Synergistic Effect of Polydeoxyribonucleotides with Low-Level Lasers on the Regeneration of Crush-Injured Facial Nerves
Abstract
1. Introduction
2. Materials and Methods
2.1. Induction of Crush Injury-Induced Facial Nerve Paralysis and Treatment
2.2. Animal Group and Each Treatment
2.3. Evaluation of Vibrissa Movement Recovery Using Slow Motion Video Analysis Software
2.4. Electrically Induced Action Potential Measurement
2.5. Using a Laser Doppler Blood Flowmeter to Quantify Nerve Blood Flow
2.6. Statistical Analysis
3. Results
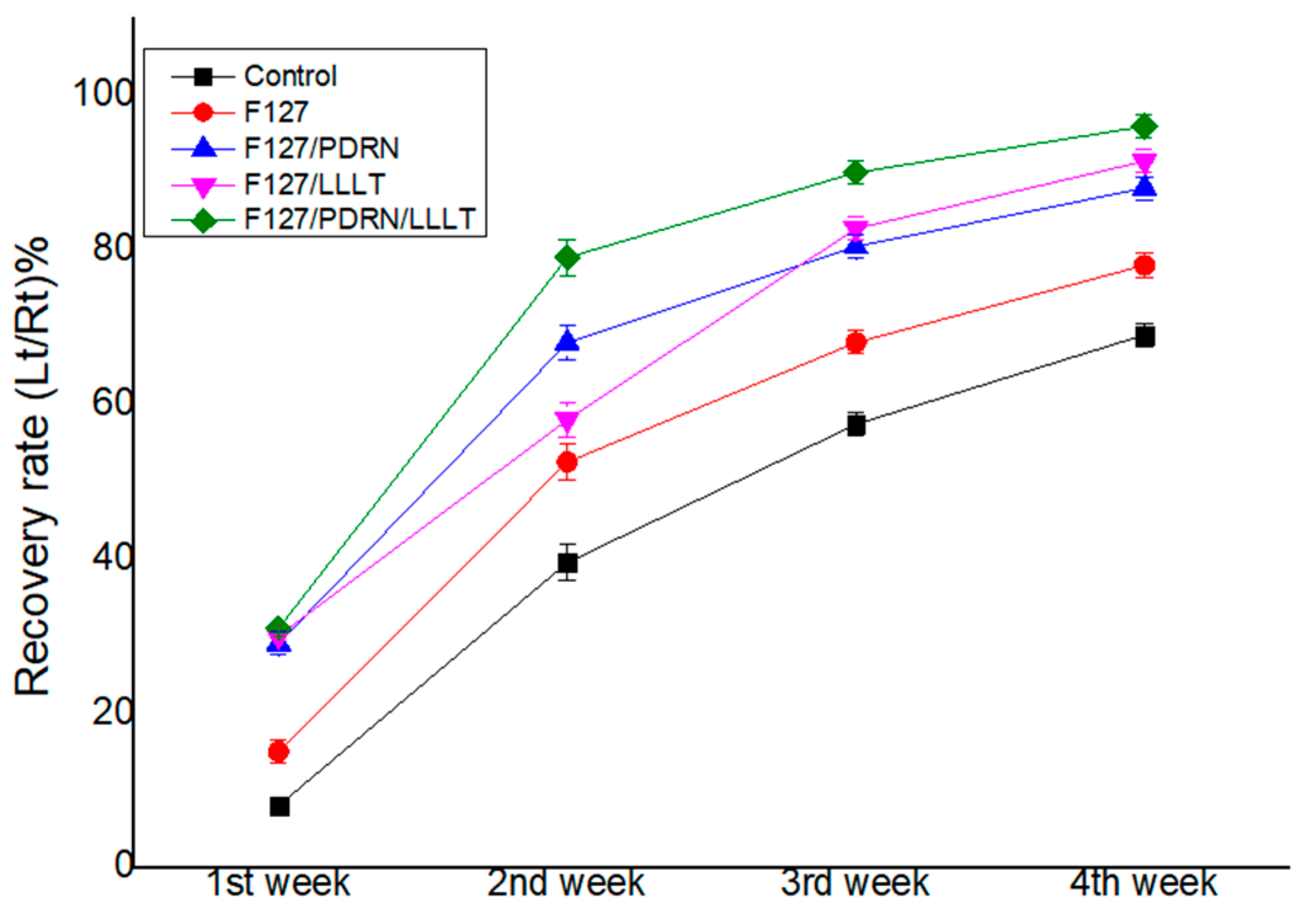
3.1. Recovery of Vibrissa Fibrillation
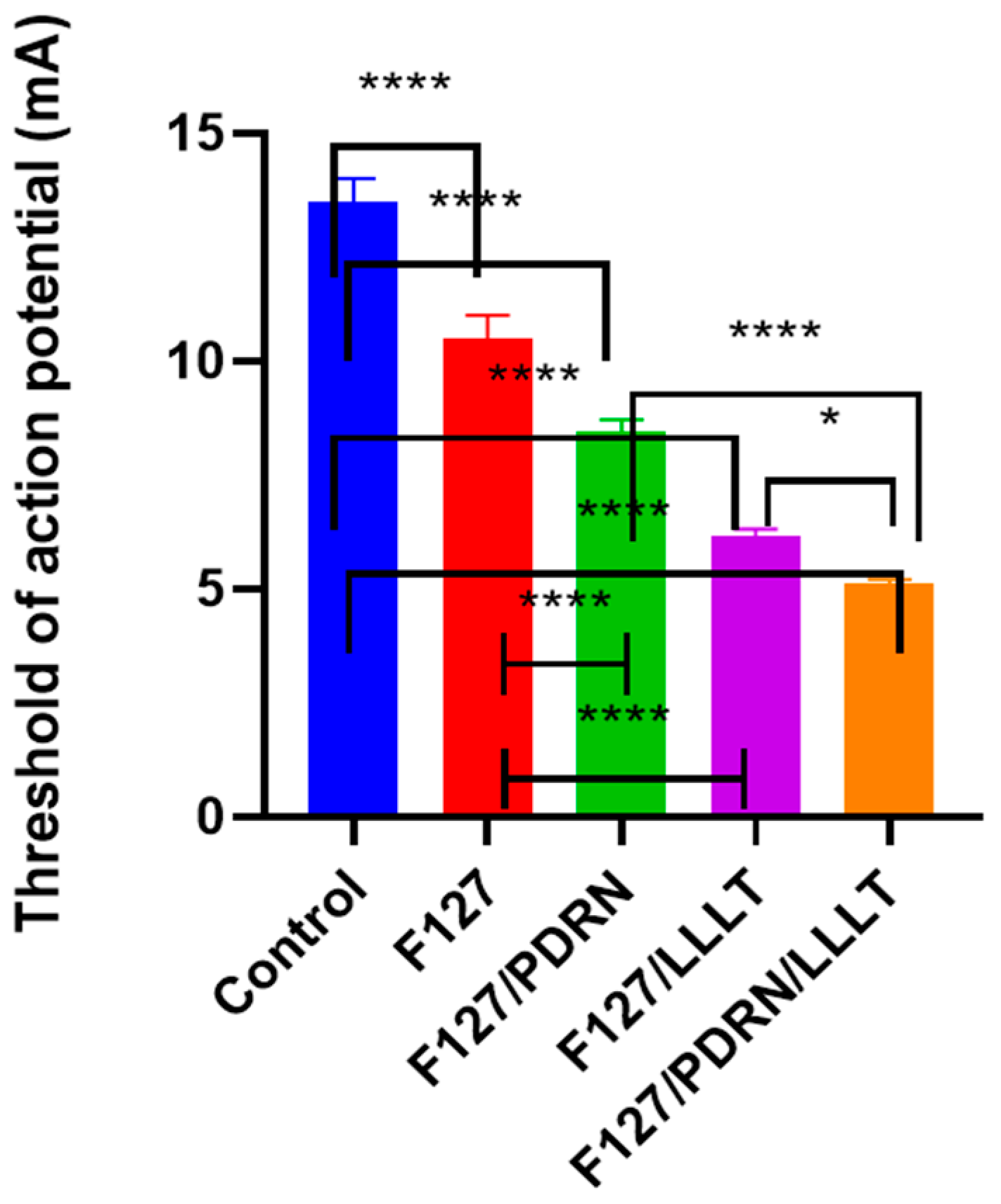
3.2. Recovery of Facial Muscle Action Potential
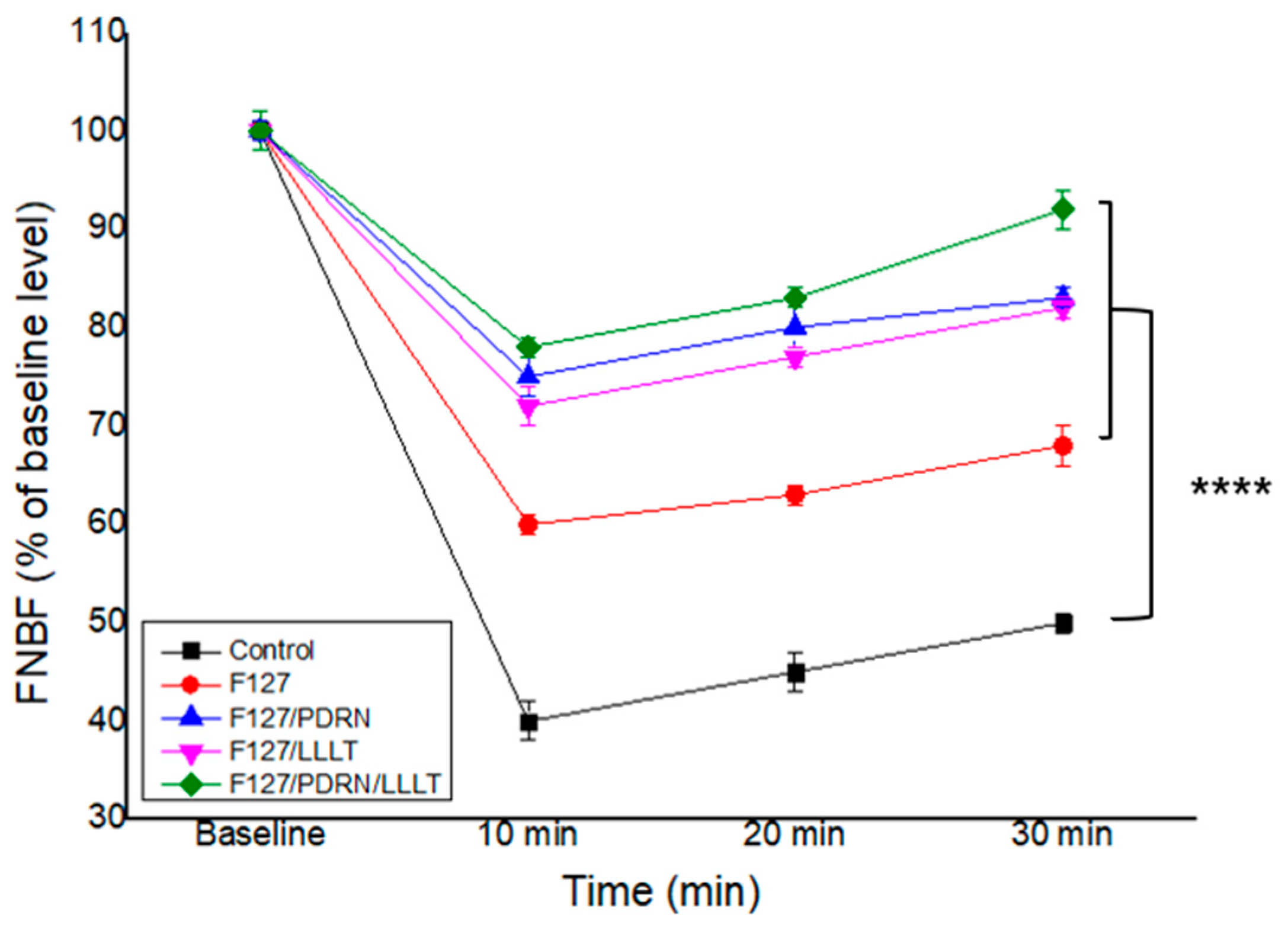
3.3. Recovery of Facial Nerve Blood Flow
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gordin, E.; Lee, T.S.; Ducic, Y.; Arnaoutakis, D. Facial nerve trauma: Evaluation and considerations in management. Craniomaxillofac. Trauma Reconstr. 2015, 8, 1–13. [Google Scholar] [CrossRef]
- Echternacht, S.R.; Chacon, M.A.; Leckenby, J.I. Central versus peripheral nervous system regeneration: Is there an exception for cranial nerves? Regen. Med. 2021, 16, 567–579. [Google Scholar] [CrossRef]
- Shen, C.C.; Yang, Y.C.; Huang, T.B.; Chan, S.C.; Liu, B.S. Low-Level Laser-Accelerated Peripheral Nerve Regeneration within a Reinforced Nerve Conduit across a Large Gap of the Transected Sciatic Nerve in Rats. Evid. Based Complement. Altern. Med. 2013, 2013, 175629. [Google Scholar] [CrossRef]
- Neves, M.; Tavares, A.L.F.; Reginato, A.; Kakihata, C.M.M.; Bertolini, G.R.F.; Ribeiro, L.F.C. Low-Level Laser Therapy in Different Wavelengths on the Tibialis Anterior Muscle of Wistar Rats After Nerve Compression Injury. J. Manip. Physiol. Ther. 2020, 43, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Liu, J.; Liu, Y.; Yu, C.; Bao, G.; Kang, H. Comparative effectiveness of low-level laser therapy with different wavelengths and transcutaneous electric nerve stimulation in the treatment of pain caused by temporomandibular disorders: A systematic review and network meta-analysis. J. Oral Rehabil. 