Physical Activity and Physical Function One Year After Hospital Discharge for COVID-19
Abstract
1. Introduction
2. Methods
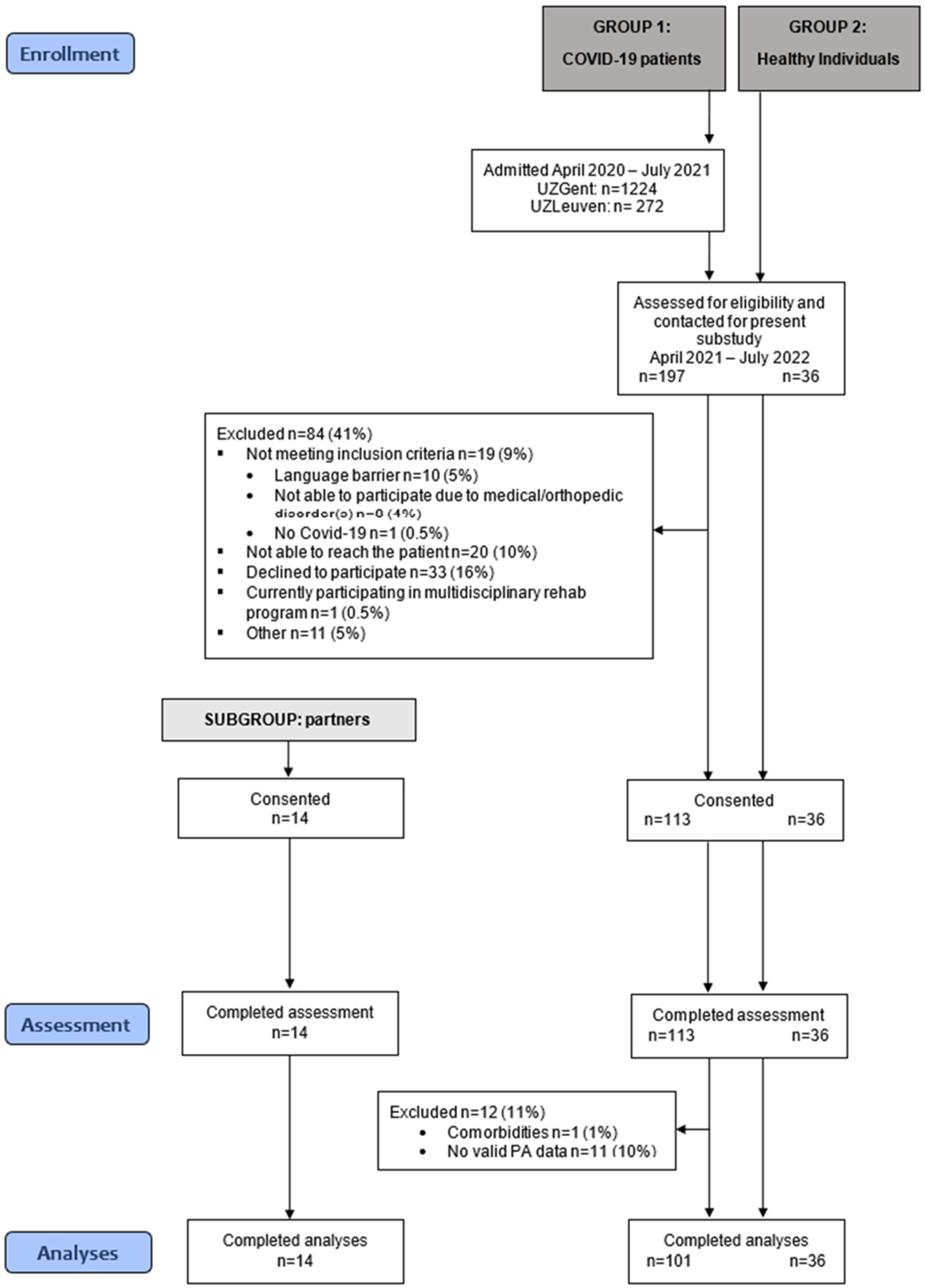
2.1. Study Design and Patient Population
2.2. Physical Activity Assessment
2.3. Clinical Outcomes and Subject Characterization
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Physical Activity
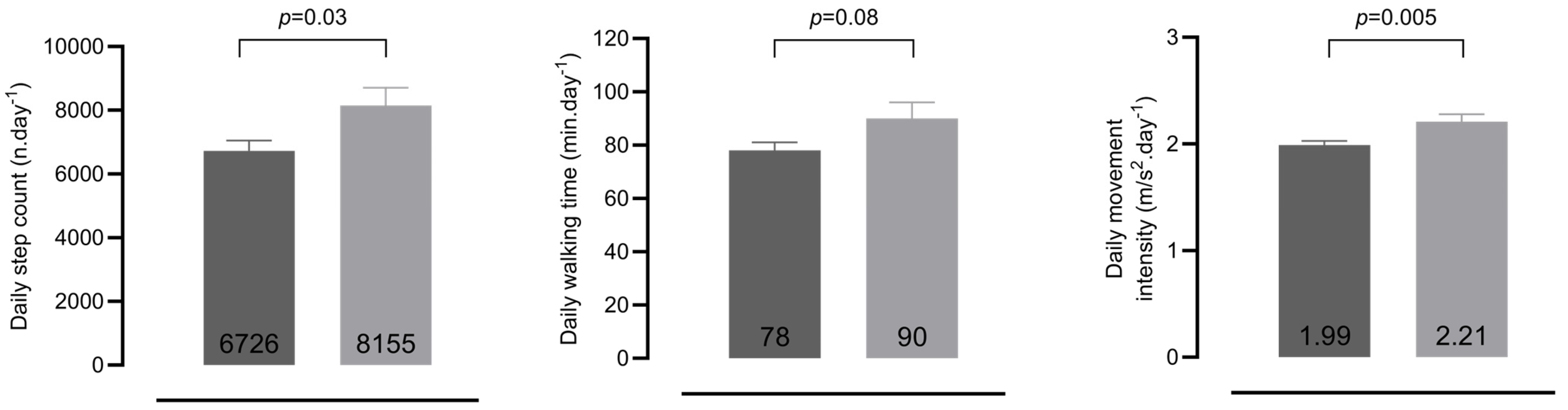
3.2.1. PA of Patients and Healthy Controls
