Evaluation of the Diagnostic Performance of the Brush/Biopsy Rapid On-Site Evaluation (B-ROSE) in Cases of Bile Duct Stricture: A Prospective, Pilot Study
Abstract
1. Introduction
2. Materials and Methods
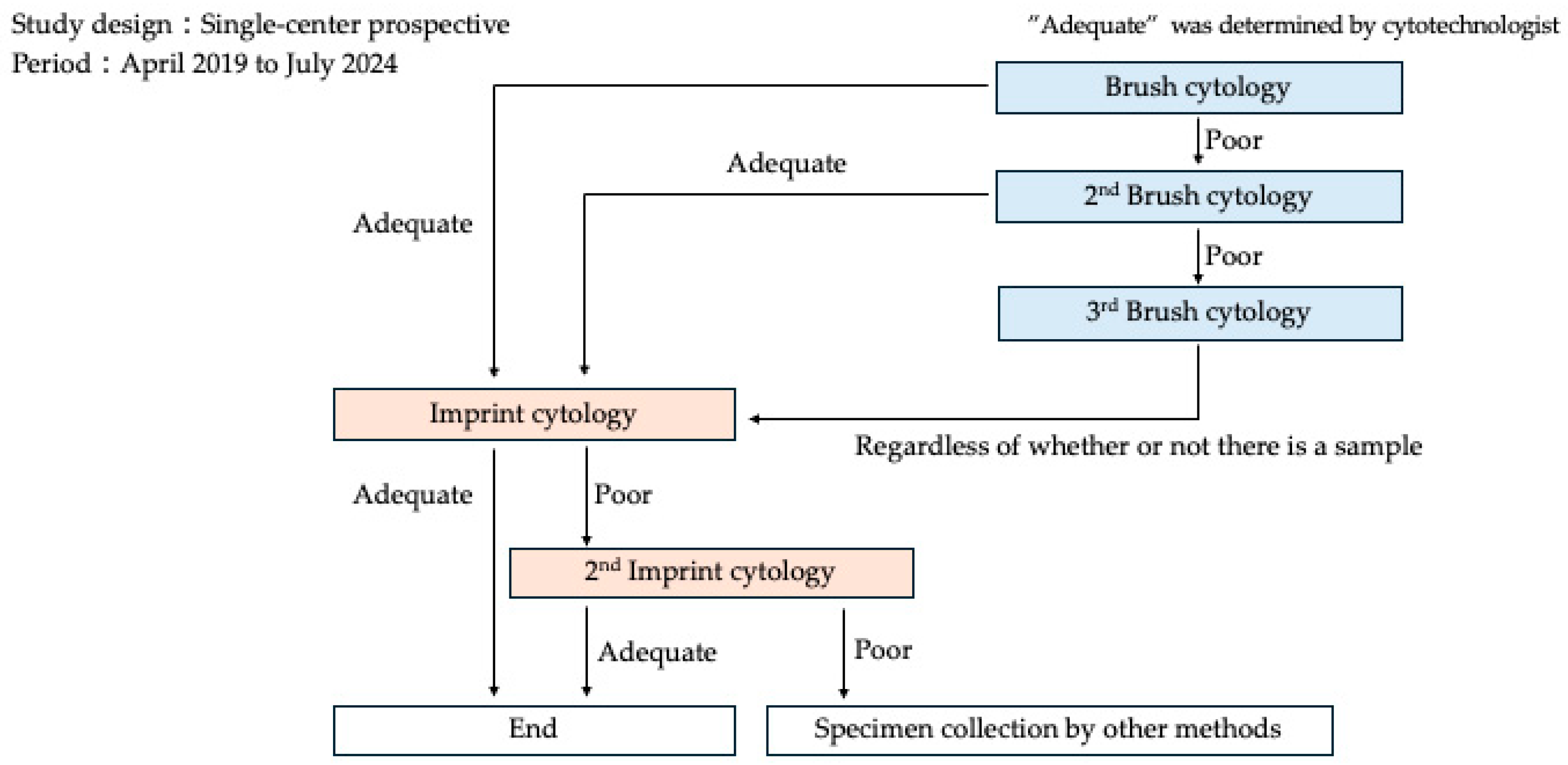
2.1. Study Design
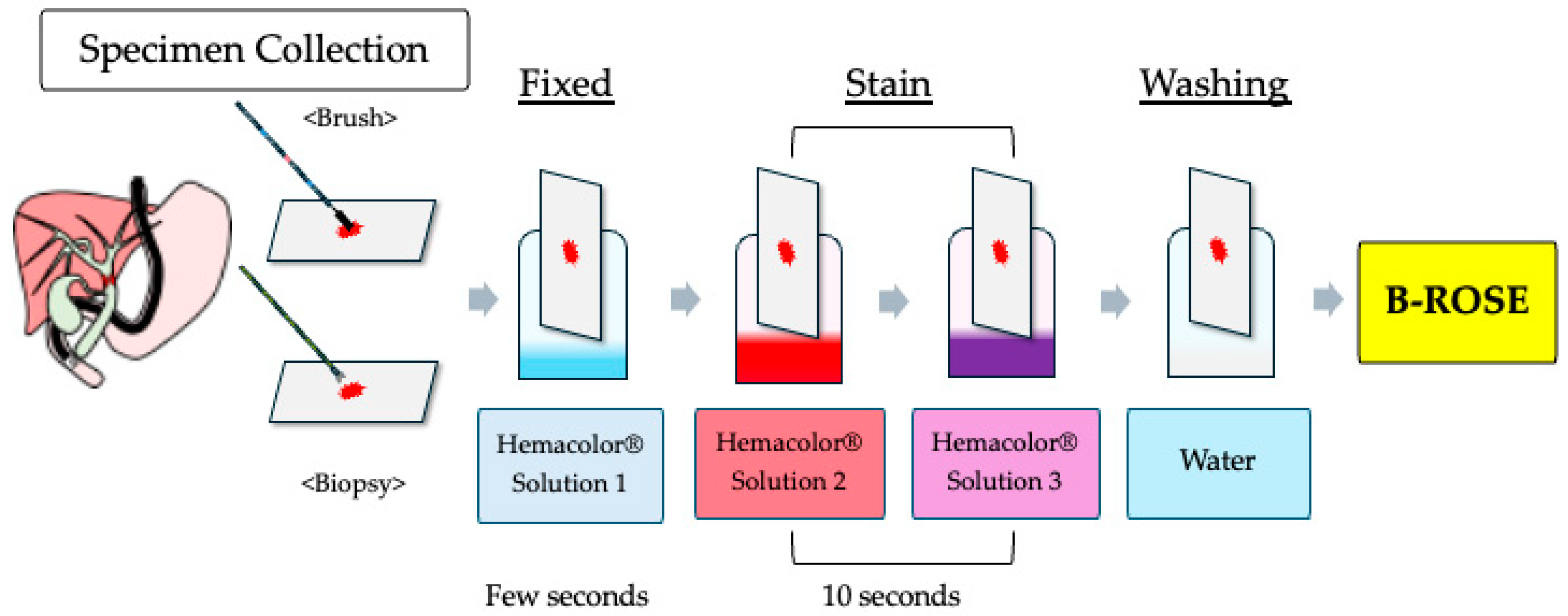
2.2. Study Procedure
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Specimen Collection and Diagnostic Accuracy
3.3. Adverse Events
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ERCP | endoscopic retrograde cholangiopancreatography |
| ROSE | rapid on-site evaluation |
| EUS-FNA | endoscopic ultrasound-guided fine needle aspiration |
| B-ROSE | brush/biopsy rapid on-site evaluation |
| POCS | peroral cholangioscopy |
| ENBD | endoscopic nasobiliary drainage |
| ROSE-TIC | ROSE for touch imprint cytology |
| EST | endoscopic sphincterotomy |
References
- Glasbrenner, B.; Ardan, M.; Boeck, W.; Preclik, G.; Möller, P.; Adler, G. Prospective evaluation of brush cytology of biliary strictures during endoscopic retrograde cholangiopancreatography. Endoscopy 1999, 31, 712–717. [Google Scholar] [CrossRef]
- Harewood, G.C.; Baron, T.H.; Stadheim, L.M.; Kipp, B.R.; Sebo, T.J.; Salomao, D.R. Prospective, blinded assessment of factors influencing the accuracy of biliary cytology interpretation. Am. J. Gastroenterol. 2004, 99, 1464–1469. [Google Scholar] [CrossRef]
- Kocjan, G.; Smith, A.N. Bile duct brushings cytology: Potential pitfalls in diagnosis. Diagn. Cytopathol. 1997, 16, 358–363. [Google Scholar] [CrossRef]
- Navaneethan, U.; Njei, B.; Lourdusamy, V.; Konjeti, R.; Vargo, J.J.; Parsi, M.A. Comparative effectiveness of biliary brush cytology and intraductal biopsy for detection of malignant biliary strictures: A systematic review and meta-analysis. Gastrointest. Endosc. 2015, 81, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Osanai, M.; Itoi, T.; Igarashi, Y.; Tanaka, K.; Kida, M.; Maguchi, H.; Yasuda, K.; Okano, N.; Imaizumi, H.; Itokawa, F. Peroral video cholangioscopy to evaluate indeterminate bile duct lesions and preoperative mucosal cancerous extension: A prospective multicenter study. Endoscopy 2013, 45, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Korrapati, P.; Ciolino, J.; Wani, S.; Shah, J.; Watson, R.; Muthusamy, V.R.; Klapman, J.; Komanduri, S. The efficacy of peroral cholangioscopy for difficult bile duct stones and indeterminate strictures: A systematic review and meta-analysis. Endosc. Int. Open 2016, 4, E263. [Google Scholar] [CrossRef] [PubMed]
