplash” and its management. Spine 1995, 20, 1s–73s. [Google Scholar]
- Salén, B.A.; Spangfort, E.V.; Nygren, A.L.; Nordemar, R. The Disability Rating Index: An instrument for the assessment of disability in clinical settings. J. Clin. Epidemiol. 1994, 47, 1423–1435. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, D.E.; Cooper, P.F.; FitzGerald, P.; Parkes, K.R. The Cognitive Failures Questionnaire (CFQ) and its correlates. Br. J. Clin. Psychol. 1982, 21, 1–16. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Bunevicius, A.; Peceliuniene, J.; Mickuviene, N.; Valius, L.; Bunevicius, R. Screening for depression and anxiety disorders in primary care patients. Depress. Anxiety 2007, 24, 455–460. [Google Scholar] [CrossRef]
- Obelieniene, D.; Schrader, H.; Bovim, G.; Miseviciene, I.; Sand, T. Pain after whiplash: A prospective controlled inception cohort study. J. Neurol. Neurosurg. Psychiatry 1999, 66, 279–283. [Google Scholar] [CrossRef]
- Ferrari, R.; Kwan, O.; Russell, A.S.; Pearce, J.M.; Schrader, H. The best approach to the problem of whiplash? One ticket to Lithuania, please. Clin. Exp. Rheumatol. 1999, 17, 321–326. [Google Scholar]
- Lietuvos Respublikos Transporto Priemonių Valdytojų Civilinės Atsakomybės Privalomojo Draudimo Įstatymas, IX-378. 2001. Available online: https://e-seimas.lrs.lt/portal/legalAct/lt/TAD/TAIS.140221?positionInSearchResults=1&searchModelUUID=7a96ef86-e498-4faf-b7da-f31bcad0e03d (accessed on 1 March 2025).
- Girotto, D.; Ledić, D.; Strenja-Linić, I.; Peharec, S.; Grubesić, A. Clinical and medicolegal characteristics of neck injuries. Coll. Antropol. 2011, 35 (Suppl. S2), 187–190. [Google Scholar] [PubMed]
- Sterner, Y.; Gerdle, B. Acute and chronic whiplash disorders—A review. J. Rehabil. Med. 2004, 36, 193–209, quiz 210. [Google Scholar] [CrossRef]
- Hoy, D.G.; Protani, M.; De, R.; Buchbinder, R. The epidemiology of neck pain. Best Pract. Res. Clin. Rheumatol. 2010, 24, 783–792. [Google Scholar] [CrossRef]
- Wu, A.-M.; Cross, M.; Elliott, J.M.; Culbreth, G.T.; Haile, L.M.; Steinmetz, J.D.; Hagins, H.; Kopec, J.A.; Brooks, P.M.; Woolf, A.D.; et al. Global, regional, and national burden of neck pain, 1990–2020, and projections to 2050: A systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2024, 6, e142–e155. [Google Scholar] [CrossRef]
- Mazaheri, M.; Abichandani, D.; Kingma, I.; Treleaven, J.; Falla, D. A meta-analysis and systematic review of changes in joint position sense and static standing balance in patients with whiplash-associated disorder. PLoS ONE 2021, 16, e0249659. [Google Scholar] [CrossRef] [PubMed]
- Treleaven, J. Dizziness, Unsteadiness, Visual Disturbances, and Sensorimotor Control in Traumatic Neck Pain. J. Orthop. Sports Phys. Ther. 2017, 47, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Goodhew, S.C.; Edwards, M. A meta-analysis on the relationship between subjective cognitive failures as measured by the cognitive failures questionnaire (CFQ) and objective performance on executive function tasks. Psychon. Bull. Rev. 2024, 32, 528–546. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, E.; Voogt, L.; Lenoir, D.; Coppieters, I.; Ickmans, K. Convergent Validity of the Central Sensitization Inventory in Chronic Whiplash-Associated Disorders; Associations with Quantitative Sensory Testing, Pain Intensity, Fatigue, and Psychosocial Factors. Pain Med. 2020, 21, 3401–3412. [Google Scholar] [CrossRef]