3.2.2. PA of Patient–Partner Dyads (n = 14)
3.3. The Long-Term Impact of an ICU-Stay
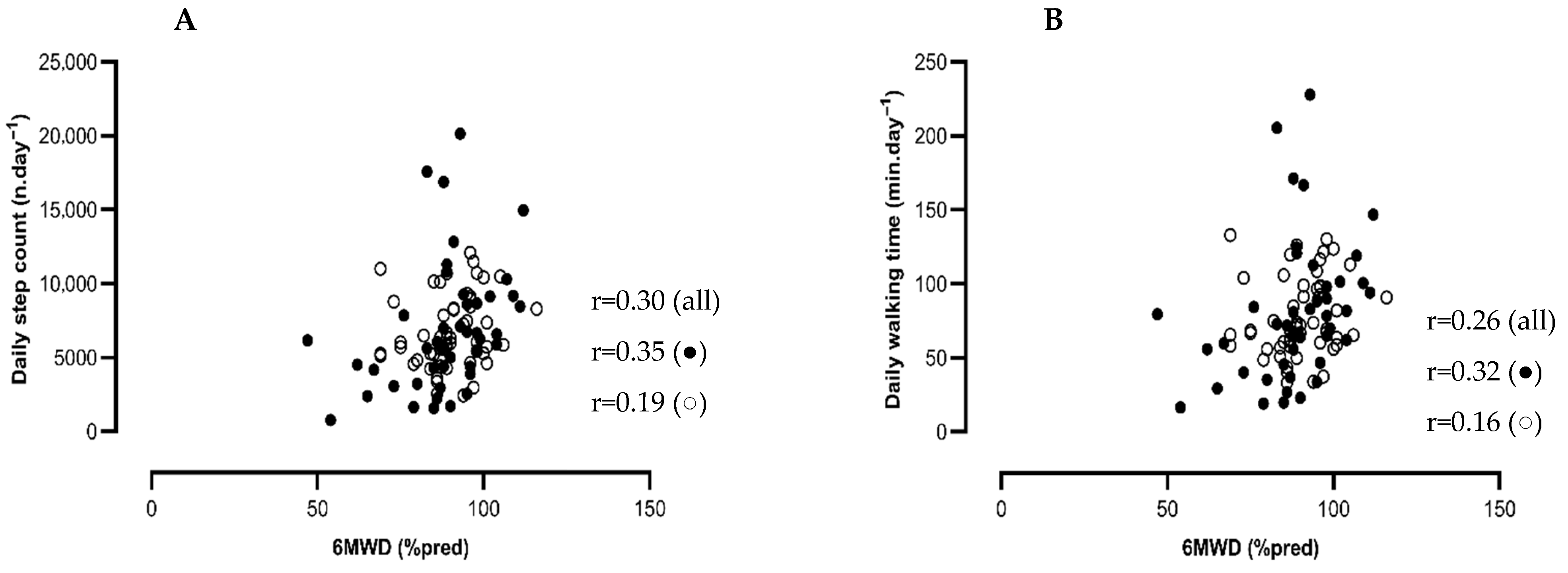
3.4. Associations Between Physical Function and Physical Activity in COVID-19 Patients
4. Discussion
5. Strength and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard Geneva: World Health Organization 2020. Available online: https://covid19.who.int/ (accessed on 13 September 2023).