- Manta, R.; Frazzoni, M.; Conigliaro, R.; Maccio, L.; Melotti, G.; Dabizzi, E.; Bertani, H.; Manno, M.; Castellani, D.; Villanacci, V.; et al. SpyGlass single-operator peroral cholangioscopy in the evaluation of indeterminate biliary lesions: A single-center, prospective, cohort study. Surg. Endosc. 2013, 27, 1569–1572. [Google Scholar] [CrossRef]
- Ramchandani, M.; Reddy, D.N.; Lakhtakia, S.; Tandan, M.; Maydeo, A.; Chandrashekhar, T.S.; Kumar, A.; Sud, R.; Rerknimitr, R.; Makmun, D.; et al. Per oral cholangiopancreatoscopy in pancreatico biliary diseases: Expert consensus statements. World J. Gastroenterol. 2015, 21, 4722–4734. [Google Scholar] [CrossRef][Green Version]
- Kalaitzakis, E.; Webster, G.J.; Oppong, K.W.; Kallis, Y.; Vlavianos, P.; Huggett, M.; Dawwas, M.F.; Lekharaju, V.; Hatfield, A.; Westaby, D.; et al. Diagnostic and therapeutic utility of single-operator peroral cholangioscopy for indeterminate biliary lesions and bile duct stones. Eur. J. Gastroenterol. Hepatol. 2012, 24, 656–664. [Google Scholar] [CrossRef]
- Matsubayashi, H.; Matsui, T.; Yabuuchi, Y.; Imai, K.; Tanaka, M.; Kakushima, N.; Sasaki, K.; Ono, H. Endoscopic ultrasonography guided-fine needle aspiration for the diagnosis of solid pancreaticobiliary lesions: Clinical aspects to improve the diagnosis. World J. Gastroenterol. 2016, 22, 628. [Google Scholar] [CrossRef]
- Eloubeidi, M.; Chen, V.; Jhala, N.; Eltoum, I.E.; Jhala, D.; Chhieng, D.C.; Syed, S.A.; Vickers, S.M.; Wilcox, C.M. Endoscopic ultrasound-guided fine needle aspiration biopsy of suspected cholangiocarcinoma. Clin. Gastroenterol. Hepatol. 2004, 2, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Onda, S.; Ogura, T.; Kurisu, Y.; Masuda, D.; Sano, T.; Takagi, W.; Fukunishi, S.; Higuchi, K. EUS-guided FNA for biliary disease as first-line modality to obtain histological evidence. Ther. Adv. Gastroenterol. 2016, 9, 302. [Google Scholar] [CrossRef]