2022, 49, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Ziago, E.K.M.; Fazan, V.P.S.; Iyomasa, M.M.; Sousa, L.G.; Yamauchi, P.Y.; da Silva, E.A.; Borie, E.; Fuentes, R.; Dias, F.J. Analysis of the variation in low-level laser energy density on the crushed sciatic nerves of rats: A morphological, quantitative, and morphometric study. Lasers Med. Sci. 2017, 32, 369–378. [Google Scholar] [CrossRef]
- Rosso, M.P.O.; Buchaim, D.V.; Kawano, N.; Furlanette, G.; Pomini, K.T.; Buchaim, R.L. Photobiomodulation Therapy (PBMT) in Peripheral Nerve Regeneration: A Systematic Review. Bioengineering 2018, 5, 44. [Google Scholar] [CrossRef] [PubMed]
- Buchaim, D.V.; Rodrigues, A.d.C.; Buchaim, R.L.; Barraviera, B.; Junior, R.S.F.; Junior, G.M.R.; Bueno, C.R.d.S.; Roque, D.D.; Dias, D.V.; Dare, L.R. The new heterologous fibrin sealant in combination with low-level laser therapy (LLLT) in the repair of the buccal branch of the facial nerve. Lasers Med. Sci. 2016, 31, 965–972. [Google Scholar] [CrossRef]
- Buchaim, D.V.; Andreo, J.C.; Ferreira Junior, R.S.; Barraviera, B.; Rodrigues, A.d.C.; Macedo, M.d.C.; Rosa Junior, G.M.; Shinohara, A.L.; Santos German, I.J.; Pomini, K.T. Efficacy of laser photobiomodulation on morphological and functional repair of the facial nerve. Photomed. Laser Surg. 2017, 35, 442–449. [Google Scholar] [CrossRef]
- Yuca, Y.; Yucesoy, T.; Tok, O.E.; Alkan, A. The efficiency of ozone therapy and low-level laser therapy in rat facial nerve injury. J. Cranio-Maxillofac. Surg. 2020, 48, 308–314. [Google Scholar] [CrossRef]