- Jain, U. Effect of COVID-19 on the Organs. Cureus 2020, 12, e9540. [Google Scholar] [CrossRef] [PubMed]
- Belli, S.; Balbi, B.; Prince, I.; Cattaneo, D.; Masocco, F.; Zaccaria, S.; Bertalli, L.; Cattini, F.; Lomazzo, A.; Dal Negro, F.; et al. Low physical functioning and impaired performance of activities of daily life in COVID-19 patients who survived hospitalisation. Eur. Respir. J. 2020, 56, 2002096. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yao, Q.; Gu, X.; Wang, Q.; Ren, L.; Wang, Y.; Hu, P.; Guo, L.; Liu, M.; Xu, J.; et al. 1-year outcomes in hospital survivors with COVID-19: A longitudinal cohort study. Lancet 2021, 398, 747–758. [Google Scholar] [CrossRef]
- Seeßle, J.; Waterboer, T.; Hippchen, T.; Simon, J.; Kirchner, M.; Lim, A.; Müller, B.; Merle, U. Persistent Symptoms in Adult Patients 1 Year After Coronavirus Disease 2019 (COVID-19): A Prospective Cohort Study. Clin. Infect. Dis. 2022, 74, 1191–1198. [Google Scholar] [CrossRef]
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav. Immun. 2022, 101, 93–135. [Google Scholar] [CrossRef]
- Evans, R.A.; McAuley, H.; Harrison, E.M.; Shikotra, A.; Singapuri, A.; Sereno, M.; Elneima, O.; Docherty, A.B.; Lone, N.I.; Leavy, O.C.; et al. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): A UK multicentre, prospective cohort study. Lancet Respir. Med. 2021, 9, 1275–1287. [Google Scholar] [CrossRef]
- Alkodaymi, M.S.; Omrani, O.A.; Fawzy, N.A.; Shaar, B.A.; Almamlouk, R.; Riaz, M.; Obeidat, M.; Obeidat, Y.; Gerberi, D.; Taha, R.M.; et al. Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2022, 28, 657–666. [Google Scholar] [CrossRef]
- Zheng, C.; Chen, X.K.; Sit, C.H.; Liang, X.; Li, M.H.; Ma, A.C.; Wong, S.H. Effect of Physical Exercise-Based Rehabilitation on Long COVID: A Systematic Review and Meta-analysis. Med. Sci. Sports Exerc. 2024, 56, 143–154. [Google Scholar] [CrossRef]
- Delbressine, J.M.; Machado, F.V.C.; Goërtz, Y.M.J.; Van Herck, M.; Meys, R.; Houben-Wilke, S.; Burtin, C.; Franssen, F.M.E.; Spies, Y.; Vijlbrief, H.; et al. The Impact of Post-COVID-19 Syndrome on Self-Reported Physical Activity. Int. J. Environ. Res. Public Health 2021, 18, 6017. [Google Scholar] [CrossRef]
- Wright, J.; Astill, S.L.; Sivan, M. The Relationship between Physical Activity and Long COVID: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2022, 19, 5093. [Google Scholar] [CrossRef]
- Carter, S.J.; Baranauskas, M.N.; Raglin, J.S.; Pescosolido, B.A.; Perry, B.L. Functional Status, Mood State, and Physical Activity Among Women With Post-Acute COVID-19 Syndrome. Int. J. Public Health 2022, 67, 1604589. [Google Scholar] [CrossRef]
- Galluzzo, V.; Zazzara, M.B.; Ciciarello, F.; Tosato, M.; Martone, A.M.; Pais, C.; Savera, G.; Calvani, R.; Picca, A.; Marzetti, E.; et al. Inadequate Physical Activity Is Associated with Worse Physical Function in a Sample of COVID-19 Survivors with Post-Acute Symptoms. J. Clin. Med. 2023, 12, 2517. [Google Scholar] [CrossRef]