- Ohshima, Y.; Yasuda, I.; Kawakami, H.; Kuwatani, M.; Mukai, T.; Iwashita, T.; Doi, S.; Nakashima, M.; Hirose, Y.; Asaka, M.; et al. EUS-FNA for suspected malignant biliary strictures after negative endoscopic transpapillary brush cytology and forceps biopsy. J. Gastroenterol. 2011, 46, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, H.; Kuwatani, M.; Onodera, M.; Haba, S.; Eto, K.; Ehira, N.; Yamato, H.; Kudo, T.; Tanaka, E.; Hirano, S.; et al. Endoscopic nasobiliary drainage is the most suitable preoperative biliary drainage method in the management of patients with hilar cholangiocarcinoma. J. Gastroenterol. 2011, 46, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, H.; Itoh, A.; Ohno, E.; Itoh, Y.; Ebata, T.; Nagino, M.; Goto, H.; Hirooka, Y. Preoperative endoscopic nasobiliary drainage in 164 consecutive patients with suspected perihilar cholangiocarcinoma: A retrospective study of efficacy and risk factors related to complications. Ann. Surg. 2013, 257, 121–127. [Google Scholar] [CrossRef]
- Klapman, J.B.; Logrono, R.; Dye, C.E.; Waxman, I. Clinical impact of on-site cytopathology interpretation on endoscopic ultrasound-guided fine needle aspiration. Am. J. Gastroenterol. 2003, 98, 1289–1294. [Google Scholar] [CrossRef]
- Dudgeon, L.S.; Patrick, C.V. A new method for the rapid microscopical diagnosis of tumours: With an account of 200 cases so examined. Br. J. Surg. 1927, 15, 250–261. [Google Scholar] [CrossRef]
- Ali, S.; Hawes, R.; Kadkhodayan, K.; Rafiq, E.; Navaneethan, U.; Bang, J.Y.; Varadarajulu, S.; Hasan, M.K. Utility of rapid onsite evaluation of touch imprint cytology from endoscopic and cholangioscopic forceps biopsy sampling (with video). Gastrointest. Endosc. 2019, 89, 340–344. [Google Scholar] [CrossRef]
- Anderson, M.A.; Appalaneni, V.; Ben-Menachem, T.; Decker, G.A.; Early, D.S.; Evans, J.A.; Fanelli, R.D.; Fisher, D.A.; Fisher, L.R.; Fukami, N.; et al. The role of endoscopy in the evaluation and treatment of patients with biliary neoplasia. Gastrointest. Endosc. 2013, 77, 167–174. [Google Scholar] [CrossRef]
- Toshiro, K.; Takeshi, F.; Shinji, S.; Chong, J.M.; Ken, K.; Yasuharu, K.; Sachiko, K.; Kazumasa, H.; Ken, S. Significant factors influencing the accuracy of PTCD bile cytology for bile duct lesions. J. Jpn. Soc. Clin. Cytol. 1998, 37, 156–161. [Google Scholar] [CrossRef]