- Lee, S.Y.; Jeon, S.I.; Sim, S.B.; Byun, Y.; Ahn, C.H. A supramolecular host-guest interaction-mediated injectable hydrogel system with enhanced stability and sustained protein release. Acta Biomater. 2021, 131, 286–301. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, M.; Xia, K.; Wang, J.; Cheng, F.; Shi, K.; Ying, L.; Yu, C.; Xu, H.; Xiao, S.; et al. A bioactive injectable self-healing anti-inflammatory hydrogel with ultralong extracellular vesicles release synergistically enhances motor functional recovery of spinal cord injury. Bioact. Mater. 2021, 6, 2523–2534. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Khan, A.R.; Yu, D.; Zhai, Y.; Ji, J.; Shi, Y.; Zhai, G. Pluronic F127-functionalized molybdenum oxide nanosheets with pH-dependent degradability for chemo-photothermal cancer therapy. J. Colloid. Interface Sci. 2019, 553, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Qi, Z.; Pei, P.; Shen, W.; Zhang, Y.; Yang, K.; Sun, L.; Liu, T. Biocompatible nanomicelles for sensitive detection and photodynamic therapy of early-stage cancer. Biomater. Sci. 2021, 9, 6227–6235. [Google Scholar] [CrossRef]
- Diniz, I.M.; Chen, C.; Xu, X.; Ansari, S.; Zadeh, H.H.; Marques, M.M.; Shi, S.; Moshaverinia, A. Pluronic F-127 hydrogel as a promising scaffold for encapsulation of dental-derived mesenchymal stem cells. J. Mater. Sci. Mater. Med. 2015, 26, 153. [Google Scholar] [CrossRef]
- Chen, J.; Chen, L.; Wu, Y.; Fang, Y.; Zeng, F.; Wu, S.; Zhao, Y. A H2O2-activatable nanoprobe for diagnosing interstitial cystitis and liver ischemia-reperfusion injury via multispectral optoacoustic tomography and NIR-II fluorescent imaging. Nat. Commun. 2021, 12, 6870. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C.; Kan, C.W.; Wang, W. Dual-responsive (pH/temperature) Pluronic F-127 hydrogel drug delivery system for textile-based transdermal therapy. Sci. Rep. 2019, 9, 11658. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C.; Wat, E.; Kan, C.W.; Leung, P.C.; Wang, W. Drug delivery system of dual-responsive PF127 hydrogel with polysaccharide-based nano-conjugate for textile-based transdermal therapy. Carbohydr. Polym. 2020, 236, 116074. [Google Scholar] [CrossRef]