- Poppele, I.; Ottiger, M.; Stegbauer, M.; Schlesinger, T.; Müller, K. Device-assessed physical activity and sleep quality of post-COVID patients undergoing a rehabilitation program. BMC Sports Sci. Med. Rehabil. 2024, 16, 122. [Google Scholar] [CrossRef]
- Plekhanova, T.; Rowlands, A.V.; Evans, R.A.; Edwardson, C.L.; Bishop, N.C.; Bolton, C.E.; Chalmers, J.D.; Davies, M.J.; Daynes, E.; Dempsey, P.C.; et al. Device-assessed sleep and physical activity in individuals recovering from a hospital admission for COVID-19: A multicentre study. Int. J. Behav. Nutr. Phys. Act. 2022, 19, 94. [Google Scholar] [CrossRef]
- Çelik, Z.; Kafa, N.; Güzel, N.A.; Köktürk, N. The effects of physical activity tele-counseling intervention on physical activity, functional performance, and quality of life in post-COVID-19 conditions: A randomized controlled trial. Expert Rev. Respir. Med. 2024, 18, 321–331. [Google Scholar] [CrossRef]
- van Bakel, B.M.A.; van den Heuvel, F.M.A.; Vos, J.L.; Rotbi, H.; Bakker, E.A.; Nijveldt, R.; Thijssen, D.H.J.; Eijsvogels, T.M.H. High Levels of Sedentary Time in Patients with COVID-19 after Hospitalisation. J. Clin. Med. 2022, 11, 1110. [Google Scholar] [CrossRef] [PubMed]
- Diciolla, N.S.; Ampuero-López, A.; Marques, A.; Jiménez-Martín, A.; García-De Villa, S.; Torres-Lacomba, M.; Yuste-Sánchez, M.J. Physical Activity and Sedentary Behaviour in People with Long COVID: A Follow-Up from 12 to 18 Months After Discharge. J. Clin. Med. 2025, 14, 3641. [Google Scholar] [CrossRef] [PubMed]
- Tryfonos, A.; Pourhamidi, K.; Jörnåker, G.; Engvall, M.; Eriksson, L.; Elhallos, S.; Asplund, N.; Mandic, M.; Sundblad, P.; Sepic, A.; et al. Functional Limitations and Exercise Intolerance in Patients With Post-COVID Condition: A Randomized Crossover Clinical Trial. JAMA Netw. Open 2024, 7, e244386. [Google Scholar] [CrossRef] [PubMed]
- Freire, A.; Lira, F.S.; Morano, A.; Pereira, T.; Coelho, E.S.M.J.; Caseiro, A.; Christofaro, D.G.D.; Marchioto Júnior, O.; Dorneles, G.P.; Minuzzi, L.G.; et al. Role of Body Mass and Physical Activity in Autonomic Function Modulation on Post-COVID-19 Condition: An Observational Subanalysis of Fit-COVID Study. Int. J. Environ. Res. Public Health 2022, 19, 2457. [Google Scholar] [CrossRef]
- Fresenko, L.E.; Rivera, Z.C.; Parry, S.M.; Mayer, K.P. Post-Intensive Care Syndrome: Physical Impairments and Function. Crit. Care Clin. 2025, 41, 1–20. [Google Scholar] [CrossRef]
- Lorent, N.; Vande Weygaerde, Y.; Claeys, E.; Guler Caamano Fajardo, I.; De Vos, N.; De Wever, W.; Salhi, B.; Gyselinck, I.; Bosteels, C.; Lambrecht, B.N.; et al. Prospective longitudinal evaluation of hospitalised COVID-19 survivors 3 and 12 months after discharge. ERJ Open Res. 2022, 8, 00004-2022. [Google Scholar] [CrossRef] [PubMed]