- Lenoir, D.; Willaert, W.; Ickmans, K.; Bernaers, L.; Nijs, J.; Malfliet, A.; Danneels, L.; Leysen, L.; De Pauw, R.; Cagnie, B.; et al. Are Reports of Pain, Disability, Quality of Life, Psychological Factors, and Central Sensitization Related to Outcomes of Quantitative Sensory Testing in Patients Suffering From Chronic Whiplash Associated Disorders? Clin. J. Pain 2021, 38, 159–172. [Google Scholar] [CrossRef]
- Bontinck, J.; Lenoir, D.; Cagnie, B.; Murillo, C.; Timmers, I.; Cnockaert, E.; Bernaers, L.; Meeus, M.; Coppieters, I. Temporal changes in pain processing after whiplash injury, based on Quantitative Sensory Testing: A systematic review. Eur. J. Pain 2022, 26, 227–245. [Google Scholar] [CrossRef]
- Guo, J.; Wang, J.; Liang, P.; Tian, E.; Liu, D.; Guo, Z.; Chen, J.; Zhang, Y.; Zhou, Z.; Kong, W.; et al. Vestibular dysfunction leads to cognitive impairments: State of knowledge in the field and clinical perspectives (Review). Int. J. Mol. Med. 2024, 53, 36. [Google Scholar] [CrossRef]
- Wenzel, H.G.; Haug, T.T.; Mykletun, A.; Dahl, A.A. A population study of anxiety and depression among persons who report whiplash traumas. J. Psychosom. Res. 2002, 53, 831–835. [Google Scholar] [CrossRef]
- Elliott, J.; Jull, G.; Noteboom, J.T.; Darnell, R.; Galloway, G.; Gibbon, W.W. Fatty infiltration in the cervical extensor muscles in persistent whiplash-associated disorders: A magnetic resonance imaging analysis. Spine 2006, 31, E847–E855. [Google Scholar] [CrossRef]
- Elliott, J.M.; O’Leary, S.; Sterling, M.; Hendrikz, J.; Pedler, A.; Jull, G. Magnetic resonance imaging findings of fatty infiltrate in the cervical flexors in chronic whiplash. Spine 2010, 35, 948–954. [Google Scholar] [CrossRef]
- Higgins, J.P.; Elliott, J.M.; Parrish, T.B. Brain Network Disruption in Whiplash. Am. J. Neuroradiol. 2020, 41, 994. [Google Scholar] [CrossRef] [PubMed]
- Higgins, D.M.; Martin, A.M.; Baker, D.G.; Vasterling, J.J.; Risbrough, V. The Relationship Between Chronic Pain and Neurocognitive Function: A Systematic Review. Clin. J. Pain 2018, 34, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Kessels, R.P.; Aleman, A.; Verhagen, W.I.; van Luijtelaar, E.L. Cognitive functioning after whiplash injury: A meta-analysis. J. Int. Neuropsychol. Soc. 2000, 6, 271–278. [Google Scholar] [CrossRef]
- Callan, B.; Kolber, M.; Cleland, J.; Elliott, J. Sleep Disturbances Effect on the Development of Disability Following a Motor Vehicle Collision: A Cohort Study. Internet J. Allied Health Sci. Pract. 2024, 22, 27. [Google Scholar]
- Radanov, B.P.; Di Stefano, G.; Schnidrig, A.; Sturzenegger, M.; Augustiny, K.F. Cognitive Functioning After Common Whiplash: A Controlled Follow-up Study. Arch. Neurol. 1993, 50, 87–91. [Google Scholar] [CrossRef]
- Marshall, C.M.; Vernon, H.; Leddy, J.J.; Baldwin, B.A. The role of the cervical spine in post-concussion syndrome. Physician Sportsmed. 2015, 43, 274–284. [Google Scholar] [CrossRef]