- Galeano, M.; Bitto, A.; Altavilla, D.; Minutoli, L.; Polito, F.; Calò, M.; Lo Cascio, P.; Stagno d’Alcontres, F.; Squadrito, F. Polydeoxyribonucleotide stimulates angiogenesis and wound healing in the genetically diabetic mouse. Wound Repair. Regen. 2008, 16, 208–217. [Google Scholar] [CrossRef]
- Bitto, A.; Galeano, M.; Squadrito, F.; Minutoli, L.; Polito, F.; Dye, J.F.; Clayton, E.A.; Calò, M.; Venuti, F.S.; Vaccaro, M.; et al. Polydeoxyribonucleotide improves angiogenesis and wound healing in experimental thermal injury. Crit. Care Med. 2008, 36, 1594–1602. [Google Scholar] [CrossRef]
- Minutoli, L.; Arena, S.; Bonvissuto, G.; Bitto, A.; Polito, F.; Irrera, N.; Arena, F.; Fragalà, E.; Romeo, C.; Nicotina, P.A.; et al. Activation of adenosine A2A receptors by polydeoxyribonucleotide increases vascular endothelial growth factor and protects against testicular damage induced by experimental varicocele in rats. Fertil. Steril. 2011, 95, 1510–1513. [Google Scholar] [CrossRef] [PubMed]
- Baek, A.; Kim, Y.; Lee, J.W.; Lee, S.C.; Cho, S.R. Effect of Polydeoxyribonucleotide on Angiogenesis and Wound Healing in an In Vitro Model of Osteoarthritis. Cell Transpl. Transplant. 2018, 27, 1623–1633. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.; Choi, J.; Jeong, W.; Kwon, S. Polydeoxyribonucleotide Improves Peripheral Tissue Oxygenation and Accelerates Angiogenesis in Diabetic Foot Ulcers. Arch. Plast. Surg. 2017, 44, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Hong, H.J.; Roh, H.; Lee, W.J. The Effect of Polydeoxyribonucleotide on Ischemic Rat Skin Flap Survival. Ann. Plast. Surg. 2015, 75, 84–90. [Google Scholar] [CrossRef]
- Cho, G.; Moon, C.; Maharajan, N.; Ang, M.J.; Kim, M.; Jang, C.H. Effect of Pre-Induced Mesenchymal Stem Cell-Coated Cellulose/Collagen Nanofibrous Nerve Conduit on Regeneration of Transected Facial Nerve. Int. J. Mol. Sci. 2022, 23, 7638. [Google Scholar] [CrossRef]