- Gore, S.; Blackwood, J.; Guyette, M.; Alsalaheen, B. Validity and Reliability of Accelerometers in Patients With COPD: A Systematic Review. J. Cardiopulm. Rehabil. Prev. 2018, 38, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Demeyer, H.; Mohan, D.; Burtin, C.; Vaes, A.W.; Heasley, M.; Bowler, R.P.; Casaburi, R.; Cooper, C.B.; Corriol-Rohou, S.; Frei, A.; et al. Objectively Measured Physical Activity in Patients with COPD: Recommendations from an International Task Force on Physical Activity. Chronic Obstr. Pulm. Dis. 2021, 8, 528–550. [Google Scholar] [CrossRef] [PubMed]
- Pitta, F.; Troosters, T.; Spruit, M.A.; Probst, V.S.; Decramer, M.; Gosselink, R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2005, 171, 972–977. [Google Scholar] [CrossRef]
- Tudor-Locke, C.; Craig, C.L.; Brown, W.J.; Clemes, S.A.; De Cocker, K.; Giles-Corti, B.; Hatano, Y.; Inoue, S.; Matsudo, S.M.; Mutrie, N.; et al. How many steps/day are enough? For adults. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 79. [Google Scholar] [CrossRef]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef]
- Heyns, A.; Dupont, J.; Gielen, E.; Flamaing, J.; Peers, K.; Gosselink, R.; Vrijsen, B.; Lorent, N.; Everaerts, S.; Janssens, W.; et al. Impact of COVID-19: Urging a need for multi-domain assessment of COVID-19 inpatients. Eur. Geriatr. Med. 2021, 12, 741–748. [Google Scholar] [CrossRef]
- Nellessen, A.G.; Donária, L.; Hernandes, N.A.; Pitta, F. Analysis of three different equations for predicting quadriceps femoris muscle strength in patients with COPD. J. Bras. Pneumol. 2015, 41, 305–312. [Google Scholar] [CrossRef]
- Ware, J.E., Jr. SF-36 health survey update. Spine 2000, 25, 3130–3139. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Sallis, R.; Young, D.R.; Tartof, S.Y.; Sallis, J.F.; Sall, J.; Li, Q.; Smith, G.N.; Cohen, D.A. Physical inactivity is associated with a higher risk for severe COVID-19 outcomes: A study in 48 440 adult patients. Br. J. Sports Med. 2021, 55, 1099–1105. [Google Scholar] [CrossRef]
- Blondeel, A.; Demeyer, H.; Janssens, W.; Troosters, T. Accuracy of consumer-based activity trackers as measuring tool and coaching device in patients with COPD and healthy controls. PLoS ONE 2020, 15, e0236676. [Google Scholar] [CrossRef]
- Wendel-Vos, W.; Droomers, M.; Kremers, S.; Brug, J.; van Lenthe, F. Potential environmental determinants of physical activity in adults: A systematic review. Obes. Rev. 2007, 8, 425–440. [Google Scholar] [CrossRef]
- Ahmed, H.; Patel, K.; Greenwood, D.C.; Halpin, S.; Lewthwaite, P.; Salawu, A.; Eyre, L.; Breen, A.; O’Connor, R.; Jones, A.; et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: A systematic review and meta-analysis. J. Rehabil. Med. 2020, 52, jrm00063. [Google Scholar] [CrossRef] [PubMed]
- Vanhorebeek, I.; Latronico, N.; Van den Berghe, G. ICU-acquired weakness. Intensive Care Med. 2020, 46, 637–653. [Google Scholar] [CrossRef]