- Oyekan, A.A.; Eagle, S.; Trbovich, A.M.; Shaw, J.D.; Schneider, M.; Collins, M.; Lee, J.Y.; Kontos, A.P. Neck Symptoms and Associated Clinical Outcomes in Patients Following Concussion. J. Head Trauma Rehabil. 2023, 38, 417–424. [Google Scholar] [CrossRef]
- Cheever, K.; McDevitt, J.; Phillips, J.; Kawata, K. The Role of Cervical Symptoms in Post-concussion Management: A Systematic Review. Sports Med. 2021, 51, 1875–1891. [Google Scholar] [CrossRef] [PubMed]
- Cnossen, M.C.; van der Naalt, J.; Spikman, J.M.; Nieboer, D.; Yue, J.K.; Winkler, E.A.; Manley, G.T.; von Steinbuechel, N.; Polinder, S.; Steyerberg, E.W.; et al. Prediction of Persistent Post-Concussion Symptoms after Mild Traumatic Brain Injury. J. Neurotrauma 2018, 35, 2691–2698. [Google Scholar] [CrossRef]
- Elkin, B.S.; Elliott, J.M.; Siegmund, G.P. Whiplash Injury or Concussion? A Possible Biomechanical Explanation for Concussion Symptoms in Some Individuals Following a Rear-End Collision. J. Orthop. Sports Phys. Ther. 2016, 46, 874–885. [Google Scholar] [CrossRef]
- Jang, S.H.; Kwon, Y.H. A Review of Traumatic Axonal Injury following Whiplash Injury As Demonstrated by Diffusion Tensor Tractography. Front. Neurol. 2018, 9, 57. [Google Scholar] [CrossRef]
- Gil, C.; Decq, P. How similar are whiplash and mild traumatic brain injury? A systematic review. Neurochirurgie 2021, 67, 238–243. [Google Scholar] [CrossRef]
- Carstensen, T.B.; Fink, P.; Oernboel, E.; Kasch, H.; Jensen, T.S.; Frostholm, L. Sick Leave within 5 Years of Whiplash Trauma Predicts Recovery: A Prospective Cohort and Register-Based Study. PLoS ONE 2015, 10, e0130298. [Google Scholar] [CrossRef]
- Buitenhuis, J.; de Jong, P.J.; Jaspers, J.P.; Groothoff, J.W. Work disability after whiplash: A prospective cohort study. Spine 2009, 34, 262–267. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Disease, G.B.D.; Injury, I.; Prevalence, C. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samusyte, G.; Ciceliene, J.; Pajediene, E.; Stasaitis, K.; Petrikonis, K.; Bileviciute-Ljungar, I. Is Chronic Whiplash-Associated Disorder Associated with Central Nervous System Impairments? A Controlled Observational Study in a Lithuanian Cohort. J. Clin. Med. 2025, 14, 6222. https://doi.org/10.3390/jcm14176222
Samusyte G, Ciceliene J, Pajediene E, Stasaitis K, Petrikonis K, Bileviciute-Ljungar I. Is Chronic Whiplash-Associated Disorder Associated with Central Nervous System Impairments? A Controlled Observational Study in a Lithuanian Cohort. Journal of Clinical Medicine. 2025; 14(17):6222. https://doi.org/10.3390/jcm14176222
Chicago/Turabian StyleSamusyte, Gintaute, Jolita Ciceliene, Evelina Pajediene, Kestutis Stasaitis, Kestutis Petrikonis, and Indre Bileviciute-Ljungar. 2025. "Is Chronic Whiplash-Associated Disorder Associated with Central Nervous System Impairments? A Controlled Observational Study in a Lithuanian Cohort" Journal of Clinical Medicine 14, no. 17: 6222. https://doi.org/10.3390/jcm14176222
APA StyleSamusyte, G., Ciceliene, J., Pajediene, E., Stasaitis, K., Petrikonis, K., & Bileviciute-Ljungar, I. (2025). Is Chronic Whiplash-Associated Disorder Associated with Central Nervous System Impairments? A Controlled Observational Study in a Lithuanian Cohort. Journal of Clinical Medicine, 14(17), 6222. https://doi.org/10.3390/jcm14176222