- Sun, K.H.; Choi, C.H.; Cho, G.W.; Jang, C.H. Effect of Metformin on the Functional and Electrophysiological Recovery of Crush Injury-Induced Facial Nerve Paralysis in Diabetic Rats. J. Pers. Med. 2023, 13, 1317. [Google Scholar] [CrossRef]
- Bengur, F.B.; Komatsu, C.; Fedor, C.N.; Loder, S.; Baker, J.S.; Totwani, A.; Irgebay, Z.; Nerone, W.V.; Solari, M.G.; Marra, K.G. Biodegradable Nerve Guide with Glial Cell Line-Derived Neurotrophic Factor Improves Recovery After Facial Nerve Injury in Rats. Facial Plast. Surg. Aesthet. Med. 2023, 25, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Li, L.; Kou, N.; Bai, Y.; Zhang, Y.; Lu, Y.; Gao, L.; Wang, F. Low level laser therapy promotes bone regeneration by coupling angiogenesis and osteogenesis. Stem Cell Res. Ther. 2021, 12, 432. [Google Scholar] [CrossRef]
- Galeano, M.; Pallio, G.; Irrera, N.; Mannino, F.; Bitto, A.; Altavilla, D.; Vaccaro, M.; Squadrito, G.; Arcoraci, V.; Colonna, M.R.; et al. Polydeoxyribonucleotide: A Promising Biological Platform to Accelerate Impaired Skin Wound Healing. Pharmaceuticals 2021, 14, 1103. [Google Scholar] [CrossRef]
- Baek, A.; Kim, M.; Kim, S.H.; Cho, S.-R.; Kim, H.J. Anti-inflammatory effect of DNA polymeric molecules in a cell model of osteoarthritis. Inflammation 2018, 41, 677–688. [Google Scholar] [CrossRef]
- Castellini, C.; Belletti, S.; Govoni, P.; Guizzardi, S. Anti inflammatory property of PDRN—An in vitro study on cultured macrophages. Adv. Biosci. Biotechnol. 2017, 8, 13–26. [Google Scholar] [CrossRef]
- Hwang, L.; Ko, I.G.; Jin, J.J.; Kim, S.H.; Kim, C.J.; Hwang, J.J.; Choi, C.W.; Chang, B.S. Attenuation effect of polydeoxyribonucleotide on inflammatory cytokines and apoptotic factors induced by particulate matter (PM10) damage in human bronchial cells. J. Biochem. Mol. Toxicol. 2021, 35, e22635. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Jung, J.; Hwang, L.; Ko, I.G.; Nam, O.H.; Kim, M.S.; Lee, J.W.; Choi, B.J.; Lee, D.W. Anti-inflammatory effect of polydeoxyribonucleotide on zoledronic acid-pretreated and lipopolysaccharide-stimulated RAW 264.7 cells. Exp. Ther. Med. 2018, 16, 400–405. [Google Scholar] [CrossRef]