- Blondeel, A.; Hermans, F.; Breuls, S.; Wuyts, M.; Everaerts, S.; Gyselinck, I.; De Maeyer, N.; Verniest, T.; Derom, E.; Janssens, W.; et al. Factors associated to physical activity in patients with COPD: An ecological approach. Respir. Med. 2023, 219, 107424. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Aymerich, J.; Serra, I.; Gómez, F.P.; Farrero, E.; Balcells, E.; Rodríguez, D.A.; de Batlle, J.; Gimeno, E.; Donaire-Gonzalez, D.; Orozco-Levi, M.; et al. Physical activity and clinical and functional status in COPD. Chest 2009, 136, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Osadnik, C.R.; Loeckx, M.; Louvaris, Z.; Demeyer, H.; Langer, D.; Rodrigues, F.M.; Janssens, W.; Vogiatzis, I.; Troosters, T. The likelihood of improving physical activity after pulmonary rehabilitation is increased in patients with COPD who have better exercise tolerance. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 3515–3527. [Google Scholar] [CrossRef]
- Manocchio, N.; Ljoka, C.; Buttarelli, L.; Giordan, L.; Sorbino, A.; Foti, C. Early motor and respiratory re-education in patients hospitalized for COVID-19. Adv. Rehabil. 2025, 39, 29–45. [Google Scholar] [CrossRef]
- Daynes, E.; Evans, R.A.; Greening, N.J.; Bishop, N.C.; Yates, T.; Lozano-Rojas, D.; Ntotsis, K.; Richardson, M.; Baldwin, M.M.; Hamrouni, M.; et al. Post-Hospitalisation COVID-19 Rehabilitation (PHOSP-R): A randomised controlled trial of exercise-based rehabilitation. Eur. Respir. J. 2025, 65, 2402152. [Google Scholar] [CrossRef]
- Carta, M.G.; Orrù, G.; Littera, R.; Firinu, D.; Chessa, L.; Cossu, G.; Primavera, D.; Del Giacco, S.; Tramontano, E.; Manocchio, N.; et al. Comparing the responses of countries and National Health Systems to the COVID-19 pandemic: A critical analysis with a case-report series. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 7868–7880. [Google Scholar]
| Characteristics | Patients (n = 101) | Healthy Controls (n = 36) | p-Value |
|---|---|---|---|
| Sex (n (% male)) | 70 (69) | 29 (58) | 0.17 |
| Age (years) | 60 ± 10 | 60 ± 9 | 0.71 |
| BMI (kg/m2) | 28.7 (26.4; 32.8) | 24.8 (23.6; 29.0) | <0.001 |
| Active smoker (n (%)) | 3 (3) | 5 (14) | 0.02 |
| Work status (n (%)) | 0.75 | ||
| 59 (58) | 20 (59) | |
| 10 (10) | 2 (6) | |
| 32 (32) | 12 (35) | |
| Season of inclusion (n (%)) | 0.10 | ||
| 19 (19) | 9 (25) | |
| 35 (35) | 5 (14) | |
| 13 (13) | 4 (11) | |
| 34 (34) | 18 (50) |
| Characteristics | Patients (n = 14) | Partner Healthy Controls (n = 14) | p-Value |
|---|---|---|---|
| Sex (n (% male)) | 11 (79) | 3 (21) | 0.002 |
| Age (years) | 58 ± 9 | 55 ± 8 | 0.36 |
| BMI (kg/m2) | 27.7 (24.7; 30.5) | 28.2 (22.5; 32.0) | 0.86 |
| Active smoker (n (%)) | 0 (0) | 2 (14) | 0.14 |
| Work status (n (%)) | 0.63 | ||
| 8 (57) | 9 (64) | |
| 2 (14) | 3 (21) | |
| 4 (28) | 2 (14) |
| All Patients | ICU | Non-ICU | Absolute Mean Difference (ICU—Non-ICU) | 95% CI | p-Value * | |
|---|---|---|---|---|---|---|
| N (n (%)) | 101 (100) | 45 (45) | 56 (55) | |||
| Male (n (%)) | 70 (71) | 39 (87) | 31 (55) | <0.001 | ||
| Clinical characteristics | ||||||
| Age (years) | 60 ± 10 | 62 ± 8 | 59 ± 10 | 0.07 | ||
| BMI (kg/m2) | 28.6 (26.4; 32.8) | 30.3 (27.2; 35.1) | 28.1 (25.3; 30.9) | 0.02 | ||
| FEV1 < 80%pred (n (%)) | 8 (8) | 5 (11) | 3 (5) | 0.29 | ||
| DLCO < 80%pred (n (%)) | 31 (31) | 19 (42) | 12 (22) | 0.03 | ||