- Weber, A.; von Weyhern, C.; Fend, F.; Schneider, J.; Neu, B.; Meining, A.; Weidenbach, H.; Schmid, R.M.; Prinz, C. Endoscopic transpapillary brush cytology and forceps biopsy in patients with hilar cholangiocarcinoma. World J. Gastroenterol. 2008, 14, 1097–1101. [Google Scholar] [CrossRef]
- Selvaggi, S. Biliary brushing cytology. Cytopathology 2004, 15, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Kubota, Y.; Takaoka, M.; Tani, K.; Ogura, M.; Kin, H.; Fujimura, K.; Mizuno, T.; Inoue, K. Endoscopic transpapillary biopsy for the diagnosis of patients with pancreaticobiliary ductal strictures. Am. J. Gastroenterol. 1993, 88, 1700–1704. [Google Scholar] [PubMed]
- Tamada, K.; Tomiyama, T.; Wada, S.; Ohashi, A.; Satoh, Y.; Ido, K.; Sugano, K. Endoscopic transpapillary bile duct biopsy with the combination of intraductal ultrasonography in the diagnosis of biliary strictures. Gut 2002, 50, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Domagk, D.; Poremba, C.; Dietl, K.; Senninger, N.; Heinecke, A.; Domaschke, W.; Menzel, J. Endoscopic transpapillary biopsies and intraductal ultrasonography in the diagnostics of bile duct strictures: A prospective study. Gut 2002, 51, 240–244. [Google Scholar] [CrossRef]
| Groups | |
|---|---|
| Number of patients | 37 |
| Age, median (IQR), years | 73(70–78.5) |
| Sex (male/female) | 27/10 |
| ECOG-PS (0/1/2) | (27/9/1) |
| Antithrombotic drug | 5 |
| Bile duct stricture (distal/portal) | 19/18 |
| Final Diagnosis | Malignant: 35 |
| Perihilar bile duct cancer: 16 | |
| Distal bile duct cancer: 12 | |
| Biliary stricture due to pancreatic ductal adenocarcinoma: 6 | |
| Biliary stricture due to lymph node metastasis of gallbladder cancer: 1 | |
| Benign: 2 | |
| Chronic pancreatitis: 1 | |
| Unknown etiology: 1 |
| Method | Number of Attempts | Specimen Collection Rate |
|---|---|---|
| Brush Cytology | 1 Attempt: 26 | Conventional: 70.3% |
| 2 Attempts: 6 | B-ROSE: 89.2% | |
| 3 Attempts: 5 | ||
| Biopsy | 1 Attempt: 35 | Conventional: 94.6% |
| 2 Attempts: 2 | B-ROSE: 97.2% |
| Group | Sensitivity | Specificity | Accuracy |
|---|---|---|---|
| Brush Cytology | 48.6% | 50.0% | 48.6% |