- Buchaim, R.L.; Andreo, J.C.; Barraviera, B.; Junior, R.S.F.; Buchaim, D.V.; Junior, G.M.R.; de Oliveira, A.L.R.; de Castro Rodrigues, A. Effect of low-level laser therapy (LLLT) on peripheral nerve regeneration using fibrin glue derived from snake venom. Injury 2015, 46, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Rochkind, S. Phototherapy in peripheral nerve injury for muscle preservation and nerve regeneration. Photomed. Laser Surg. 2009, 27, 219–221. [Google Scholar] [CrossRef]
- Salama, A.H. PLURONIC F127 and ITS applications. Pharmacologyonline 2021, 2, 1393–1403. [Google Scholar]
- Pušnik, L.; Radochová, B.; Janáček, J.; Saudek, F.; Serša, I.; Cvetko, E.; Umek, N.; Snoj, Ž. Fascicle differentiation of upper extremity nerves on high-resolution ultrasound with multimodal microscopic verification. Sci. Rep. 2025, 15, 557. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, K.H.; Choi, C.H.; Jang, C.H. Synergistic Effect of Polydeoxyribonucleotides with Low-Level Lasers on the Regeneration of Crush-Injured Facial Nerves. J. Clin. Med. 2025, 14, 1678. https://doi.org/10.3390/jcm14051678
Sun KH, Choi CH, Jang CH. Synergistic Effect of Polydeoxyribonucleotides with Low-Level Lasers on the Regeneration of Crush-Injured Facial Nerves. Journal of Clinical Medicine. 2025; 14(5):1678. https://doi.org/10.3390/jcm14051678
Chicago/Turabian StyleSun, Kyung Hoon, Cheol Hee Choi, and Chul Ho Jang. 2025. "Synergistic Effect of Polydeoxyribonucleotides with Low-Level Lasers on the Regeneration of Crush-Injured Facial Nerves" Journal of Clinical Medicine 14, no. 5: 1678. https://doi.org/10.3390/jcm14051678
APA StyleSun, K. H., Choi, C. H., & Jang, C. H. (2025). Synergistic Effect of Polydeoxyribonucleotides with Low-Level Lasers on the Regeneration of Crush-Injured Facial Nerves. Journal of Clinical Medicine, 14(5), 1678. https://doi.org/10.3390/jcm14051678