| LOS (days) | 11.0 (8.0; 21.5) | 23.5 (12.0; 39.0) | 8.5 (6.0; 10.3) | <0.001 | ||
| NIVM (n (%)) | 55 (56) | 30 (68.2) | 25 (46) | 0.02 | ||
| NIVM (days) | 7 (5; 11) | 9 (5; 15) | 7 (5; 10) | 0.14 | ||
| IVM (n (%)) | 26 (27) | 26 (61) | 0 | <0.001 | ||
| Rehabilitation (n (%)) | 42 (42) | 22 (49) | 20 (36) | 0.13 | ||
| Physical activity | ||||||
| Daily step count (n.day−1) | 6800 ± 337 | 6759 ± 451 | 6841 ± 503 | −82 | −268 to 104 | 0.90 |
| <7500 daily step count (n, %) | 68 (67) | 30 (67) | 38 (68) | 0.90 | ||
| Daily walking time (min.day−1) | 78 ± 4 | 80 ± 5 | 77 ± 5 | −3.6 | −18.5 to 11.3 | 0.76 |
| Daily MI during walking (m/s2.day−1) | 1.99 ± 0.04 | 1.92 ± 0.05 | 2.04 ± 0.47 | −0.12 | −0.40 to 0.17 | 0.09 |
| Physical function | ||||||
| 6 MWD (m) | 591 ± 108 | 582 ± 119 | 599 ± 98 | −17 | −61 to 27 | 0.44 |
| 6 MWD ≤ 70%pred (n, %) | 8 (8) | 5 (11) | 3 (6) | 0.30 | ||
| QF (Nm/kg) | 1.70 ± 0.50 | 1.66 ± 0.49 | 1.73 ± 0.50 | −0.07 | −0.28 to 0.14 | 0.51 |
| QF ≤ 70%pred (n, %) | 24 (27) | 13 (32) | 11 (23) | 0.35 | ||
| HGF (kg) | 42 ± 14 | 43 ± 11 | 41 ± 16 | 2.0 | −2.7 to 6.7 | 0.38 |
| HGF ≤ 70%pred (n, %) | 6 (6) | 3 (7) | 3 (6) | 0.78 | ||
| Symptom experience | ||||||
| mMRC dyspnea score < 2 (n (%)) | 66 (84) | 30 (83) | 36 (84) | 0.60 | ||
| HADS Anxiety (score) | 4 (1.0; 7.0) | 3.0 (1.3; 7.0) | 4.0 (1.0; 7.0) | −1.0 | −1.4 to −0.6 | 0.88 |
| HADS Depression (score) | 2.0 (0.0; 4.3) | 2.0 (0.0; 5.0) | 2.0 (0.0; 4.0) | 0.0 | −0.2 to 0.2 | 0.92 |
| SF-36 | ||||||
| - Physical component (score) | 47 ± 10 | 1 | 47 ± 11 | 0 | −3.9 to 3.9 | 0.93 |
| - Mental component (score) | 50 ± 9 | 1 | 51 ± 9 | 2 | −1.8 to 5.8 | 0.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arents, E.; Hermans, F.; Glorie, L.; Salhi, B.; Bosteels, C.; Derom, E.; Janssens, W.; Van Braeckel, E.; Lorent, N.; Vande Weygaerde, Y.; et al. Physical Activity and Physical Function One Year After Hospital Discharge for COVID-19. J. Clin. Med. 2025, 14, 6206. https://doi.org/10.3390/jcm14176206
Arents E, Hermans F, Glorie L, Salhi B, Bosteels C, Derom E, Janssens W, Van Braeckel E, Lorent N, Vande Weygaerde Y, et al. Physical Activity and Physical Function One Year After Hospital Discharge for COVID-19. Journal of Clinical Medicine. 2025; 14(17):6206. https://doi.org/10.3390/jcm14176206
Chicago/Turabian StyleArents, Eva, Fien Hermans, Lies Glorie, Bihiyga Salhi, Cedric Bosteels, Eric Derom, Wim Janssens, Eva Van Braeckel, Natalie Lorent, Yannick Vande Weygaerde, and et al. 2025. "Physical Activity and Physical Function One Year After Hospital Discharge for COVID-19" Journal of Clinical Medicine 14, no. 17: 6206. https://doi.org/10.3390/jcm14176206
APA StyleArents, E., Hermans, F., Glorie, L., Salhi, B., Bosteels, C., Derom, E., Janssens, W., Van Braeckel, E., Lorent, N., Vande Weygaerde, Y., Troosters, T., & Demeyer, H. (2025). Physical Activity and Physical Function One Year After Hospital Discharge for COVID-19. Journal of Clinical Medicine, 14(17), 6206. https://doi.org/10.3390/jcm14176206