| Biopsy | 68.6% | 100.0% | 70.3% |
| B-ROSE (Combined) | 71.4% | 100.0% | 73.0% |
| Age | Sex | Papilla | Biliary Stricture | Brush Cytology (Times) | Biopsy (Times) | Final Diagnosis | Adverse Events | Therapy |
|---|---|---|---|---|---|---|---|---|
| 76 | F | Post EST | Distal | 1 | 1 | Bile duct cancer | Hyperamylasemia | Conservative |
| 77 | M | Post EST | Distal | 1 | 1 | Bile duct cancer | Hyperamylasemia | Conservative |
| 78 | F | Intact | Distal | 1 | 1 | Pancreatic cancer | Pancreatitis (moderate) | Conservative |
| 73 | M | Post EST | Distal | 1 | 1 | Chronic pancreatitis | Pancreatitis (mild) | Conservative |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hattori, N.; Uchida, D.; Harada, K.; Sato, R.; Obata, T.; Matsumi, A.; Miyamoto, K.; Terasawa, H.; Fujii, Y.; Tsutsumi, K.; et al. Evaluation of the Diagnostic Performance of the Brush/Biopsy Rapid On-Site Evaluation (B-ROSE) in Cases of Bile Duct Stricture: A Prospective, Pilot Study. J. Clin. Med. 2025, 14, 6207. https://doi.org/10.3390/jcm14176207
Hattori N, Uchida D, Harada K, Sato R, Obata T, Matsumi A, Miyamoto K, Terasawa H, Fujii Y, Tsutsumi K, et al. Evaluation of the Diagnostic Performance of the Brush/Biopsy Rapid On-Site Evaluation (B-ROSE) in Cases of Bile Duct Stricture: A Prospective, Pilot Study. Journal of Clinical Medicine. 2025; 14(17):6207. https://doi.org/10.3390/jcm14176207
Chicago/Turabian StyleHattori, Nao, Daisuke Uchida, Kei Harada, Ryosuke Sato, Taisuke Obata, Akihiro Matsumi, Kazuya Miyamoto, Hiroyuki Terasawa, Yuki Fujii, Koichiro Tsutsumi, and et al. 2025. "Evaluation of the Diagnostic Performance of the Brush/Biopsy Rapid On-Site Evaluation (B-ROSE) in Cases of Bile Duct Stricture: A Prospective, Pilot Study" Journal of Clinical Medicine 14, no. 17: 6207. https://doi.org/10.3390/jcm14176207
APA StyleHattori, N., Uchida, D., Harada, K., Sato, R., Obata, T., Matsumi, A., Miyamoto, K., Terasawa, H., Fujii, Y., Tsutsumi, K., Horiguchi, S., Matsumoto, K., & Otsuka, M. (2025). Evaluation of the Diagnostic Performance of the Brush/Biopsy Rapid On-Site Evaluation (B-ROSE) in Cases of Bile Duct Stricture: A Prospective, Pilot Study. Journal of Clinical Medicine, 14(17), 6207. https://doi.org/10.3390/jcm14176207






